Epigenetics and Cancer: Mechanisms, Aberrations, and Reprogramming
VerifiedAdded on 2023/06/03
|16
|3968
|241
Report
AI Summary
This report provides a comprehensive overview of the role of epigenetics in cancer development. It begins with an introduction to epigenetic programming and its importance in maintaining cell function, highlighting the significance of epigenetic alterations in cancer alongside genetic changes. The literature review covers key epigenetic mechanisms, including DNA methylation, histone modifications, nucleosome positioning, and micro-RNAs, and their influence on gene expression. The report then delves into aberrant reprogramming of the epigenome in cancer, examining changes in histone modifications, DNA methylation abnormalities, and epigenetic switching. It discusses how alterations in these mechanisms contribute to the initiation and progression of cancer, emphasizing the potential for epigenetic-based therapeutic interventions. The report includes figures illustrating epigenetic gene silencing mechanisms and DNA methylation changes in cancer, along with a list of abbreviations to aid understanding. The conclusion synthesizes the findings, underscoring the importance of understanding epigenetic processes for developing novel cancer treatments.

Epigenetics 1
Epigenetics and Cancer
By:
Student ID:
Course No:
Tutor:
Date:
Epigenetics and Cancer
By:
Student ID:
Course No:
Tutor:
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Epigenetics 2
Table of Contents
List of Figures............................................................................................................................2
List of Abbreviations..................................................................................................................3
Abstract......................................................................................................................................4
1.0 Introduction..........................................................................................................................5
2.0 Literature review..................................................................................................................6
2.1 Epigenetic mechanisms in Cancer....................................................................................6
2.1.1 DNA methylation.......................................................................................................6
2.1.2 Histone modifications................................................................................................6
2.1.3 Nucleosome positioning............................................................................................8
2.1.4 Micro-RNAs..............................................................................................................9
2.2 Aberrant reprogramming of the epigenome in cancer....................................................10
2.2.1 Changes in histone modifications in cancer............................................................10
2.2.2 DNA methylation abnormalities in cancer..............................................................10
2.2.3 Epigenetic switching in cancer................................................................................11
Conclusion................................................................................................................................12
References................................................................................................................................14
List of Figures
Figure 2.1 Epigenetic gene silencing mechanisms in mammals................................................8
Figure 2.2 DNA methylation changes in cancer........................................................................9
Table of Contents
List of Figures............................................................................................................................2
List of Abbreviations..................................................................................................................3
Abstract......................................................................................................................................4
1.0 Introduction..........................................................................................................................5
2.0 Literature review..................................................................................................................6
2.1 Epigenetic mechanisms in Cancer....................................................................................6
2.1.1 DNA methylation.......................................................................................................6
2.1.2 Histone modifications................................................................................................6
2.1.3 Nucleosome positioning............................................................................................8
2.1.4 Micro-RNAs..............................................................................................................9
2.2 Aberrant reprogramming of the epigenome in cancer....................................................10
2.2.1 Changes in histone modifications in cancer............................................................10
2.2.2 DNA methylation abnormalities in cancer..............................................................10
2.2.3 Epigenetic switching in cancer................................................................................11
Conclusion................................................................................................................................12
References................................................................................................................................14
List of Figures
Figure 2.1 Epigenetic gene silencing mechanisms in mammals................................................8
Figure 2.2 DNA methylation changes in cancer........................................................................9

Epigenetics 3
List of Abbreviations
miRNA - Micro-Ribonucleic acid
DNMTs - DNA methyltransferases
LOI - loss of imprinting
NFRs - Nucleosome-free regions
HDACs - Histone deacetylases
HATs - histone acetyltransferase
List of Abbreviations
miRNA - Micro-Ribonucleic acid
DNMTs - DNA methyltransferases
LOI - loss of imprinting
NFRs - Nucleosome-free regions
HDACs - Histone deacetylases
HATs - histone acetyltransferase
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Epigenetics 4
Abstract
Epigenetic processes are necessary for the general progression and sustenance of the tissue-
specific gene manifestation arrays in mammals. The interruption of the epigenetic mechanism
is likely to result in modified gene function and harmful cellular alterations. Recent studies
have attributed cancer to epigenetic abnormalities in addition to the conventional view of it
being a genetic disease only. As a result, there is evidence of widespread reprogramming of
each aspect of the epigenetic mechanism such as DNA methylation, histone modifications,
nucleosome positioning and miRNA expression. This review covers the epigenetic gene
control as influenced by these expressions in relation to cancer.
Abstract
Epigenetic processes are necessary for the general progression and sustenance of the tissue-
specific gene manifestation arrays in mammals. The interruption of the epigenetic mechanism
is likely to result in modified gene function and harmful cellular alterations. Recent studies
have attributed cancer to epigenetic abnormalities in addition to the conventional view of it
being a genetic disease only. As a result, there is evidence of widespread reprogramming of
each aspect of the epigenetic mechanism such as DNA methylation, histone modifications,
nucleosome positioning and miRNA expression. This review covers the epigenetic gene
control as influenced by these expressions in relation to cancer.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Epigenetics 5
1.0 Introduction
Epigenetic programming is key in the progression of mammals, and constant inheritance of
epigenetic structures is fundamental for the sustenance of the specific functions of cells and
tissues (Verhoeven et al., 2016). Almost all processes of differentiation except regulated
genomic alterations, e.g. the immunoglobulin and T-cell receptor genes in B and T cells are
triggered and sustained via the process of epigenetics. This, therefore, implies that epigenetic
gene control is marked by an elevated level of stability and integrity. Several studies have
consented that the inherent stability is as a result, numerous interlocking response
mechanisms between epigenetic layers that are not associated with function-wise and histone
altering enzymes. Cancer has largely been described as the expression of both epigenetic and
genetic alterations (Hatano et al., 2015; Plass et al., 2013). Even though the commencement
of cancer and development are primarily determined by acquired genetic changes, it is now
more evident that significant roles are played in neoplastic progression by the
microenvironment facilitated epigenetic distresses (Shukla et al., 2014). Januar et al. (2015)
define epigenetics as inherited alterations in gene manifestation activity and manifestation
that takes place without making modifying the DNA sequences, yet adequate to control the
dynamics of gene manifestation. The fundamental procedures involved in epigenetic control
included DNA methylation, alterations in chromatin, physical alterations (nucleosome
positioning), and micro-RNAs (Matzke and Mosher, 2014). Multiple and organised
epigenetic changes are related with abnormal gene roles and modified patterns of gene
manifestation that have significant functions in the pathology of cancer. This review covers
the epigenetic gene control as influenced by DNA methylation, alterations in chromatin,
physical modifications (nucleosome positioning), and micro-RNAs, in addition to aberrant
reprogramming of the epigenome in cancer. A proper insight into the molecular details
1.0 Introduction
Epigenetic programming is key in the progression of mammals, and constant inheritance of
epigenetic structures is fundamental for the sustenance of the specific functions of cells and
tissues (Verhoeven et al., 2016). Almost all processes of differentiation except regulated
genomic alterations, e.g. the immunoglobulin and T-cell receptor genes in B and T cells are
triggered and sustained via the process of epigenetics. This, therefore, implies that epigenetic
gene control is marked by an elevated level of stability and integrity. Several studies have
consented that the inherent stability is as a result, numerous interlocking response
mechanisms between epigenetic layers that are not associated with function-wise and histone
altering enzymes. Cancer has largely been described as the expression of both epigenetic and
genetic alterations (Hatano et al., 2015; Plass et al., 2013). Even though the commencement
of cancer and development are primarily determined by acquired genetic changes, it is now
more evident that significant roles are played in neoplastic progression by the
microenvironment facilitated epigenetic distresses (Shukla et al., 2014). Januar et al. (2015)
define epigenetics as inherited alterations in gene manifestation activity and manifestation
that takes place without making modifying the DNA sequences, yet adequate to control the
dynamics of gene manifestation. The fundamental procedures involved in epigenetic control
included DNA methylation, alterations in chromatin, physical alterations (nucleosome
positioning), and micro-RNAs (Matzke and Mosher, 2014). Multiple and organised
epigenetic changes are related with abnormal gene roles and modified patterns of gene
manifestation that have significant functions in the pathology of cancer. This review covers
the epigenetic gene control as influenced by DNA methylation, alterations in chromatin,
physical modifications (nucleosome positioning), and micro-RNAs, in addition to aberrant
reprogramming of the epigenome in cancer. A proper insight into the molecular details

Epigenetics 6
surrounding epigenetic cancer illnesses is significant for medical interventions because it
permits novel strategies for the development of drugs (Hon et al., 2013).
2.0 Literature review
2.1 Epigenetic mechanisms in Cancer
2.1.1 DNA methylation
DNA methylation is a significant element of the epigenetic mechanism in controlling gene
manifestation and co-operating with nucleosomes that regulate DNA packaging influencing
all the domains of DNA (Delpu et al., 2013). The DNA methylation in mammal cells takes
place at the fifth place of the cytosine ring in the CpG dinucleotides through an accumulation
of a methyl group leading to the formation of 5-methylcytosine (Smith and Meissner, 2013).
The DNA methyltransferases (DNMTs) enzymes catalyse the alterations that occur at 5-
methylcytosine. Horvath (2013) observed that three major DNMTs exist namely DNMT1,
DNMT3a, and DNMT3b. According to Suzuki and Bird (2008), the DNMT1 is a primary
sustaining enzyme that protects the available methylation arrays after DNA imitation by
integrating methyl groups to the CpG spots that are hemimethylated. Both DNMT3a and
DNMT3b start methylation by targeting unmethylated CpGs and their significant
manifestations occur during embryogenesis and are least manifested in adult tissues (Horvath,
2013). Even though DNA methylation controls the manifestation of genes in normal cells via
genomic imprinting and the inactivation of the female X-chromosome, these mechanisms are
substantially modified in cancer due to the loss of imprinting (LOI) (Jeltsch and Jurkowska,
2014).
2.1.2 Histone modifications
Histone proteins are made up of the nucleosome core that has a globular C-terminal area and
the N- end tail that is not structured (Deindl et al., 2013). The N-end tails of the histones go
through several posttranslational covalent alterations in addition to methylation and
ubiquitylation (Zentner and Henikoff, 2013). It is these alterations that are responsible for the
surrounding epigenetic cancer illnesses is significant for medical interventions because it
permits novel strategies for the development of drugs (Hon et al., 2013).
2.0 Literature review
2.1 Epigenetic mechanisms in Cancer
2.1.1 DNA methylation
DNA methylation is a significant element of the epigenetic mechanism in controlling gene
manifestation and co-operating with nucleosomes that regulate DNA packaging influencing
all the domains of DNA (Delpu et al., 2013). The DNA methylation in mammal cells takes
place at the fifth place of the cytosine ring in the CpG dinucleotides through an accumulation
of a methyl group leading to the formation of 5-methylcytosine (Smith and Meissner, 2013).
The DNA methyltransferases (DNMTs) enzymes catalyse the alterations that occur at 5-
methylcytosine. Horvath (2013) observed that three major DNMTs exist namely DNMT1,
DNMT3a, and DNMT3b. According to Suzuki and Bird (2008), the DNMT1 is a primary
sustaining enzyme that protects the available methylation arrays after DNA imitation by
integrating methyl groups to the CpG spots that are hemimethylated. Both DNMT3a and
DNMT3b start methylation by targeting unmethylated CpGs and their significant
manifestations occur during embryogenesis and are least manifested in adult tissues (Horvath,
2013). Even though DNA methylation controls the manifestation of genes in normal cells via
genomic imprinting and the inactivation of the female X-chromosome, these mechanisms are
substantially modified in cancer due to the loss of imprinting (LOI) (Jeltsch and Jurkowska,
2014).
2.1.2 Histone modifications
Histone proteins are made up of the nucleosome core that has a globular C-terminal area and
the N- end tail that is not structured (Deindl et al., 2013). The N-end tails of the histones go
through several posttranslational covalent alterations in addition to methylation and
ubiquitylation (Zentner and Henikoff, 2013). It is these alterations that are responsible for the
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Epigenetics 7
regulation of the primary processes that take place in the cells such as imitation, transcription
and restoration (Zentner and Henikoff, 2013). It is suggested that complements of alterations
reserve the epigenetic memory in a cell in the form of a ‘histocode’ that regulates the
organization and function of varying areas of the chromatin (Kalashnikova, 2016). The
alterations of the histone are achieved through altering chromatin accessibility or by
integrating non-histone effector protein, which decodes the response encoded by the changing
arrays. However, the inheritance process of this histone code is yet to be understood
comprehensively. Histone alteration can result in domination or initiation unlike DNA
methylation, but this is dependent on the type of residues that are changed and the nature of
existing modifications (Zentner and Henikoff, 2013). For instance, H3K27 (H3K27me3) and
H3K9 (H3K9me3) exists at transcriptionally suppressed gene promoters, and both of their
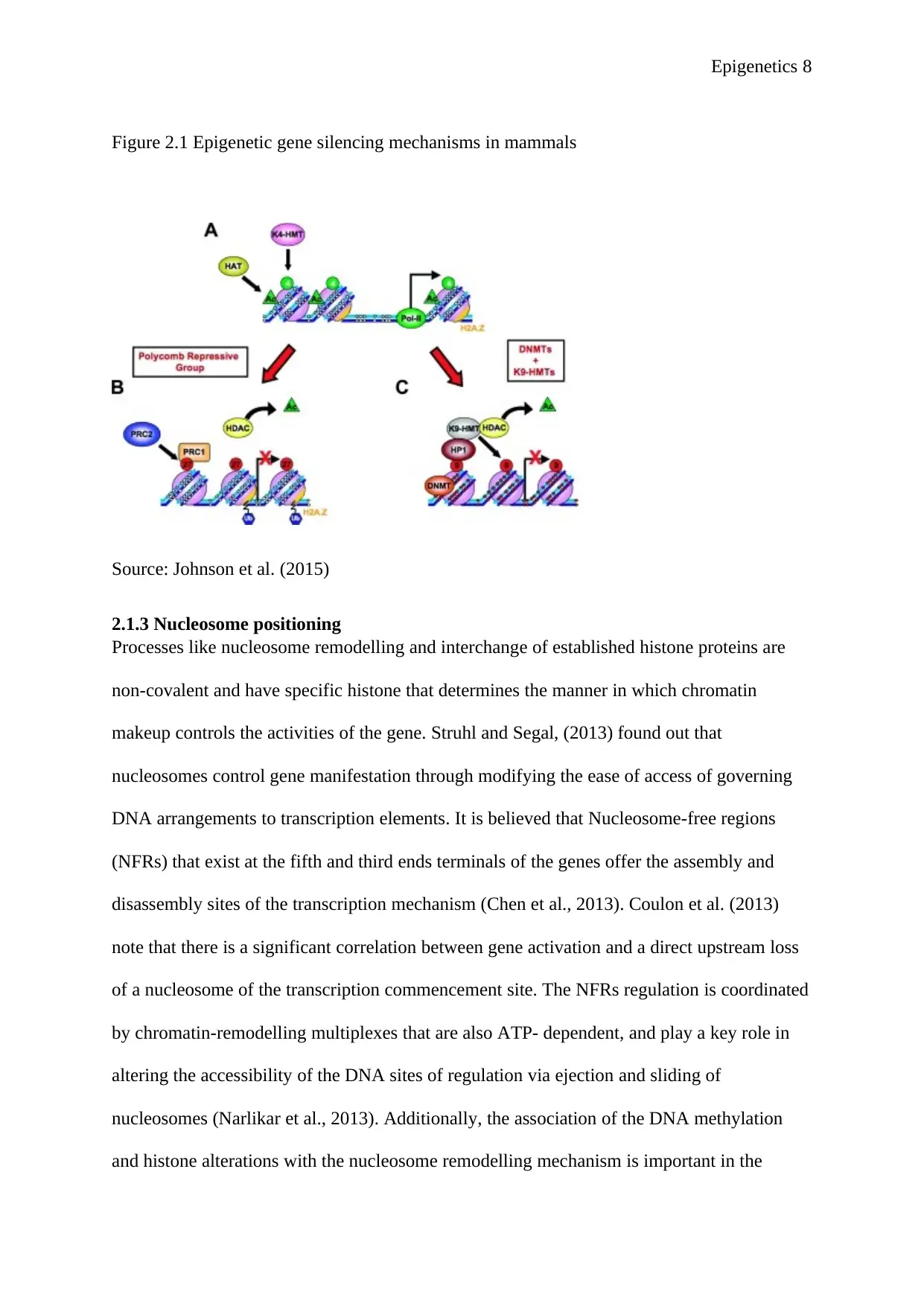
alterations make up the two major silencing process in mammalian cells (Figure 2.1). Histone
alternations have some patterns that exist in specific cell types and are suggested to have a
significant function in ascertaining cellular identity (Ramos et al., 2013).
regulation of the primary processes that take place in the cells such as imitation, transcription
and restoration (Zentner and Henikoff, 2013). It is suggested that complements of alterations
reserve the epigenetic memory in a cell in the form of a ‘histocode’ that regulates the
organization and function of varying areas of the chromatin (Kalashnikova, 2016). The
alterations of the histone are achieved through altering chromatin accessibility or by
integrating non-histone effector protein, which decodes the response encoded by the changing
arrays. However, the inheritance process of this histone code is yet to be understood
comprehensively. Histone alteration can result in domination or initiation unlike DNA
methylation, but this is dependent on the type of residues that are changed and the nature of
existing modifications (Zentner and Henikoff, 2013). For instance, H3K27 (H3K27me3) and
H3K9 (H3K9me3) exists at transcriptionally suppressed gene promoters, and both of their
alterations make up the two major silencing process in mammalian cells (Figure 2.1). Histone
alternations have some patterns that exist in specific cell types and are suggested to have a
significant function in ascertaining cellular identity (Ramos et al., 2013).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Epigenetics 8
Figure 2.1 Epigenetic gene silencing mechanisms in mammals
Figure 1 Figure 2.1 Epigenetic gene silencing mechanisms in mammals
Source: Johnson et al. (2015)
2.1.3 Nucleosome positioning
Processes like nucleosome remodelling and interchange of established histone proteins are
non-covalent and have specific histone that determines the manner in which chromatin
makeup controls the activities of the gene. Struhl and Segal, (2013) found out that
nucleosomes control gene manifestation through modifying the ease of access of governing
DNA arrangements to transcription elements. It is believed that Nucleosome-free regions
(NFRs) that exist at the fifth and third ends terminals of the genes offer the assembly and
disassembly sites of the transcription mechanism (Chen et al., 2013). Coulon et al. (2013)
note that there is a significant correlation between gene activation and a direct upstream loss
of a nucleosome of the transcription commencement site. The NFRs regulation is coordinated
by chromatin-remodelling multiplexes that are also ATP- dependent, and play a key role in
altering the accessibility of the DNA sites of regulation via ejection and sliding of
nucleosomes (Narlikar et al., 2013). Additionally, the association of the DNA methylation
and histone alterations with the nucleosome remodelling mechanism is important in the
Figure 2.1 Epigenetic gene silencing mechanisms in mammals
Figure 1 Figure 2.1 Epigenetic gene silencing mechanisms in mammals
Source: Johnson et al. (2015)
2.1.3 Nucleosome positioning
Processes like nucleosome remodelling and interchange of established histone proteins are
non-covalent and have specific histone that determines the manner in which chromatin
makeup controls the activities of the gene. Struhl and Segal, (2013) found out that
nucleosomes control gene manifestation through modifying the ease of access of governing
DNA arrangements to transcription elements. It is believed that Nucleosome-free regions
(NFRs) that exist at the fifth and third ends terminals of the genes offer the assembly and
disassembly sites of the transcription mechanism (Chen et al., 2013). Coulon et al. (2013)
note that there is a significant correlation between gene activation and a direct upstream loss
of a nucleosome of the transcription commencement site. The NFRs regulation is coordinated
by chromatin-remodelling multiplexes that are also ATP- dependent, and play a key role in
altering the accessibility of the DNA sites of regulation via ejection and sliding of
nucleosomes (Narlikar et al., 2013). Additionally, the association of the DNA methylation
and histone alterations with the nucleosome remodelling mechanism is important in the
Epigenetics 9
developing worldwide patterns of gene expression and chromatin structure (Figure 2.2 )
(Milagro et al., 2013). Lawrence et al. (2016) also showed that the integration of histone
alternatives such as H2A.Z and H3.3, into nucleosomes, alongside physical changes in
nucleosomal settings through nucleosome remodelers, affect nucleosome occupancy and
hence gene activity.
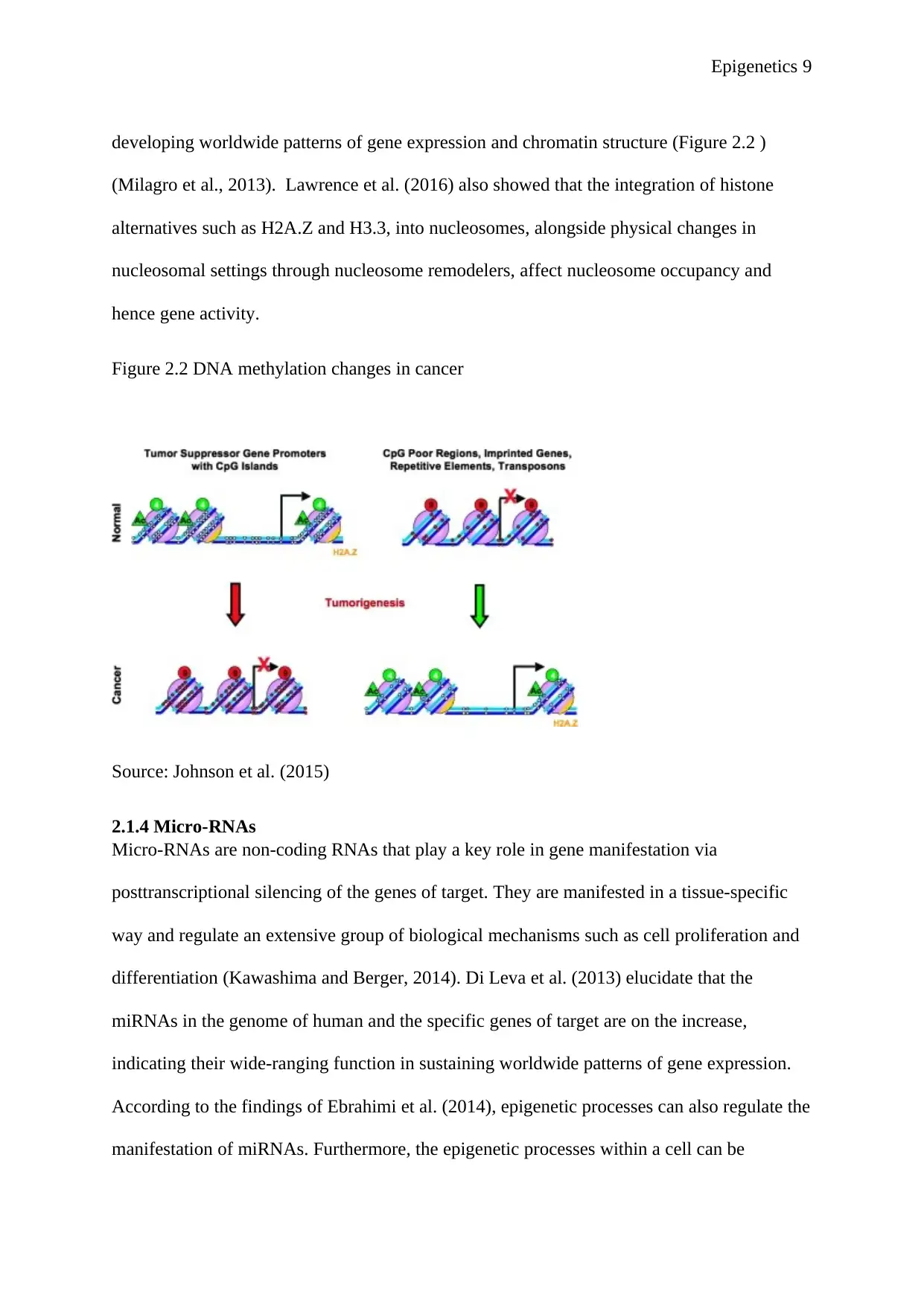
Figure 2.2 DNA methylation changes in cancer
Figure 2 Figure 2.2 DNA methylation changes in cancer
Source: Johnson et al. (2015)
2.1.4 Micro-RNAs
Micro-RNAs are non-coding RNAs that play a key role in gene manifestation via
posttranscriptional silencing of the genes of target. They are manifested in a tissue-specific
way and regulate an extensive group of biological mechanisms such as cell proliferation and
differentiation (Kawashima and Berger, 2014). Di Leva et al. (2013) elucidate that the
miRNAs in the genome of human and the specific genes of target are on the increase,
indicating their wide-ranging function in sustaining worldwide patterns of gene expression.
According to the findings of Ebrahimi et al. (2014), epigenetic processes can also regulate the
manifestation of miRNAs. Furthermore, the epigenetic processes within a cell can be
developing worldwide patterns of gene expression and chromatin structure (Figure 2.2 )
(Milagro et al., 2013). Lawrence et al. (2016) also showed that the integration of histone
alternatives such as H2A.Z and H3.3, into nucleosomes, alongside physical changes in
nucleosomal settings through nucleosome remodelers, affect nucleosome occupancy and
hence gene activity.
Figure 2.2 DNA methylation changes in cancer
Figure 2 Figure 2.2 DNA methylation changes in cancer
Source: Johnson et al. (2015)
2.1.4 Micro-RNAs
Micro-RNAs are non-coding RNAs that play a key role in gene manifestation via
posttranscriptional silencing of the genes of target. They are manifested in a tissue-specific
way and regulate an extensive group of biological mechanisms such as cell proliferation and
differentiation (Kawashima and Berger, 2014). Di Leva et al. (2013) elucidate that the
miRNAs in the genome of human and the specific genes of target are on the increase,
indicating their wide-ranging function in sustaining worldwide patterns of gene expression.
According to the findings of Ebrahimi et al. (2014), epigenetic processes can also regulate the
manifestation of miRNAs. Furthermore, the epigenetic processes within a cell can be
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Epigenetics 10
regulated by the miRNAs by targeting the enzymes associated with DNA methylation and
histone alterations (Xu et al., 2014). The association among the diverse elements of the
epigenetic mechanism underscores the cohesive type of epigenetic processes that are utilised
in sustaining universal gene manifestation patterns.
2.2 Aberrant reprogramming of the epigenome in cancer
The specific epigenomic setting that exists in normal cells goes through a widespread
alteration in cancer (Shen and Laird, 2013). It is such transformations in addition to the
extensive genetic modifications that have a significant purpose in the instigation and
progression of cancer.
2.2.1 Changes in histone modifications in cancer
The advancement in studies on sequencing has led to extensive screening of chromatin
alterations that take place during tumorigenesis. As a result, there is evidence of the massive
loss of various forms of histone acetylation including acetylated H4-lysine 16 (Johnson et al.,
2015). This big depletion of histone acetylation, facilitated by Histone deacetylases
(HDACs), has led to gene repression. Tang et al. (2013) observed overexpression of HDACs
in multiple cancer forms, and have consequently been the main focus of epigenetic therapy.
The levels of histone acetylation are maintained by both HDACs and histone
acetyltransferase (HATs). However, these levels are changed in cancer. The development of
abnormal fusion proteins via chromosomal alteration of HAT and associated genes takes
place in leukaemia (Mar et al., 2014). Cancer cells similarly indicate extensive modifications
in the patters of histone methylation besides the alterations in histone acetylation.
2.2.2 DNA methylation abnormalities in cancer
The commencement and development of cancer go along with substantial alterations in DNA
methylation which formed the initial epigenetic modifications noticed in cancer (Yang et al.,
2013). An epigenome that is cancerous can be identified by an extensive genome
regulated by the miRNAs by targeting the enzymes associated with DNA methylation and
histone alterations (Xu et al., 2014). The association among the diverse elements of the
epigenetic mechanism underscores the cohesive type of epigenetic processes that are utilised
in sustaining universal gene manifestation patterns.
2.2 Aberrant reprogramming of the epigenome in cancer
The specific epigenomic setting that exists in normal cells goes through a widespread
alteration in cancer (Shen and Laird, 2013). It is such transformations in addition to the
extensive genetic modifications that have a significant purpose in the instigation and
progression of cancer.
2.2.1 Changes in histone modifications in cancer
The advancement in studies on sequencing has led to extensive screening of chromatin
alterations that take place during tumorigenesis. As a result, there is evidence of the massive
loss of various forms of histone acetylation including acetylated H4-lysine 16 (Johnson et al.,
2015). This big depletion of histone acetylation, facilitated by Histone deacetylases
(HDACs), has led to gene repression. Tang et al. (2013) observed overexpression of HDACs
in multiple cancer forms, and have consequently been the main focus of epigenetic therapy.
The levels of histone acetylation are maintained by both HDACs and histone
acetyltransferase (HATs). However, these levels are changed in cancer. The development of
abnormal fusion proteins via chromosomal alteration of HAT and associated genes takes
place in leukaemia (Mar et al., 2014). Cancer cells similarly indicate extensive modifications
in the patters of histone methylation besides the alterations in histone acetylation.
2.2.2 DNA methylation abnormalities in cancer
The commencement and development of cancer go along with substantial alterations in DNA
methylation which formed the initial epigenetic modifications noticed in cancer (Yang et al.,
2013). An epigenome that is cancerous can be identified by an extensive genome
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Epigenetics 11
hypomethylation and CpG island promoter hypermethylation that is site-specific (Matzke and
Mosher, 2014) as indicated in figure 2.2 above. Research has also shown that some
modifications take place during the initial stages of the progression of cancer and are likely to
foster the beginning of cancer (Matzke and Mosher, 2014). The authors point out that the
continual repetition of DNA hypomethylation causes an increase in the instability of the
genome by enhancing chromosomal re-positioning. Moreover, genomic instability can be
increased by the activation and translocation processes caused by the hypomethylation of
retrotransposons (Chénais et al., 2013). Most cancers in human have also indicated the loss of
DNA methylation and genomic instability. Additionally, DNA hypomethylation can result in
the activation of genes that foster growth such as MAPSIN found in gastric cancer and loss of
imprinting (LOI) in tumours (Rouhani et al., 2014).
Unlike hypothymelation which promotes genomic instability and triggers proto-oncogenes,
the hyperthymelation which are also site-specific play a vital function in tumorigenesis
through silencing the genes that subdue tumours. Examples of discovered genes that suppress
tumours are p16, MLH1 and BRCA1. Studies by Plass et al. (2013) indicate that these genes
also go through silencing that is tumour-specific and fostered through hypermethylation.
Furthermore, the genes are active in various processes within the cells, which are central to
the initiation and growth of cancer, as well to DNA repair, cell linkage and cell cycle.
2.2.3 Epigenetic switching in cancer
It is clearly understood that genetic changes in neoplastic cells only cannot independently be
used to explore the numerous steps of carcinogenesis through which tumour cells manifest
different phenotypes during the intricate mechanism of the initiation and development of
tumours. Cancer cells possess a modified epigenotype different from the one they originate
from. The epigenetic switch comprises alterations in the level and location of DNA
methylation and histone changes, and these modifications affect the phenotype of the
hypomethylation and CpG island promoter hypermethylation that is site-specific (Matzke and
Mosher, 2014) as indicated in figure 2.2 above. Research has also shown that some
modifications take place during the initial stages of the progression of cancer and are likely to
foster the beginning of cancer (Matzke and Mosher, 2014). The authors point out that the
continual repetition of DNA hypomethylation causes an increase in the instability of the
genome by enhancing chromosomal re-positioning. Moreover, genomic instability can be
increased by the activation and translocation processes caused by the hypomethylation of
retrotransposons (Chénais et al., 2013). Most cancers in human have also indicated the loss of
DNA methylation and genomic instability. Additionally, DNA hypomethylation can result in
the activation of genes that foster growth such as MAPSIN found in gastric cancer and loss of
imprinting (LOI) in tumours (Rouhani et al., 2014).
Unlike hypothymelation which promotes genomic instability and triggers proto-oncogenes,
the hyperthymelation which are also site-specific play a vital function in tumorigenesis
through silencing the genes that subdue tumours. Examples of discovered genes that suppress
tumours are p16, MLH1 and BRCA1. Studies by Plass et al. (2013) indicate that these genes
also go through silencing that is tumour-specific and fostered through hypermethylation.
Furthermore, the genes are active in various processes within the cells, which are central to
the initiation and growth of cancer, as well to DNA repair, cell linkage and cell cycle.
2.2.3 Epigenetic switching in cancer
It is clearly understood that genetic changes in neoplastic cells only cannot independently be
used to explore the numerous steps of carcinogenesis through which tumour cells manifest
different phenotypes during the intricate mechanism of the initiation and development of
tumours. Cancer cells possess a modified epigenotype different from the one they originate
from. The epigenetic switch comprises alterations in the level and location of DNA
methylation and histone changes, and these modifications affect the phenotype of the

Epigenetics 12
neoplastic cells (Suvà et al., 2013). Most of the cancer cells obtain modified levels of
manifestation of epigenetic enzymes. However, the outcomes of their feedback do not tally
the phenotype, indicating the presence of other factors that influence their activity (Esteller,
2008). There has existed a consensus that tumour cells have a universal hypomethylated
genome, whereas there is evidence of a simultaneous increase in focal cytosine methylation
in some areas of the genome (Hatano et al., 2015). The CpGs in the duplicative DNA
components and regions of coding of the genes are methylated in normal cells, while in
tumour cells LINE-1 duplicates, averagely duplicate DNA structures are not methylated,
making them transcriptionally silent. There are also relative variations in histone alterations
in cancer (Plass et al., 2013). The deamination of methylated cytosine develops thymine,
leading to the development of a laceration that is challenging to reverse because the DNA
repair processes are unable to identify the correct base in the final G: T mismatch. Epigenetic
changes in cancer are likely to influence genome stability, providing a connection between
the genome’s structure and its imitation and reparation. Most of the theoretical work has
explored the features of such modifications, but, the specific cause of the epigenetic switch in
cancer is yet to be fully explored.
Conclusion
The significance of epigenetics in cancer has received a lot of emphasis and research has
equally advanced over the last decades. The latest breakthrough in epigenomic methods
enables screening of the methylation/acetylation state and miRNA states with great
exactitude, which is fundamental in the determination of the biomarkers of other illnesses. s
Furthermore, the inherent change of epigenetic modifications is a better chance for the
innovation of improved approaches for the prevention and management of cancer
neoplastic cells (Suvà et al., 2013). Most of the cancer cells obtain modified levels of
manifestation of epigenetic enzymes. However, the outcomes of their feedback do not tally
the phenotype, indicating the presence of other factors that influence their activity (Esteller,
2008). There has existed a consensus that tumour cells have a universal hypomethylated
genome, whereas there is evidence of a simultaneous increase in focal cytosine methylation
in some areas of the genome (Hatano et al., 2015). The CpGs in the duplicative DNA
components and regions of coding of the genes are methylated in normal cells, while in
tumour cells LINE-1 duplicates, averagely duplicate DNA structures are not methylated,
making them transcriptionally silent. There are also relative variations in histone alterations
in cancer (Plass et al., 2013). The deamination of methylated cytosine develops thymine,
leading to the development of a laceration that is challenging to reverse because the DNA
repair processes are unable to identify the correct base in the final G: T mismatch. Epigenetic
changes in cancer are likely to influence genome stability, providing a connection between
the genome’s structure and its imitation and reparation. Most of the theoretical work has
explored the features of such modifications, but, the specific cause of the epigenetic switch in
cancer is yet to be fully explored.
Conclusion
The significance of epigenetics in cancer has received a lot of emphasis and research has
equally advanced over the last decades. The latest breakthrough in epigenomic methods
enables screening of the methylation/acetylation state and miRNA states with great
exactitude, which is fundamental in the determination of the biomarkers of other illnesses. s
Furthermore, the inherent change of epigenetic modifications is a better chance for the
innovation of improved approaches for the prevention and management of cancer
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



