Monitoring Corrosion
VerifiedAdded on 2022/11/27
|33
|7396
|181
AI Summary
This report discusses the monitoring and limiting of corrosion in marine environments. It covers factors affecting corrosion, such as humidity and pollutants, as well as the nature and reactivity of metals. The presence of an electrolyte is also explored as a trigger for corrosion. The report provides insights into the impact of corrosion on marine structures and the importance of monitoring and prevention.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running Header: Monitoring Corrosion 1
Report on Monitoring and Limiting Corrosion in Marine Environments.
Student’s Name
University Affiliation
Date
Report on Monitoring and Limiting Corrosion in Marine Environments.
Student’s Name
University Affiliation
Date
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Monitoring Corrosion 2
Part One: Monitoring and Limiting Corrosion in Marine Environments.
Introduction
Corrosion is a steady deterioration of metallic material by a chemical reaction with the
surroundings.
Corrosion oxidizes the metal into a more chemically stable form; the stable chemical
form could be its oxide, chloride, hydroxide, or sulphide. The corrosion of metals involves bond
formation with the nonmetals like oxygen and release of energy in the form of heat, hence
increasing the stability of the formed compounds (Talbort D.E & Talbort J.D., 2018). The
chemical process occurring entails the interaction of chemicals to form new compounds with
different compositions. Another mechanism which causes corrosion is the electrochemical
transfer of electrons between metals with a different potential difference in the presence of an
electrolyte. Corrosion of the less reactive metal will occur as a result of electrolytic reaction
which will develop
Oxidation reaction chemically
4 Fe (s) + 3O2 (g) → 2Fe2O3 (s)
Oxidation of zinc electrochemically
Zn (s) + 2H+ (aq.) → Zn2+ (aq.) + H2 (g)
Corrosion by oxidation interferes with the metal composition hence compromising the
structure of the metal. Metallic decay due to corrosion accounts for a major loss of equipment's,
machinery and structures. The destruction of structure in the marine environment has a
significant impact on the environment, which might be catastrophic (Morcillo et al., 2013).
Part One: Monitoring and Limiting Corrosion in Marine Environments.
Introduction
Corrosion is a steady deterioration of metallic material by a chemical reaction with the
surroundings.
Corrosion oxidizes the metal into a more chemically stable form; the stable chemical
form could be its oxide, chloride, hydroxide, or sulphide. The corrosion of metals involves bond
formation with the nonmetals like oxygen and release of energy in the form of heat, hence
increasing the stability of the formed compounds (Talbort D.E & Talbort J.D., 2018). The
chemical process occurring entails the interaction of chemicals to form new compounds with
different compositions. Another mechanism which causes corrosion is the electrochemical
transfer of electrons between metals with a different potential difference in the presence of an
electrolyte. Corrosion of the less reactive metal will occur as a result of electrolytic reaction
which will develop
Oxidation reaction chemically
4 Fe (s) + 3O2 (g) → 2Fe2O3 (s)
Oxidation of zinc electrochemically
Zn (s) + 2H+ (aq.) → Zn2+ (aq.) + H2 (g)
Corrosion by oxidation interferes with the metal composition hence compromising the
structure of the metal. Metallic decay due to corrosion accounts for a major loss of equipment's,
machinery and structures. The destruction of structure in the marine environment has a
significant impact on the environment, which might be catastrophic (Morcillo et al., 2013).

Monitoring Corrosion 3
Exposure of ships and all kind of sea locomotives to harsh sea conditions at sea, these
conditions are high humidity, relatively high temperature, salinity, winds, abrasion due to
movement in saline water and sea disturbance. All the named factors above have a significant
effect on the rate of corrosion in sea structures.
Factors Affecting Corrosion.
Humidity
Humidity is the amount of water vapour suspended in the air, water vapour is the gaseous
state of water. There are three ways of measuring humidity; relative, absolute, and specific.
Humidity at sea is high due to the continuous vaporization of the seawater by the sun's heat
during the day, and the relative warmth at night resulting from the heat absorbed by the water
masses during the day.
Vaporization process
H2O (l) + Heat energy → H2O (g)
Water vapour condenses on the metallic surface of the ships or metallic structures late at
night due lagging in temperature as the surrounding air rise, thus making them act as condensers.
Condensation occurs when warm rich with water vapor cools down, causing it to lose its capacity
to hold water.
Condensation process
H2O (g) → H2O (l) + Heat energy
A film of dew resulting from condensation of water vapour on the surface of the ship
coupled with sea salt or acid sulphates and acid chlorides present in the atmosphere provides a
Exposure of ships and all kind of sea locomotives to harsh sea conditions at sea, these
conditions are high humidity, relatively high temperature, salinity, winds, abrasion due to
movement in saline water and sea disturbance. All the named factors above have a significant
effect on the rate of corrosion in sea structures.
Factors Affecting Corrosion.
Humidity
Humidity is the amount of water vapour suspended in the air, water vapour is the gaseous
state of water. There are three ways of measuring humidity; relative, absolute, and specific.
Humidity at sea is high due to the continuous vaporization of the seawater by the sun's heat
during the day, and the relative warmth at night resulting from the heat absorbed by the water
masses during the day.
Vaporization process
H2O (l) + Heat energy → H2O (g)
Water vapour condenses on the metallic surface of the ships or metallic structures late at
night due lagging in temperature as the surrounding air rise, thus making them act as condensers.
Condensation occurs when warm rich with water vapor cools down, causing it to lose its capacity
to hold water.
Condensation process
H2O (g) → H2O (l) + Heat energy
A film of dew resulting from condensation of water vapour on the surface of the ship
coupled with sea salt or acid sulphates and acid chlorides present in the atmosphere provides a

Monitoring Corrosion 4
destructive electric conducting substance known as electrolyte, when the substances dissolve in
water they form anions and cations which facilitate electron transfer during corrosion (Leygraf et
al., 2016). Due to air pollution by industrial plants, chlorides are found in atmospheric gases and
suspended salt in the atmosphere. Chlorides have a corrosive effect on metals and are therefore
considered to be a harmful pollutant. The marine steel structure surfaces which slant horizontally
are affected the most than those which slant horizontally due to the retention of moisture and
atmospheric particle for a longer period (Talbot D & Talbot J., 2018).
The water vapor in the skies absorbs pollutants by reacting chemically to form weak acid
rains. Either way, the condensed water on the metallic surfaces of the structures combines with
the pollutants chemically. An example is Sulphur (IV) oxide gas, which dissolves in water or
water vapour to form a weak sulphuric (VI) acid.
Sulphur (VI) oxide gas dissolves in water form sulphuric acid.
SO3 (g) + H2O (l) → H2SO4 (aq.)
The weak acid reacts with the metallic parts of the structures to form their metallic salt,
which is soluble.
An example is an iron which is a component of steel which react with sulphuric (VI) acid
to give out iron (II) sulphate and hydrogen gas as products.
H2SO4 (aq.) + Fe (s) → FeSO4 (s) + H2 (g)
Aluminum reacts with sulphuric (VI) acid to give out aluminum sulphate and hydrogen gas.
H2SO4 (aq.) + Al2 (SO4)3 (s) + H2 (g)
destructive electric conducting substance known as electrolyte, when the substances dissolve in
water they form anions and cations which facilitate electron transfer during corrosion (Leygraf et
al., 2016). Due to air pollution by industrial plants, chlorides are found in atmospheric gases and
suspended salt in the atmosphere. Chlorides have a corrosive effect on metals and are therefore
considered to be a harmful pollutant. The marine steel structure surfaces which slant horizontally
are affected the most than those which slant horizontally due to the retention of moisture and
atmospheric particle for a longer period (Talbot D & Talbot J., 2018).
The water vapor in the skies absorbs pollutants by reacting chemically to form weak acid
rains. Either way, the condensed water on the metallic surfaces of the structures combines with
the pollutants chemically. An example is Sulphur (IV) oxide gas, which dissolves in water or
water vapour to form a weak sulphuric (VI) acid.
Sulphur (VI) oxide gas dissolves in water form sulphuric acid.
SO3 (g) + H2O (l) → H2SO4 (aq.)
The weak acid reacts with the metallic parts of the structures to form their metallic salt,
which is soluble.
An example is an iron which is a component of steel which react with sulphuric (VI) acid
to give out iron (II) sulphate and hydrogen gas as products.
H2SO4 (aq.) + Fe (s) → FeSO4 (s) + H2 (g)
Aluminum reacts with sulphuric (VI) acid to give out aluminum sulphate and hydrogen gas.
H2SO4 (aq.) + Al2 (SO4)3 (s) + H2 (g)
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Monitoring Corrosion 5
This product gets off the metal surface leaving behind a corroded surface which is
subjected to further corrosion, hence, depletion of the metal with time.
Pollutants
Pollutants are substances that pollute the environment by introducing undesirable
outcomes. They can be in solid, liquid, or gaseous form. These are either gases from motor
vehicles, poisonous waste from industrial. Gaseous pollution results majorly from the
combustion of fuels in power plants, factories, and automobiles (Morcillo et al., 2013). The gas
which is majorly released and found in industrialized countries are sulphur (IV) oxide, ozone,
nitrogen (IV) oxide. Others are chlorides (hydrogen chloride and chlorine gas).
These pollutants react with oxygen and water to form acids. An acid is a chemical
substance that gives protons or hydrogen ions and or accepts electrons, an acid dissociates in
water to yield a cation and anion.
An acid dissociation in water
HCl (aq.) + H2O (l) → H3O+ (aq.) + Cl- (aq.)
Oxides of carbon form carbonic acid, oxides of sulphur form sulphuric acid, and the
oxides of nitrogen form nitric acids (Leygraf et al., 2016)
Oxides of carbon reacting with water to form carbonic acid
CO2 (g) + H2O (l) → H2CO3 (aq.)
Cation Anion
This product gets off the metal surface leaving behind a corroded surface which is
subjected to further corrosion, hence, depletion of the metal with time.
Pollutants
Pollutants are substances that pollute the environment by introducing undesirable
outcomes. They can be in solid, liquid, or gaseous form. These are either gases from motor
vehicles, poisonous waste from industrial. Gaseous pollution results majorly from the
combustion of fuels in power plants, factories, and automobiles (Morcillo et al., 2013). The gas
which is majorly released and found in industrialized countries are sulphur (IV) oxide, ozone,
nitrogen (IV) oxide. Others are chlorides (hydrogen chloride and chlorine gas).
These pollutants react with oxygen and water to form acids. An acid is a chemical
substance that gives protons or hydrogen ions and or accepts electrons, an acid dissociates in
water to yield a cation and anion.
An acid dissociation in water
HCl (aq.) + H2O (l) → H3O+ (aq.) + Cl- (aq.)
Oxides of carbon form carbonic acid, oxides of sulphur form sulphuric acid, and the
oxides of nitrogen form nitric acids (Leygraf et al., 2016)
Oxides of carbon reacting with water to form carbonic acid
CO2 (g) + H2O (l) → H2CO3 (aq.)
Cation Anion

Monitoring Corrosion 6
The sulphur (IV) oxide released by the car exhaust and industrial plants get oxidized to
sulphur (VI) oxide first.
SO2 + O2 (g) → SO3 (g)
The formed oxide of sulphur (sulphur (VI) oxide gas) reacts with water to sulphuric (IV)
acid.
SO3 (g) + H2O (l) → H2SO4 (aq.)
Oxide of nitrogen forming nitric acid
3NO2 (g) + H2O (l) → 2HNO3 (aq.) + NO (g)
Oxides of chlorine reacting with water to form acid, chlorine (VII) oxide reacts with
water to form chloric (VII) acid, which is a powerful acid.
Cl2O7 (g) + H2O (l) → 2HClO4 (aq.)
A compound capable of donating free chlorine ions (Cl-) to a water-based aqueous
solution is potential to cause corrosion in steel. Chlorine is highly electronegative. It has a
high tendency to attract electrons from other elements to itself hence resulting in corrosion.
Chlorine ions form a compound with iron which is soluble in water resulting in degradation of
the metal with time. The pollutants in the form of chlorine gas and its oxides dissolve in water
to form acid, which reacts with metals (Speight, 2015).
Fe (s) + 3Cl+ (aq.) → FeCl3 (aq.)
Iron (III) chloride formed is soluble in water.
The sulphur (IV) oxide released by the car exhaust and industrial plants get oxidized to
sulphur (VI) oxide first.
SO2 + O2 (g) → SO3 (g)
The formed oxide of sulphur (sulphur (VI) oxide gas) reacts with water to sulphuric (IV)
acid.
SO3 (g) + H2O (l) → H2SO4 (aq.)
Oxide of nitrogen forming nitric acid
3NO2 (g) + H2O (l) → 2HNO3 (aq.) + NO (g)
Oxides of chlorine reacting with water to form acid, chlorine (VII) oxide reacts with
water to form chloric (VII) acid, which is a powerful acid.
Cl2O7 (g) + H2O (l) → 2HClO4 (aq.)
A compound capable of donating free chlorine ions (Cl-) to a water-based aqueous
solution is potential to cause corrosion in steel. Chlorine is highly electronegative. It has a
high tendency to attract electrons from other elements to itself hence resulting in corrosion.
Chlorine ions form a compound with iron which is soluble in water resulting in degradation of
the metal with time. The pollutants in the form of chlorine gas and its oxides dissolve in water
to form acid, which reacts with metals (Speight, 2015).
Fe (s) + 3Cl+ (aq.) → FeCl3 (aq.)
Iron (III) chloride formed is soluble in water.

Monitoring Corrosion 7
The acid formed from various types of pollutant gases combines chemically with the
different parts of the metallic structures to form various salts.
The reaction of nitric acid with; zinc, aluminum, and iron.
Zn (s) + 2HNO3 (aq.) → Zn(NO3)2 (aq.)+ H2 (g)
Fe (s) + 2HNO3 (aq.) → Fe(NO3)2 (aq.)+ H2 (g)
2Al (s) + 6HNO3 (aq.) → 2Al(NO3)3 (aq.)+ 3H2 (g)
The other acid too resulting from pollutant gasses react with the metals to form their
respective oxides.
Metals nature and reactivity.
There are two properties which the elements largely depend on when taking part in
chemical reactions. These are the tendency to gain or lose electrons. The tendency to loss or gain
electrons is mostly dependent on the structure of the element's atom. The two factors that affect
the stability of an element is ionization energy and electron affinity.
Ionization energy
Ionization energy is the energy required to remove one or more electron from a neutral
atom to form a positively charged particle in its gaseous state. Ionization energy is a physical
property that affects the chemical behavior of an atom. There are two types of ionization energy.
First ionization energy and second ionization energy.
The tendency of a metal to undergo corrosion majorly depends on its nature. Some metals
easily get oxidized while others are stable and do not readily get oxidized. The bigger the size of
The acid formed from various types of pollutant gases combines chemically with the
different parts of the metallic structures to form various salts.
The reaction of nitric acid with; zinc, aluminum, and iron.
Zn (s) + 2HNO3 (aq.) → Zn(NO3)2 (aq.)+ H2 (g)
Fe (s) + 2HNO3 (aq.) → Fe(NO3)2 (aq.)+ H2 (g)
2Al (s) + 6HNO3 (aq.) → 2Al(NO3)3 (aq.)+ 3H2 (g)
The other acid too resulting from pollutant gasses react with the metals to form their
respective oxides.
Metals nature and reactivity.
There are two properties which the elements largely depend on when taking part in
chemical reactions. These are the tendency to gain or lose electrons. The tendency to loss or gain
electrons is mostly dependent on the structure of the element's atom. The two factors that affect
the stability of an element is ionization energy and electron affinity.
Ionization energy
Ionization energy is the energy required to remove one or more electron from a neutral
atom to form a positively charged particle in its gaseous state. Ionization energy is a physical
property that affects the chemical behavior of an atom. There are two types of ionization energy.
First ionization energy and second ionization energy.
The tendency of a metal to undergo corrosion majorly depends on its nature. Some metals
easily get oxidized while others are stable and do not readily get oxidized. The bigger the size of
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Monitoring Corrosion 8
the atom, the less tightly the electrons are held by the nucleus; hence, the ionization energy will
be less.
Metals have different properties from each other, which influence the choice of its use.
For a given metal to attain stability, electrons must be lost or given out. This electron loss or gain
takes place during a chemical reaction. Metals with the list ionization energy are the most
unstable. The following factors affect ionization energy; nuclear charge, electrons shell number,
effective nuclear charge, and type of orbital (Sanderson, 2012). Atoms having stable electronic
configurations have a lesser tendency to lose electrons hence have higher ionization energy.
First ionization energy increases from left to right across the rows of a periodic table,
meaning that the elements (metals) on the left side of a periodic table are the most unstable
because they have lesser ionization energy. Low ionization energy makes them vulnerable to
oxidation. The first ionization too decreases as we go down the periodic table, which is the
column, signifying the same, easiness to get oxidized.
Increasing ionization energies in (kJ/mol.)
Least stable elements (metals) more stable elements (metals)
Examples (first ionization energies)
Na (g) → Na + (g) + e- Energy= 495.8 kJ/ mol.
Li (g) → Li+ (g) + e- Energy= 520.3 kJ/ mol. Increasing metal stability.
Fe (g) → Fe+ (g) + e- Energy= 762 kJ/ mol.
Au (g) → Au+ (g) + e- Energy= 890.3 kJ/mol.
the atom, the less tightly the electrons are held by the nucleus; hence, the ionization energy will
be less.
Metals have different properties from each other, which influence the choice of its use.
For a given metal to attain stability, electrons must be lost or given out. This electron loss or gain
takes place during a chemical reaction. Metals with the list ionization energy are the most
unstable. The following factors affect ionization energy; nuclear charge, electrons shell number,
effective nuclear charge, and type of orbital (Sanderson, 2012). Atoms having stable electronic
configurations have a lesser tendency to lose electrons hence have higher ionization energy.
First ionization energy increases from left to right across the rows of a periodic table,
meaning that the elements (metals) on the left side of a periodic table are the most unstable
because they have lesser ionization energy. Low ionization energy makes them vulnerable to
oxidation. The first ionization too decreases as we go down the periodic table, which is the
column, signifying the same, easiness to get oxidized.
Increasing ionization energies in (kJ/mol.)
Least stable elements (metals) more stable elements (metals)
Examples (first ionization energies)
Na (g) → Na + (g) + e- Energy= 495.8 kJ/ mol.
Li (g) → Li+ (g) + e- Energy= 520.3 kJ/ mol. Increasing metal stability.
Fe (g) → Fe+ (g) + e- Energy= 762 kJ/ mol.
Au (g) → Au+ (g) + e- Energy= 890.3 kJ/mol.

Monitoring Corrosion 9
Gold (Au) is the most stable and inert metal. Its stability results from large amounts of
energy required to remove electrons in its atom from its most outermost electrons (Sanderson,
2012). These metals would have been the best in construction of marine structures, but, its
availability, strength, and the cost is a challenge. Iron is made up of strong metallic bonds,
which are relatively stable due to its high ionization energy, iron will, therefore, be the most
desirable choice in this case. Sodium and lithium have lesser ionization energy than iron;
hence, they are more prone to corrosion.
Electron affinity
Electron affinity is the energy change of neutral atom in its gaseous state when an
electron is added to form a negatively charged ion. It is a measure of the likelihood of an atom
to gain an electron (Sanderson, 2012). The nonmetals attain stability by acquiring electrons
from the metals into its outermost electron shell; by doing so, they attain stability. The
elements which gain electrons from other elements to fill its outermost electron shell achieve
what is known as the octet rule.
The energy in the form of heat is released to attain stability. Nonmetals gain electrons
to attain eight electrons in its outermost shell. On the contrary, metals lose electrons to
nonmetals to achieve stability, and by doing so, they get corroded. So both the metals and
non-metals take part in a chemical reaction which involves the transfer of electrons to form
product compound, which is more stable.
Examples
Lithium (Li) 60 KJ/mol.
Sodium (Na) 53 KJ/mol.
Gold (Au) is the most stable and inert metal. Its stability results from large amounts of
energy required to remove electrons in its atom from its most outermost electrons (Sanderson,
2012). These metals would have been the best in construction of marine structures, but, its
availability, strength, and the cost is a challenge. Iron is made up of strong metallic bonds,
which are relatively stable due to its high ionization energy, iron will, therefore, be the most
desirable choice in this case. Sodium and lithium have lesser ionization energy than iron;
hence, they are more prone to corrosion.
Electron affinity
Electron affinity is the energy change of neutral atom in its gaseous state when an
electron is added to form a negatively charged ion. It is a measure of the likelihood of an atom
to gain an electron (Sanderson, 2012). The nonmetals attain stability by acquiring electrons
from the metals into its outermost electron shell; by doing so, they attain stability. The
elements which gain electrons from other elements to fill its outermost electron shell achieve
what is known as the octet rule.
The energy in the form of heat is released to attain stability. Nonmetals gain electrons
to attain eight electrons in its outermost shell. On the contrary, metals lose electrons to
nonmetals to achieve stability, and by doing so, they get corroded. So both the metals and
non-metals take part in a chemical reaction which involves the transfer of electrons to form
product compound, which is more stable.
Examples
Lithium (Li) 60 KJ/mol.
Sodium (Na) 53 KJ/mol.

Monitoring Corrosion 10
Potassium (K) 48 KJ/mol.
Rubidium (Rb) 47 KJ/mol.
Cesium (Cs) 46 KJ/mol.
Metals have a low likelihood of gaining electrons because it is easier to lose their
outermost electrons and form cations. The metals with higher electron affinities are not easily
reduced hence are strong oxidizing agents.
Presence of an electrolyte
An electrolyte is a substance that can be dissolved to make a solution which conducts
electricity. Electrolytic substances dissolve in polar solvents, like water. It, therefore, undergoes
ionization upon dissolving in water to give out free ions which are responsible for electric
current conduction. The electrolyte triggers corrosion of metals of different types which come
into contact with each other.
A marine structure made up of different types of metals such as iron and zinc, or iron and
copper will be triggered to undergo electrochemical reaction. This electrochemical reaction is
also known as a galvanic reaction. The potential difference resulting from the reactivity
difference of the various parts of the structures worsened by the presence of an electrolyte will
initiate an electrochemical reaction which is another form of corrosion (Talbot D. & Talbot J.,
2018).
Two metals of different elements (like copper and iron) brought together and in contact
with electrolyte will behave like a typical car battery or a dry cell battery in a closed circuit. This
corrosion will not occur without the presence of an electrolyte.
Potassium (K) 48 KJ/mol.
Rubidium (Rb) 47 KJ/mol.
Cesium (Cs) 46 KJ/mol.
Metals have a low likelihood of gaining electrons because it is easier to lose their
outermost electrons and form cations. The metals with higher electron affinities are not easily
reduced hence are strong oxidizing agents.
Presence of an electrolyte
An electrolyte is a substance that can be dissolved to make a solution which conducts
electricity. Electrolytic substances dissolve in polar solvents, like water. It, therefore, undergoes
ionization upon dissolving in water to give out free ions which are responsible for electric
current conduction. The electrolyte triggers corrosion of metals of different types which come
into contact with each other.
A marine structure made up of different types of metals such as iron and zinc, or iron and
copper will be triggered to undergo electrochemical reaction. This electrochemical reaction is
also known as a galvanic reaction. The potential difference resulting from the reactivity
difference of the various parts of the structures worsened by the presence of an electrolyte will
initiate an electrochemical reaction which is another form of corrosion (Talbot D. & Talbot J.,
2018).
Two metals of different elements (like copper and iron) brought together and in contact
with electrolyte will behave like a typical car battery or a dry cell battery in a closed circuit. This
corrosion will not occur without the presence of an electrolyte.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Monitoring Corrosion 11
Some of the electrolytes are, seawater- which is rich in free sodium and chloride ions,
cleaning agents, table salts, oxides of nitrogen- which is one of the pollutants, among others.
Example
Insertion of two metals (zinc and copper) in the same piece of fruit (orange) and attaching
both with copper wire or any other electrical conducting wire passing through a voltmeter, will
cause deflection of the voltmeter pointer. The zinc metal on the process gets eaten away
(corroded), and the fruits become watery as time goes by (Singh, 2014). The orange contains
citric acid, and this acts as an electrolyte.
The reaction at the zinc metal (corrosion)
Zn (s) → Zn2+ (aq.) + 2e-
The reaction at the copper metal
2H2 (g) + O2 (g) → 2H2O (l)
Similarly, this happens when the metallic parts of sea structures come in contact with the
sea salt, which is rich in Na+ and Cl- ions. It is a perfect electrolyte, hence will trigger metallic
corrosion and sustain it as long as the loop is complete.
Temperature
In all chemical reaction case, the rate of a chemical reaction increases with increase in
temperature. The reaction rate of a chemical of success in particle collisions is highly dependent
on the frequency of a successful collision (Saito & Maruyama, 2012). Increasing the
temperature of the reactants causes the following; the particles of the reactants move very
quickly, the energy of the particles increases, the frequency of fruitful collision between the
Some of the electrolytes are, seawater- which is rich in free sodium and chloride ions,
cleaning agents, table salts, oxides of nitrogen- which is one of the pollutants, among others.
Example
Insertion of two metals (zinc and copper) in the same piece of fruit (orange) and attaching
both with copper wire or any other electrical conducting wire passing through a voltmeter, will
cause deflection of the voltmeter pointer. The zinc metal on the process gets eaten away
(corroded), and the fruits become watery as time goes by (Singh, 2014). The orange contains
citric acid, and this acts as an electrolyte.
The reaction at the zinc metal (corrosion)
Zn (s) → Zn2+ (aq.) + 2e-
The reaction at the copper metal
2H2 (g) + O2 (g) → 2H2O (l)
Similarly, this happens when the metallic parts of sea structures come in contact with the
sea salt, which is rich in Na+ and Cl- ions. It is a perfect electrolyte, hence will trigger metallic
corrosion and sustain it as long as the loop is complete.
Temperature
In all chemical reaction case, the rate of a chemical reaction increases with increase in
temperature. The reaction rate of a chemical of success in particle collisions is highly dependent
on the frequency of a successful collision (Saito & Maruyama, 2012). Increasing the
temperature of the reactants causes the following; the particles of the reactants move very
quickly, the energy of the particles increases, the frequency of fruitful collision between the

Monitoring Corrosion 12
reactant particles rises, the amount of fruitful collisions also rises, and the rate of the reaction
ultimately increase.
The temperature increases heat energy to the reactants. Heat energy causes an increase in
kinetic energy between the particles of the reacting and non-reacting species. This kinetic energy
is manifested in the rise in the randomness of collision (Saito & Maruyama, 2012).
The seas and oceans conserve and hold heat very well; it absorbs more heat from the sun
during the day time than it losses during the night. Marine water has the highest heat capacity
and volumes. This makes it have a relatively stable temperature, which is relatively higher than
the land temperature. Unsurprisingly, this relatively higher temperatures of the marine
environment make metallic structures in marine environments undergo corrosion faster than
those found inside the land. These are a structure which are around 15 kilometers away from the
seashore, which experiences fewer temperature changes due to the nearness to the seas.
Presence of oxygen
Oxygen is a highly reactive nonmetal element with an atomic number eight. It exists in
various isotopes; hence, its neutrons vary. It falls in the chalcogen group on the periodic table.
Its chemical symbol is O. oxygen is the 3rd the most abundant element in the galaxy.
Oxygen reacts with metals and nonmetals to form its oxides (Sanderson, 2012). The
dissolved oxygen is the oxygen atoms that are responsible for corrosion of marine structure.
Dissolved oxygen is the volume of oxygen contained in water. In the marine environment,
oxygen enters the water as a result of water plants photosynthesis, and by transfer the water
surface in contact with the atmosphere. The solubility of the oxygen in water decreases with
reactant particles rises, the amount of fruitful collisions also rises, and the rate of the reaction
ultimately increase.
The temperature increases heat energy to the reactants. Heat energy causes an increase in
kinetic energy between the particles of the reacting and non-reacting species. This kinetic energy
is manifested in the rise in the randomness of collision (Saito & Maruyama, 2012).
The seas and oceans conserve and hold heat very well; it absorbs more heat from the sun
during the day time than it losses during the night. Marine water has the highest heat capacity
and volumes. This makes it have a relatively stable temperature, which is relatively higher than
the land temperature. Unsurprisingly, this relatively higher temperatures of the marine
environment make metallic structures in marine environments undergo corrosion faster than
those found inside the land. These are a structure which are around 15 kilometers away from the
seashore, which experiences fewer temperature changes due to the nearness to the seas.
Presence of oxygen
Oxygen is a highly reactive nonmetal element with an atomic number eight. It exists in
various isotopes; hence, its neutrons vary. It falls in the chalcogen group on the periodic table.
Its chemical symbol is O. oxygen is the 3rd the most abundant element in the galaxy.
Oxygen reacts with metals and nonmetals to form its oxides (Sanderson, 2012). The
dissolved oxygen is the oxygen atoms that are responsible for corrosion of marine structure.
Dissolved oxygen is the volume of oxygen contained in water. In the marine environment,
oxygen enters the water as a result of water plants photosynthesis, and by transfer the water
surface in contact with the atmosphere. The solubility of the oxygen in water decreases with

Monitoring Corrosion 13
temperature increase. A rise in temperature increases the kinetic energy in oxygen molecules,
which breaks intermolecular bonds and escape from the water.
The nature of disturbance experienced by the marine waters as a result of winds
provide conducive state for air to dissolve in water. It increases the surface area of the seas in
contact with air for a better dissolving of oxygen gas.
The corrosion illustration chemically.
4Fe (S) + 3O2 (g) → 2Fe2O3 (s)
First step: Oxidation of solid iron (Fe)
Firstly, the solid iron metal dissolves into a solution in the presence of water. The water is
paramount for rusting to occur; it facilitates the movement of electrons.
Fe(s) → Fe2+ (aq.) + 2e-
The electrons produced in the reaction above combines with hydrogen ions in the
water and the dissolved oxygen to give out water.
4e- + 4H+ (aq.) + O2 (aq.) → 2H2O (l)
The above chemical equations produce water and the iron (II) ions. Rusting has not
taken place, and for that to occur another chemical reaction has to occur.
Second Step: Rust formation
temperature increase. A rise in temperature increases the kinetic energy in oxygen molecules,
which breaks intermolecular bonds and escape from the water.
The nature of disturbance experienced by the marine waters as a result of winds
provide conducive state for air to dissolve in water. It increases the surface area of the seas in
contact with air for a better dissolving of oxygen gas.
The corrosion illustration chemically.
4Fe (S) + 3O2 (g) → 2Fe2O3 (s)
First step: Oxidation of solid iron (Fe)
Firstly, the solid iron metal dissolves into a solution in the presence of water. The water is
paramount for rusting to occur; it facilitates the movement of electrons.
Fe(s) → Fe2+ (aq.) + 2e-
The electrons produced in the reaction above combines with hydrogen ions in the
water and the dissolved oxygen to give out water.
4e- + 4H+ (aq.) + O2 (aq.) → 2H2O (l)
The above chemical equations produce water and the iron (II) ions. Rusting has not
taken place, and for that to occur another chemical reaction has to occur.
Second Step: Rust formation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Monitoring Corrosion 14
The use of hydrogen ions as the iron dissolves results in excess hydroxyl ions (OH -) in
the water. The iron (II) ions react with these hydroxide ions to form green rust.
Fe2+ (aq.) + 2OH- (aq.) → Fe(OH)2 (s)
The iron (II) ions also react with hydrogen and oxygen in the presence of water to give
iron (III) ions as the product
4Fe2+ (aq.) + 4H+ (aq.) + O2 (aq.) → 4Fe3+ (aq.) + 2H2O (l)
Iron (III) ions are responsible for the reddish coloration solid deposits that gradually
eats up weakens the steel and ironic materials worldwide. Iron (III) combines with the extra
hydroxide ion to give iron (III) hydroxide.
Fe3+ (aq.) + 3OH-(aq.) → Fe(OH)3 (aq.)
The compound above loses water by dehydration to form rust (Fe2O3.H2O).
Balanced equation
4Fe (s) + 3O2 (g) + 6H2O (l) → 4Fe(OH)3 (aq.)
Impurities
Impurities are elements or chemical compounds which are found in small quantities in
other substances. Impurities can be intentionally added or can occur in nature. These
substances can be beneficial or destructive. They can be destructive when undesirable effects
result from the metal, such as altering the normal functionality of a given metal ( Singh J. &
The use of hydrogen ions as the iron dissolves results in excess hydroxyl ions (OH -) in
the water. The iron (II) ions react with these hydroxide ions to form green rust.
Fe2+ (aq.) + 2OH- (aq.) → Fe(OH)2 (s)
The iron (II) ions also react with hydrogen and oxygen in the presence of water to give
iron (III) ions as the product
4Fe2+ (aq.) + 4H+ (aq.) + O2 (aq.) → 4Fe3+ (aq.) + 2H2O (l)
Iron (III) ions are responsible for the reddish coloration solid deposits that gradually
eats up weakens the steel and ironic materials worldwide. Iron (III) combines with the extra
hydroxide ion to give iron (III) hydroxide.
Fe3+ (aq.) + 3OH-(aq.) → Fe(OH)3 (aq.)
The compound above loses water by dehydration to form rust (Fe2O3.H2O).
Balanced equation
4Fe (s) + 3O2 (g) + 6H2O (l) → 4Fe(OH)3 (aq.)
Impurities
Impurities are elements or chemical compounds which are found in small quantities in
other substances. Impurities can be intentionally added or can occur in nature. These
substances can be beneficial or destructive. They can be destructive when undesirable effects
result from the metal, such as altering the normal functionality of a given metal ( Singh J. &

Monitoring Corrosion 15
Singh D., 2012). Most of the impurities are added to bring out superior qualities in the
resultant metal. The metals are melted in a precise combination to form a specific material.
An example is a carbon which is added to steel to harden it and make it tough for construction
purposes.
Elimination of impurities in metals is done chemically or physically. An example of
impurity which is separated physically is a mixture of water and salt. Salt is obtained by
evaporation or distillation of water. However, how accurate the method is, it is virtually
impossible to eliminate it 100%. That's the case with the impurities. They can only be
minimized to reduce their effects significantly.
An example, the presence or introduction of impurities of ions belonging to metals
such as copper, lead, or mercury can cause severe corrosion of the materials made of
aluminum and iron. Copper, lead, and mercury are less reactive than aluminum and iron.
Hence, it will make iron or aluminum act as a sacrificial anode to protect them (Singh, 2014).
Process, the named metals will cause aluminum and iron to get oxidized
Al (s) → Al+3 (aq.) + 3e-
Fe (s) → Fe2+ (aq.) + 2e-
Presence of microbes
Microbes or microorganisms are tiny forms of life which are too small to be seen by a
naked eye. They are found in all environments; water, air, and soil. Some of these organisms
require oxygen to live, but others do not.
Singh D., 2012). Most of the impurities are added to bring out superior qualities in the
resultant metal. The metals are melted in a precise combination to form a specific material.
An example is a carbon which is added to steel to harden it and make it tough for construction
purposes.
Elimination of impurities in metals is done chemically or physically. An example of
impurity which is separated physically is a mixture of water and salt. Salt is obtained by
evaporation or distillation of water. However, how accurate the method is, it is virtually
impossible to eliminate it 100%. That's the case with the impurities. They can only be
minimized to reduce their effects significantly.
An example, the presence or introduction of impurities of ions belonging to metals
such as copper, lead, or mercury can cause severe corrosion of the materials made of
aluminum and iron. Copper, lead, and mercury are less reactive than aluminum and iron.
Hence, it will make iron or aluminum act as a sacrificial anode to protect them (Singh, 2014).
Process, the named metals will cause aluminum and iron to get oxidized
Al (s) → Al+3 (aq.) + 3e-
Fe (s) → Fe2+ (aq.) + 2e-
Presence of microbes
Microbes or microorganisms are tiny forms of life which are too small to be seen by a
naked eye. They are found in all environments; water, air, and soil. Some of these organisms
require oxygen to live, but others do not.

Monitoring Corrosion 16
Chemotrophs are responsible for corrosion of metals in the marine environment. These
are microbes that get energy by oxidation of electron donors, hence significant effects on the
lifespan of certain metals which donate electrons. These microorganisms are present in nearly
all the habitats. They are found too in seawater and freshwater bodies. It has been established
that a good number of steel pipes buried in the earth have undergone severe corrosion because
of the microbes.
The mechanism on how the microorganisms cause corrosion has never be understood
clearly. There is no specific microorganism which has been connected to the single
biochemical reaction (Venzlaff et al., 2013). The complexity of the reactions results from
different microbes executing different electrochemical reactions by secreting proteins and
metabolic products, which have effects on the metals.
Corrosion occurring to metal deeply buried in the soil is attributed to the sulphate
reducing bacteria which survives without requiring oxygen. Cathodic depolarization and
attack by hydrogen sulphide occur due to this type of microbes (Venzlaff et al., 2013).
The existence of aggressive microbes has been severe in the industrial systems,
ranging from piping systems, cooling water and injection systems, storage vessels, among
others. Alloy corrosion has been experienced too.
Salinity
Salinity is the concentration of dissolved salt in a given amount of water, and it is
measured in salt grams dissolved per kilogram of water. If you have 10 grams of salt dissolved in
1000 grams of water, then the salinity is 10 g/ kg. Marine water is an aqueous solution of salts.
Chemotrophs are responsible for corrosion of metals in the marine environment. These
are microbes that get energy by oxidation of electron donors, hence significant effects on the
lifespan of certain metals which donate electrons. These microorganisms are present in nearly
all the habitats. They are found too in seawater and freshwater bodies. It has been established
that a good number of steel pipes buried in the earth have undergone severe corrosion because
of the microbes.
The mechanism on how the microorganisms cause corrosion has never be understood
clearly. There is no specific microorganism which has been connected to the single
biochemical reaction (Venzlaff et al., 2013). The complexity of the reactions results from
different microbes executing different electrochemical reactions by secreting proteins and
metabolic products, which have effects on the metals.
Corrosion occurring to metal deeply buried in the soil is attributed to the sulphate
reducing bacteria which survives without requiring oxygen. Cathodic depolarization and
attack by hydrogen sulphide occur due to this type of microbes (Venzlaff et al., 2013).
The existence of aggressive microbes has been severe in the industrial systems,
ranging from piping systems, cooling water and injection systems, storage vessels, among
others. Alloy corrosion has been experienced too.
Salinity
Salinity is the concentration of dissolved salt in a given amount of water, and it is
measured in salt grams dissolved per kilogram of water. If you have 10 grams of salt dissolved in
1000 grams of water, then the salinity is 10 g/ kg. Marine water is an aqueous solution of salts.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Monitoring Corrosion 17
Salinity is obtained by calculation of chloride ions concentrations in water. Salinity= 1.80655 x
[Cl-]. Ocean water is composed of the following salts. Sodium chloride, magnesium chloride,
calcium chloride, sodium hydrogen carbonate, potassium bromide, and many more.
Water in the freshwater lakes and rivers have minimal salts which do not exceed salinity
0.5 g/ kg. Salinity along and across the marine environments varies significantly due to various
factors such as the rate of evaporation, precipitation, freezing, and nearness to the river source.
Saline water allows fast oxidation of iron because it contains salts which act as catalysts
in the corrosion process (Goyal et al., 2018). Nonetheless, salt water has a lot of dissociated ions;
this makes it a good conductor. This results in electrolysis reaction that immensely speeds up the
corrosion of steel or iron in salty water and saline environment.
Seawater corrosion increases proportionally with an increase in salinity and reduces when
salinity surpasses 3%. There is an inverse relationship between salinity and the oxygen dissolved
in water. Increase in salinity minimizes the ability of water to dissolve more oxygen, lowers
solubility of oxygen (Saito & Maruyama, 2012). Salts dissolved in marine water increases the
conductivity of water, which boosts corrosion.
If iron is in contact with another metal and salty water, assuming the metal was
aluminum, you will effectively get a battery due to the potential differences between the two
metals.
Chemical illustration.
Salinity is obtained by calculation of chloride ions concentrations in water. Salinity= 1.80655 x
[Cl-]. Ocean water is composed of the following salts. Sodium chloride, magnesium chloride,
calcium chloride, sodium hydrogen carbonate, potassium bromide, and many more.
Water in the freshwater lakes and rivers have minimal salts which do not exceed salinity
0.5 g/ kg. Salinity along and across the marine environments varies significantly due to various
factors such as the rate of evaporation, precipitation, freezing, and nearness to the river source.
Saline water allows fast oxidation of iron because it contains salts which act as catalysts
in the corrosion process (Goyal et al., 2018). Nonetheless, salt water has a lot of dissociated ions;
this makes it a good conductor. This results in electrolysis reaction that immensely speeds up the
corrosion of steel or iron in salty water and saline environment.
Seawater corrosion increases proportionally with an increase in salinity and reduces when
salinity surpasses 3%. There is an inverse relationship between salinity and the oxygen dissolved
in water. Increase in salinity minimizes the ability of water to dissolve more oxygen, lowers
solubility of oxygen (Saito & Maruyama, 2012). Salts dissolved in marine water increases the
conductivity of water, which boosts corrosion.
If iron is in contact with another metal and salty water, assuming the metal was
aluminum, you will effectively get a battery due to the potential differences between the two
metals.
Chemical illustration.

Monitoring Corrosion 18
The various salts present in water dissociates to form ions which enhance the
conductivity of water.
NaCl (aq.) → Na+ (aq.) + Cl- (aq.)
CaCl2 (aq.) → Ca+ (aq.) + 2Cl- (aq.)
KBr (aq.) → K+ (aq.) + Br- (aq.)
Experiment 1 on Part One: Experiments Investigating Factors Affecting Corrosion
Aim: To investigate the rate of corrosion of various ironic nail in different salinity (salt
concentrations).
Hypothesis: The higher the salinity, the higher the corrosion rate.
Table 1
Variables
Dependent Independent Controls
Salinity Nails mass Temperature
Oxygen Time (3 days)
Apparatus
Glass beakers labeled
Clean iron nails.
The various salts present in water dissociates to form ions which enhance the
conductivity of water.
NaCl (aq.) → Na+ (aq.) + Cl- (aq.)
CaCl2 (aq.) → Ca+ (aq.) + 2Cl- (aq.)
KBr (aq.) → K+ (aq.) + Br- (aq.)
Experiment 1 on Part One: Experiments Investigating Factors Affecting Corrosion
Aim: To investigate the rate of corrosion of various ironic nail in different salinity (salt
concentrations).
Hypothesis: The higher the salinity, the higher the corrosion rate.
Table 1
Variables
Dependent Independent Controls
Salinity Nails mass Temperature
Oxygen Time (3 days)
Apparatus
Glass beakers labeled
Clean iron nails.

Monitoring Corrosion 19
Tap water.
Sodium chloride salt.
Spatula.
Measuring electronic balance for measuring mass.
Method
Two clean iron nails were put in four glass beakers, to the 1st glass beaker 100 milliliters
of distilled water was added, to the 2nd glass beaker 100 milliliters of tap water was added, to the
3rd glass beaker 1 gram of sodium chloride salt was put and 100 grams of water added, salt was
then stirred gently to dissolve. To the 4th and 5th glass beaker 2 and 3 grams of sodium chloride
salt respectively was added, followed by 100 grams of water and stirred to dissolve.
Observations and results
The observations taken three days later after the setup has been left undisturbed were
recorded as per the visual examination conducted.
Physical appearance.
It was observed that the nails in the first glass beaker rusted slightly. The nails in second
glass beaker significantly rusted and were well noticeable. The rusting in the third, fourth, and
fifth beakers was intense, with severity in rusting increasing from 3rd, 4th, to 5th beaker
respectively.
Quantitative analysis
Tap water.
Sodium chloride salt.
Spatula.
Measuring electronic balance for measuring mass.
Method
Two clean iron nails were put in four glass beakers, to the 1st glass beaker 100 milliliters
of distilled water was added, to the 2nd glass beaker 100 milliliters of tap water was added, to the
3rd glass beaker 1 gram of sodium chloride salt was put and 100 grams of water added, salt was
then stirred gently to dissolve. To the 4th and 5th glass beaker 2 and 3 grams of sodium chloride
salt respectively was added, followed by 100 grams of water and stirred to dissolve.
Observations and results
The observations taken three days later after the setup has been left undisturbed were
recorded as per the visual examination conducted.
Physical appearance.
It was observed that the nails in the first glass beaker rusted slightly. The nails in second
glass beaker significantly rusted and were well noticeable. The rusting in the third, fourth, and
fifth beakers was intense, with severity in rusting increasing from 3rd, 4th, to 5th beaker
respectively.
Quantitative analysis
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
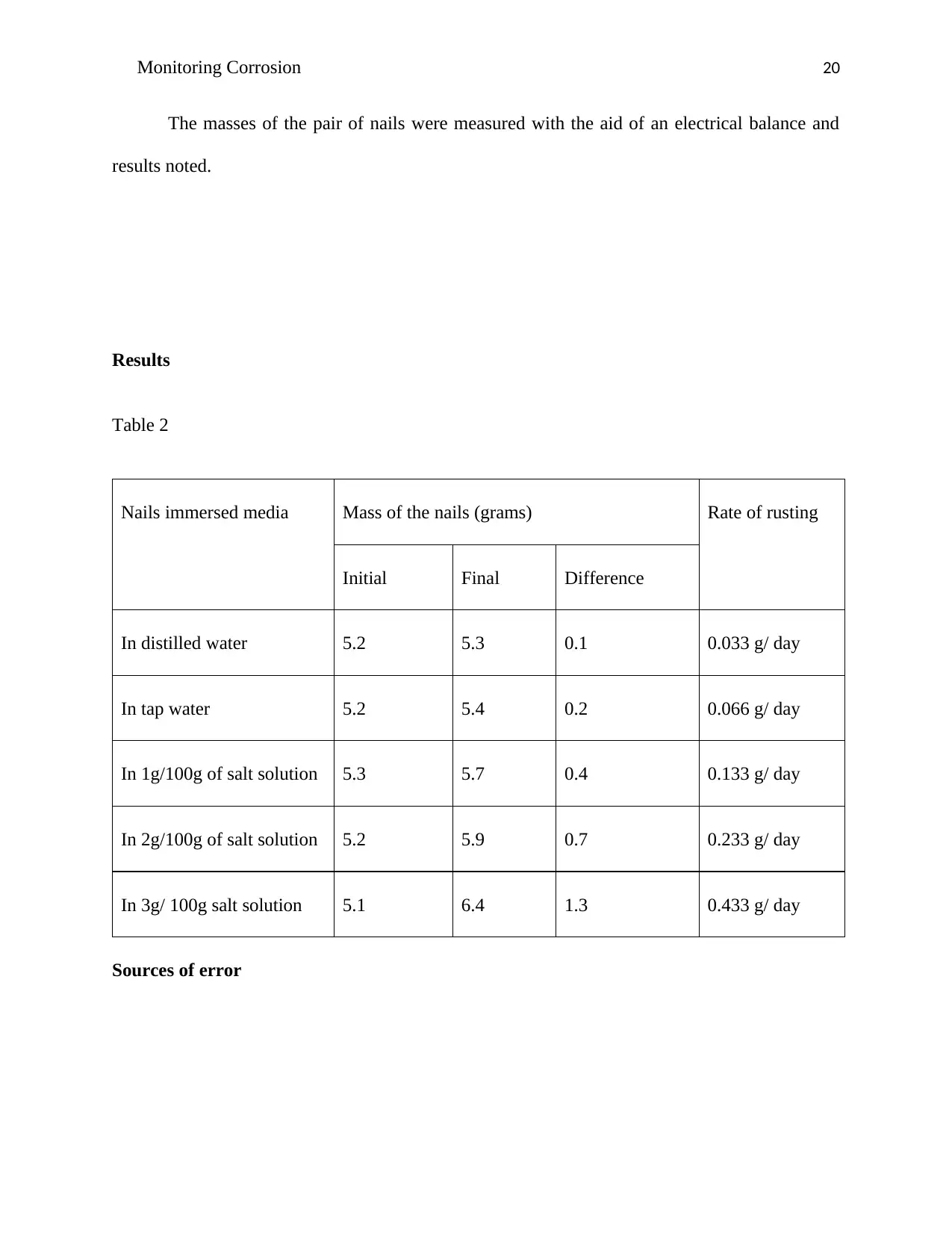
Monitoring Corrosion 20
The masses of the pair of nails were measured with the aid of an electrical balance and
results noted.
Results
Table 2
Nails immersed media Mass of the nails (grams) Rate of rusting
Initial Final Difference
In distilled water 5.2 5.3 0.1 0.033 g/ day
In tap water 5.2 5.4 0.2 0.066 g/ day
In 1g/100g of salt solution 5.3 5.7 0.4 0.133 g/ day
In 2g/100g of salt solution 5.2 5.9 0.7 0.233 g/ day
In 3g/ 100g salt solution 5.1 6.4 1.3 0.433 g/ day
Sources of error
The masses of the pair of nails were measured with the aid of an electrical balance and
results noted.
Results
Table 2
Nails immersed media Mass of the nails (grams) Rate of rusting
Initial Final Difference
In distilled water 5.2 5.3 0.1 0.033 g/ day
In tap water 5.2 5.4 0.2 0.066 g/ day
In 1g/100g of salt solution 5.3 5.7 0.4 0.133 g/ day
In 2g/100g of salt solution 5.2 5.9 0.7 0.233 g/ day
In 3g/ 100g salt solution 5.1 6.4 1.3 0.433 g/ day
Sources of error

Monitoring Corrosion 21
Human errors resulting from improper reading of instruments, or addition of exact
volume water into the beakers. Also, the low accuracy of the electric balance due to the minute
nature of the increase in the mass of the materials measured.
Human errors can be eliminated or minimized by paying attention to experimental
procedures and a proper reading of instruments. An electric balance with better calibration and
accuracy can be procured for the next use.
Explanations
Rusting occurred in beaker 1 because both water and oxygen were present which is
essential for rusting to take place. Rusting in the second glass beaker was greater than in the first
glass beaker due to the presence of salt in tap water. Its quantities estimated to be less than 0.5%,
this is the primary catalyst in the rusting process (Singh D & Singh J, 2012). Rusting in glass
beaker 3 to 6 is intense with severity increase as the salt concentrations (salinity) per unit volume
increase. The increase in salt concentrations increases the number of free ions in the solution.
These free ions are responsible for conductivity in the solution.
The masses of the metals increase with an increase in salinity. Rusting is positively
favored by salinity.
Conclusions
The hypothesis was supported. It was noted that the severity of the corrosion and the rate
of corrosion increased with increase in salinity.
Recommendations
Human errors resulting from improper reading of instruments, or addition of exact
volume water into the beakers. Also, the low accuracy of the electric balance due to the minute
nature of the increase in the mass of the materials measured.
Human errors can be eliminated or minimized by paying attention to experimental
procedures and a proper reading of instruments. An electric balance with better calibration and
accuracy can be procured for the next use.
Explanations
Rusting occurred in beaker 1 because both water and oxygen were present which is
essential for rusting to take place. Rusting in the second glass beaker was greater than in the first
glass beaker due to the presence of salt in tap water. Its quantities estimated to be less than 0.5%,
this is the primary catalyst in the rusting process (Singh D & Singh J, 2012). Rusting in glass
beaker 3 to 6 is intense with severity increase as the salt concentrations (salinity) per unit volume
increase. The increase in salt concentrations increases the number of free ions in the solution.
These free ions are responsible for conductivity in the solution.
The masses of the metals increase with an increase in salinity. Rusting is positively
favored by salinity.
Conclusions
The hypothesis was supported. It was noted that the severity of the corrosion and the rate
of corrosion increased with increase in salinity.
Recommendations

Monitoring Corrosion 22
Metallic structures exposed to a saline condition should be protected by isolating it from
in contact with saline solutions. The corrosion rate in such an environment is faster, which
results in accelerated wasting away of the structural components.
Experiment 2 on Part One: Effects of Temperature on Corrosion rate.
Aim: To investigate the effect of temperature on the corrosion of iron nails immersed in saline
conditions.
Hypothesis: The increase in temperature accelerates corrosion rate.
Table 3
Variables
Dependent Independent Controls
Temperature Nails mass Salinity
Oxygen Time (7 days)
Apparatus, reagents, and chemicals
Beakers
Regulated oven with a thermostat
3% saline solution
Metallic structures exposed to a saline condition should be protected by isolating it from
in contact with saline solutions. The corrosion rate in such an environment is faster, which
results in accelerated wasting away of the structural components.
Experiment 2 on Part One: Effects of Temperature on Corrosion rate.
Aim: To investigate the effect of temperature on the corrosion of iron nails immersed in saline
conditions.
Hypothesis: The increase in temperature accelerates corrosion rate.
Table 3
Variables
Dependent Independent Controls
Temperature Nails mass Salinity
Oxygen Time (7 days)
Apparatus, reagents, and chemicals
Beakers
Regulated oven with a thermostat
3% saline solution
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Monitoring Corrosion 23
Electric weighing balance
Iron nails
Method
Every two pairs of iron nails were measured and date recorded. The iron nails were then
inserted into salts solutions of the same concentrations. The first beaker was left at room
temperature, the second to the fourth beaker with salt solutions containing iron nails were put in
a well-ventilated oven with regulated temperature with the following degrees, 25, 30, 35, and 40
degrees respectively.
The setup was left for a week. After a week, the nails were then removed, observed, and
their masses measured.
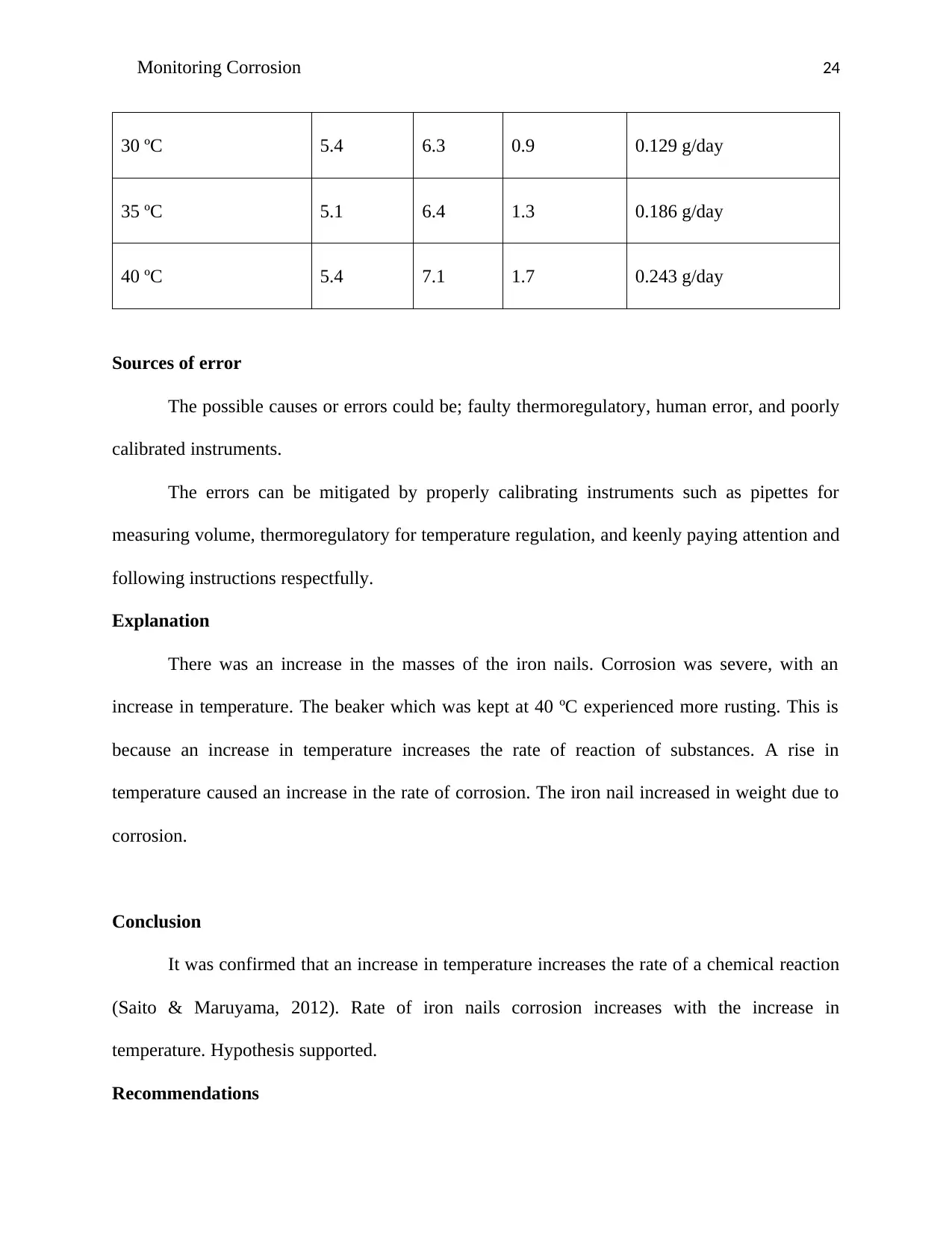
Results
Table 4
Nails media
temperature
Mass of the nails (grams) Rate of rusting
Initial Final Difference
22 ºC 5.3 5.6 0.3 0.043 g/day
25 ºC 5.2 5.8 0.6 0.086 g/day
Electric weighing balance
Iron nails
Method
Every two pairs of iron nails were measured and date recorded. The iron nails were then
inserted into salts solutions of the same concentrations. The first beaker was left at room
temperature, the second to the fourth beaker with salt solutions containing iron nails were put in
a well-ventilated oven with regulated temperature with the following degrees, 25, 30, 35, and 40
degrees respectively.
The setup was left for a week. After a week, the nails were then removed, observed, and
their masses measured.
Results
Table 4
Nails media
temperature
Mass of the nails (grams) Rate of rusting
Initial Final Difference
22 ºC 5.3 5.6 0.3 0.043 g/day
25 ºC 5.2 5.8 0.6 0.086 g/day
Monitoring Corrosion 24
30 ºC 5.4 6.3 0.9 0.129 g/day
35 ºC 5.1 6.4 1.3 0.186 g/day
40 ºC 5.4 7.1 1.7 0.243 g/day
Sources of error
The possible causes or errors could be; faulty thermoregulatory, human error, and poorly
calibrated instruments.
The errors can be mitigated by properly calibrating instruments such as pipettes for
measuring volume, thermoregulatory for temperature regulation, and keenly paying attention and
following instructions respectfully.
Explanation
There was an increase in the masses of the iron nails. Corrosion was severe, with an
increase in temperature. The beaker which was kept at 40 ºC experienced more rusting. This is
because an increase in temperature increases the rate of reaction of substances. A rise in
temperature caused an increase in the rate of corrosion. The iron nail increased in weight due to
corrosion.
Conclusion
It was confirmed that an increase in temperature increases the rate of a chemical reaction
(Saito & Maruyama, 2012). Rate of iron nails corrosion increases with the increase in
temperature. Hypothesis supported.
Recommendations
30 ºC 5.4 6.3 0.9 0.129 g/day
35 ºC 5.1 6.4 1.3 0.186 g/day
40 ºC 5.4 7.1 1.7 0.243 g/day
Sources of error
The possible causes or errors could be; faulty thermoregulatory, human error, and poorly
calibrated instruments.
The errors can be mitigated by properly calibrating instruments such as pipettes for
measuring volume, thermoregulatory for temperature regulation, and keenly paying attention and
following instructions respectfully.
Explanation
There was an increase in the masses of the iron nails. Corrosion was severe, with an
increase in temperature. The beaker which was kept at 40 ºC experienced more rusting. This is
because an increase in temperature increases the rate of reaction of substances. A rise in
temperature caused an increase in the rate of corrosion. The iron nail increased in weight due to
corrosion.
Conclusion
It was confirmed that an increase in temperature increases the rate of a chemical reaction
(Saito & Maruyama, 2012). Rate of iron nails corrosion increases with the increase in
temperature. Hypothesis supported.
Recommendations

Monitoring Corrosion 25
The materials which are subjected to high temperatures which are used to make industrial
boilers, cooling systems, among others should be protected from corrosion. Corrosion in such
environments is very high due to high temperatures.
Part Two: Corrosion Control Methods
Painting
Another way to protect iron and steel structures from corrosion is to keep it painted.
The layer of the paint isolates the structure from coming into contact with oxygen and water
which is essential for rusting to occur. The protection remains as long as the paint remains
intact and unbroken (Knudsen & Forsgren 2017). Oiling is a temporary solution to prevent
corrosion; it does the same thing as the paints. The problem with oil is less durable; it
vaporizes slowly, especially at relatively warm or hot environmental conditions. It forms a
weak bond (intermolecular force) with the metal surface in contact; hence, its coat can be
removed easily from the surface of the metal. The oil-based paints are the most durable and
very effective.
Alloying
An alloy is a metal combined chemically with one or more substances by mixing to
obtain a new metal with superior qualities. The alloy may have the following attributes than
The materials which are subjected to high temperatures which are used to make industrial
boilers, cooling systems, among others should be protected from corrosion. Corrosion in such
environments is very high due to high temperatures.
Part Two: Corrosion Control Methods
Painting
Another way to protect iron and steel structures from corrosion is to keep it painted.
The layer of the paint isolates the structure from coming into contact with oxygen and water
which is essential for rusting to occur. The protection remains as long as the paint remains
intact and unbroken (Knudsen & Forsgren 2017). Oiling is a temporary solution to prevent
corrosion; it does the same thing as the paints. The problem with oil is less durable; it
vaporizes slowly, especially at relatively warm or hot environmental conditions. It forms a
weak bond (intermolecular force) with the metal surface in contact; hence, its coat can be
removed easily from the surface of the metal. The oil-based paints are the most durable and
very effective.
Alloying
An alloy is a metal combined chemically with one or more substances by mixing to
obtain a new metal with superior qualities. The alloy may have the following attributes than
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Monitoring Corrosion 26
the predecessor metals: stronger, harder, resistant to corrosion, or malleable (Goyal et al.,
2018).
Alloys which are resistant to corrosion are made from one or more of these metals;
chrome, titanium, stainless steel, cobalt, nickel, titanium, iron, and molybdenum. When mixed
such metals will produce corrosion-resistant metal, and as a result of that, the expensive
maintenance and repair of structures would have been minimized.
An example is steel, an alloy created from iron and carbon. This metal is suitable for
construction as opposed to the brittle iron. Steel is an alloy of iron and carbon; the mixture of
the two allows a small degree of stretching in their bonds, making steel tough and tensile
(Sanderson, 2012).
The stainless steel which more resistant to corrosion and staining is an alloy made
from chromium and iron. This new metal is more resistant to corrosion than steel hence
suitable for construction of structures which are subjected to harsh conditions in the sea.
Cathodic protection
Cathodic protection is achievable by connecting the less reactive metal to be protected
with a relatively reactive metal. Steel and ironic structures can be protected from the agents of
corrosion by attaching these kind metals on them (Singh, 2014). Zinc or magnesium can be
sacrificed to guard the steel and ironic structures in the sea from corrosion, which is
accelerated by the saline conditions. The sacrificial anodes are metals like zinc, which are
highly reactive; they are used to guard less reactive metals like iron and steel. Sacrificial
anodes keep steel structures free of oxygen and water, which are necessary for rusting
the predecessor metals: stronger, harder, resistant to corrosion, or malleable (Goyal et al.,
2018).
Alloys which are resistant to corrosion are made from one or more of these metals;
chrome, titanium, stainless steel, cobalt, nickel, titanium, iron, and molybdenum. When mixed
such metals will produce corrosion-resistant metal, and as a result of that, the expensive
maintenance and repair of structures would have been minimized.
An example is steel, an alloy created from iron and carbon. This metal is suitable for
construction as opposed to the brittle iron. Steel is an alloy of iron and carbon; the mixture of
the two allows a small degree of stretching in their bonds, making steel tough and tensile
(Sanderson, 2012).
The stainless steel which more resistant to corrosion and staining is an alloy made
from chromium and iron. This new metal is more resistant to corrosion than steel hence
suitable for construction of structures which are subjected to harsh conditions in the sea.
Cathodic protection
Cathodic protection is achievable by connecting the less reactive metal to be protected
with a relatively reactive metal. Steel and ironic structures can be protected from the agents of
corrosion by attaching these kind metals on them (Singh, 2014). Zinc or magnesium can be
sacrificed to guard the steel and ironic structures in the sea from corrosion, which is
accelerated by the saline conditions. The sacrificial anodes are metals like zinc, which are
highly reactive; they are used to guard less reactive metals like iron and steel. Sacrificial
anodes keep steel structures free of oxygen and water, which are necessary for rusting

Monitoring Corrosion 27
occurrence (Singh, 2014). The sacrificial metals which act as an anode are monitored
regularly and replaced because they get used up with time. For example, the hull of the ship
has been protected from corrosion by attachment of zinc bars.
Galvanizing
Galvanizing is the process of immersing hot iron or steel in a molten solution of zinc
to protect it from corrosion (Goyal et al., 2018). The obtained material metallurgically will be
of superior qualities, one being corrosion resistant.
The zinc on the surface of the iron is readily oxidized. This is because zinc has a lower
reduction potential than iron (Singh, 2014). When iron is coated with zinc metal, the iron is
therefore isolated from the agents which cause corrosion. The protection is assured even if the
zinc coating is scratched accidentally to expose the steel or iron to the agents of corrosion.
Chemical illustration
Zn2+ (aq) + 2e- → Zn(s) E0red = -0.76 V
Fe2+ (aq) + 2e- → Fe(s) E0red = -0.44 V
The Iron is preferentially reduced over the Zinc
As shown above, the zinc metal will keep oxygen and water away from the iron, and if
accidentally the metallic zinc coating is scratched, the zinc coating will electrochemically guard
the Iron against oxidation.
Experiment 3 on Part Two: Investigating Corrosion control methods
occurrence (Singh, 2014). The sacrificial metals which act as an anode are monitored
regularly and replaced because they get used up with time. For example, the hull of the ship
has been protected from corrosion by attachment of zinc bars.
Galvanizing
Galvanizing is the process of immersing hot iron or steel in a molten solution of zinc
to protect it from corrosion (Goyal et al., 2018). The obtained material metallurgically will be
of superior qualities, one being corrosion resistant.
The zinc on the surface of the iron is readily oxidized. This is because zinc has a lower
reduction potential than iron (Singh, 2014). When iron is coated with zinc metal, the iron is
therefore isolated from the agents which cause corrosion. The protection is assured even if the
zinc coating is scratched accidentally to expose the steel or iron to the agents of corrosion.
Chemical illustration
Zn2+ (aq) + 2e- → Zn(s) E0red = -0.76 V
Fe2+ (aq) + 2e- → Fe(s) E0red = -0.44 V
The Iron is preferentially reduced over the Zinc
As shown above, the zinc metal will keep oxygen and water away from the iron, and if
accidentally the metallic zinc coating is scratched, the zinc coating will electrochemically guard
the Iron against oxidation.
Experiment 3 on Part Two: Investigating Corrosion control methods
Monitoring Corrosion 28
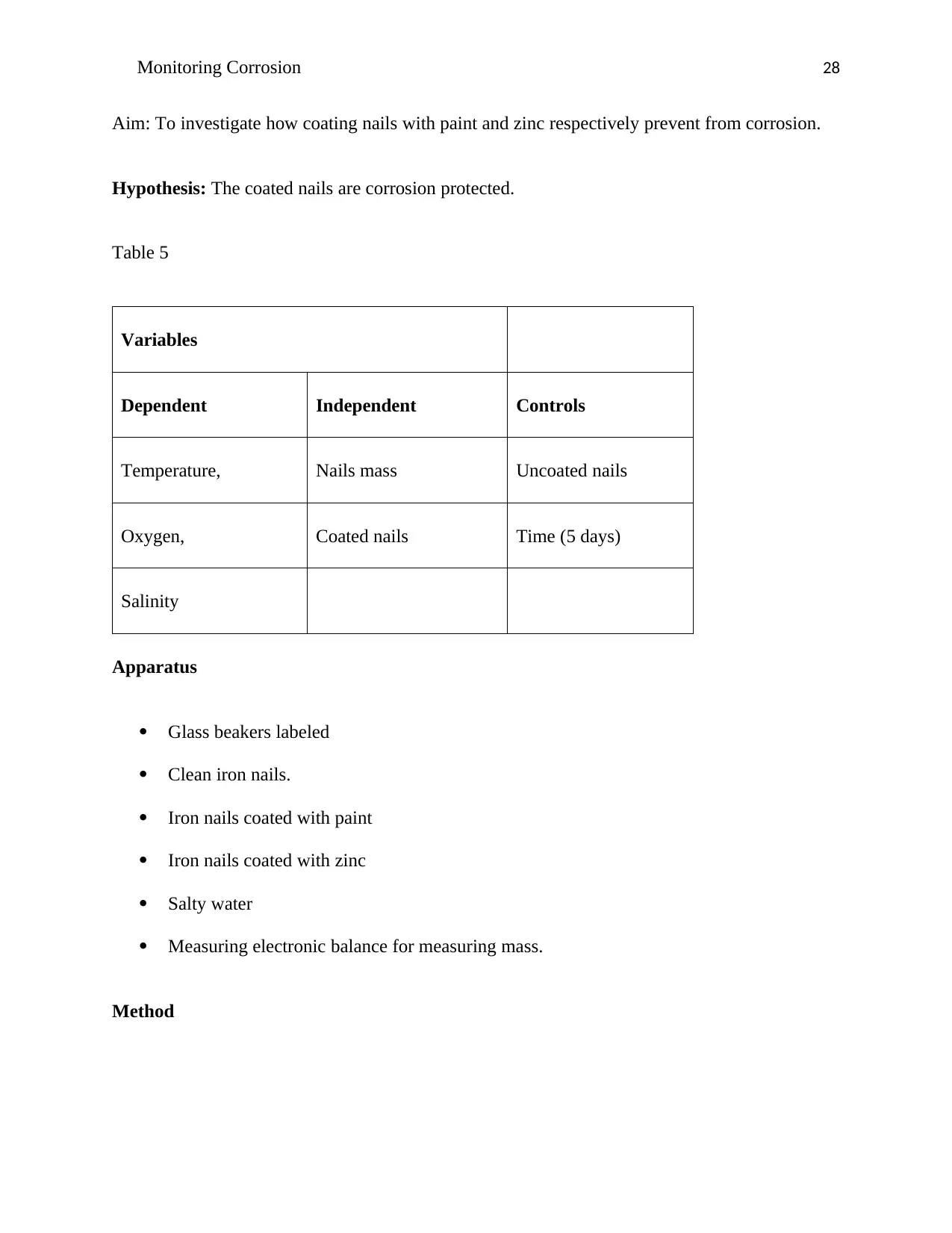
Aim: To investigate how coating nails with paint and zinc respectively prevent from corrosion.
Hypothesis: The coated nails are corrosion protected.
Table 5
Variables
Dependent Independent Controls
Temperature, Nails mass Uncoated nails
Oxygen, Coated nails Time (5 days)
Salinity
Apparatus
Glass beakers labeled
Clean iron nails.
Iron nails coated with paint
Iron nails coated with zinc
Salty water
Measuring electronic balance for measuring mass.
Method
Aim: To investigate how coating nails with paint and zinc respectively prevent from corrosion.
Hypothesis: The coated nails are corrosion protected.
Table 5
Variables
Dependent Independent Controls
Temperature, Nails mass Uncoated nails
Oxygen, Coated nails Time (5 days)
Salinity
Apparatus
Glass beakers labeled
Clean iron nails.
Iron nails coated with paint
Iron nails coated with zinc
Salty water
Measuring electronic balance for measuring mass.
Method
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Monitoring Corrosion 29
The masses of the three pair of nails were measured and put in different beakers. Saline
(salty) water was then added to the beakers till the iron nails were fully submerged. The iron
nails were then left in the same environment. Subjected to the same (equal) temperature, salinity,
and air circulation.
Observations and results
The observations taken five days later after the setup has been left undisturbed were
recorded as per the visual examination conducted.
Physical appearance.
It was observed that the nails coated with paint in the first glass beaker were not changed;
it had the same colour and texture. The nails coated with zinc in second glass beaker was
unchanged too, it had original texture and colour. The nails in the third beaker rusted severely; it
lost its smooth texture to form a rough surface and shiny characteristics to form a reddish-brown
colour.
Quantitative analysis
The masses of the pair of nails were measured with the aid of an electrical balance. There
was a significant increase in the mass of the uncoated nails. The coated nails mass remained
constant.
Results
The masses of the three pair of nails were measured and put in different beakers. Saline
(salty) water was then added to the beakers till the iron nails were fully submerged. The iron
nails were then left in the same environment. Subjected to the same (equal) temperature, salinity,
and air circulation.
Observations and results
The observations taken five days later after the setup has been left undisturbed were
recorded as per the visual examination conducted.
Physical appearance.
It was observed that the nails coated with paint in the first glass beaker were not changed;
it had the same colour and texture. The nails coated with zinc in second glass beaker was
unchanged too, it had original texture and colour. The nails in the third beaker rusted severely; it
lost its smooth texture to form a rough surface and shiny characteristics to form a reddish-brown
colour.
Quantitative analysis
The masses of the pair of nails were measured with the aid of an electrical balance. There
was a significant increase in the mass of the uncoated nails. The coated nails mass remained
constant.
Results
Monitoring Corrosion 30
Table 6
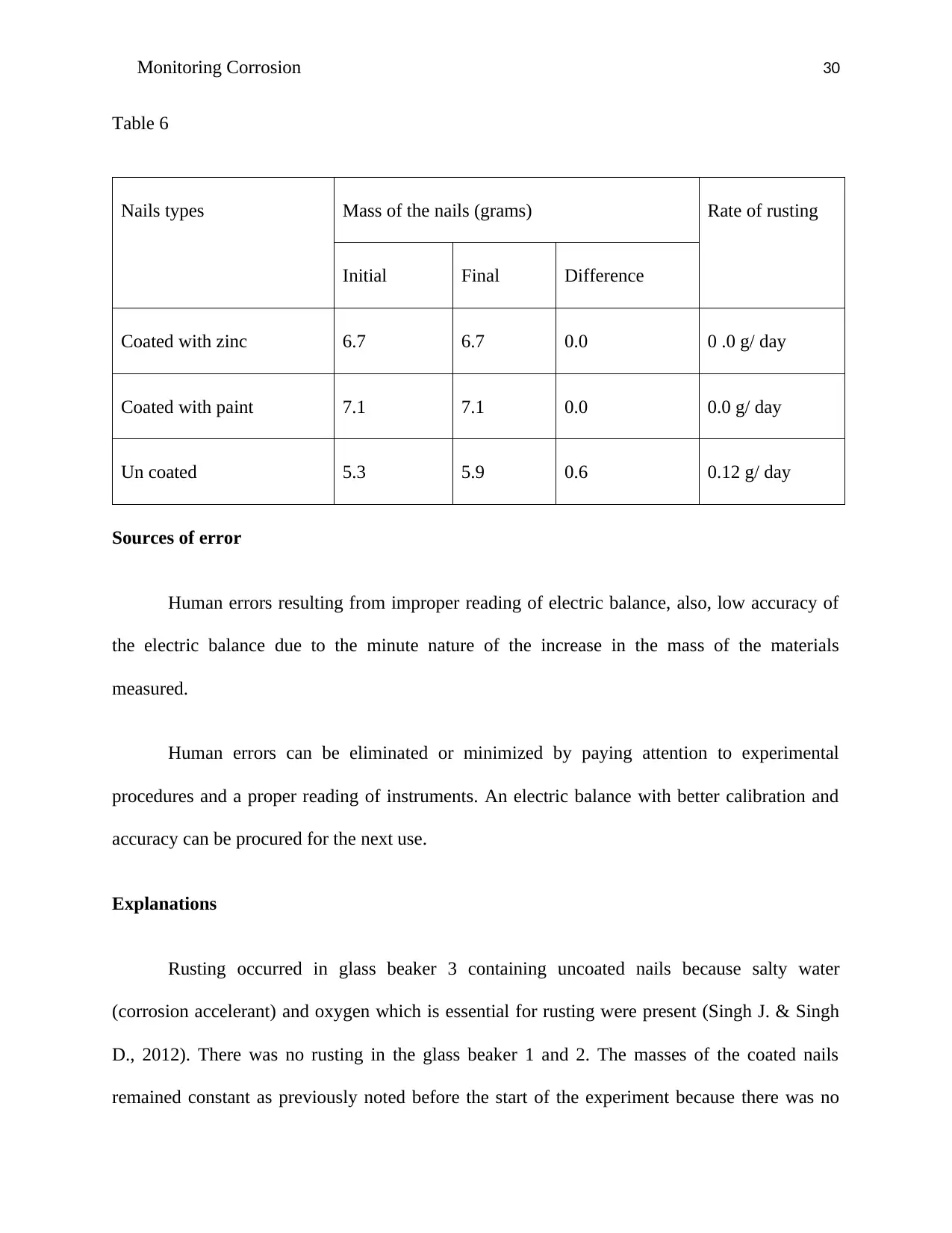
Nails types Mass of the nails (grams) Rate of rusting
Initial Final Difference
Coated with zinc 6.7 6.7 0.0 0 .0 g/ day
Coated with paint 7.1 7.1 0.0 0.0 g/ day
Un coated 5.3 5.9 0.6 0.12 g/ day
Sources of error
Human errors resulting from improper reading of electric balance, also, low accuracy of
the electric balance due to the minute nature of the increase in the mass of the materials
measured.
Human errors can be eliminated or minimized by paying attention to experimental
procedures and a proper reading of instruments. An electric balance with better calibration and
accuracy can be procured for the next use.
Explanations
Rusting occurred in glass beaker 3 containing uncoated nails because salty water
(corrosion accelerant) and oxygen which is essential for rusting were present (Singh J. & Singh
D., 2012). There was no rusting in the glass beaker 1 and 2. The masses of the coated nails
remained constant as previously noted before the start of the experiment because there was no
Table 6
Nails types Mass of the nails (grams) Rate of rusting
Initial Final Difference
Coated with zinc 6.7 6.7 0.0 0 .0 g/ day
Coated with paint 7.1 7.1 0.0 0.0 g/ day
Un coated 5.3 5.9 0.6 0.12 g/ day
Sources of error
Human errors resulting from improper reading of electric balance, also, low accuracy of
the electric balance due to the minute nature of the increase in the mass of the materials
measured.
Human errors can be eliminated or minimized by paying attention to experimental
procedures and a proper reading of instruments. An electric balance with better calibration and
accuracy can be procured for the next use.
Explanations
Rusting occurred in glass beaker 3 containing uncoated nails because salty water
(corrosion accelerant) and oxygen which is essential for rusting were present (Singh J. & Singh
D., 2012). There was no rusting in the glass beaker 1 and 2. The masses of the coated nails
remained constant as previously noted before the start of the experiment because there was no

Monitoring Corrosion 31
rusting. The coats on the surface of the nails form a barrier which prevents oxygen and water
from getting in contact with the iron nails
Conclusions
The hypothesis was supported, the two types of coat protected the nails from getting
corroded. Painting and galvanizing iron nails protect the iron nails from coming in contact with
water and oxygen, which are essential for rusting to occur.
Recommendations
Metallic structures exposed to a saline condition should be protected by isolating it from
in contact with saline solutions. Coating the structures by painting or galvanizing makes them
corrosion free (Knudsen & Forsgren, 2017). It isolates them from saline water and oxygen gas,
which are necessary for corrosion to occur.
rusting. The coats on the surface of the nails form a barrier which prevents oxygen and water
from getting in contact with the iron nails
Conclusions
The hypothesis was supported, the two types of coat protected the nails from getting
corroded. Painting and galvanizing iron nails protect the iron nails from coming in contact with
water and oxygen, which are essential for rusting to occur.
Recommendations
Metallic structures exposed to a saline condition should be protected by isolating it from
in contact with saline solutions. Coating the structures by painting or galvanizing makes them
corrosion free (Knudsen & Forsgren, 2017). It isolates them from saline water and oxygen gas,
which are necessary for corrosion to occur.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Monitoring Corrosion 32
References
Bertolini, L., Elsener, B., Pedeferri, P., Redaelli, E., & Polder, R. (2013). Corrosion of steel in
concrete (Vol. 392). Weinheim, Germany: Wiley-Vch.
Goyal, A., Pouya, H. S., Ganjian, E., & Claisse, P. (2018). A Review of Corrosion and
Protection of Steel in Concrete. Arabian Journal for Science and Engineering, 43(10),
5035-5055. doi:10.1007/s13369-018-3303-2
Hartt, W. H. (2012). Cathodic Protection of Offshore Structures – History and Current Status.
Corrosion, 120711075009003. doi:10.5006/0718
Knudsen, O. Ø., & Forsgren, A. (2017). Corrosion control through organic coatings. CRC Press.
Leygraf, C., Wallinder, I. O., Tidblad, J., & Graedel, T. (2016). Atmospheric corrosion. John
Wiley & Sons.
Morcillo, M., Chico, B., Díaz, I., Cano, H., & De la Fuente, D. (2013). Atmospheric corrosion
data of weathering steels. A review. Corrosion Science, 77, 6-24.
Saito, Y., Önay, B., & Maruyama, T. (Eds.). (2012). High temperature corrosion of advanced
materials and protective coatings. Elsevier.
Sanderson, R. (2012). Chemical bonds and bonds energy (Vol. 21). Elsevier.
Singh, J. K., & Singh, D. D. N. (2012). The nature of rusts and corrosion characteristics of low
alloy and plain carbon steels in three kinds of concrete pore solution with salinity and
different pH. Corrosion Science, 56, 129-142.
Singh, R. (2014). Corrosion control for offshore structures: cathodic protection and high-
efficiency Coating. Gulf Professional Publishing.
References
Bertolini, L., Elsener, B., Pedeferri, P., Redaelli, E., & Polder, R. (2013). Corrosion of steel in
concrete (Vol. 392). Weinheim, Germany: Wiley-Vch.
Goyal, A., Pouya, H. S., Ganjian, E., & Claisse, P. (2018). A Review of Corrosion and
Protection of Steel in Concrete. Arabian Journal for Science and Engineering, 43(10),
5035-5055. doi:10.1007/s13369-018-3303-2
Hartt, W. H. (2012). Cathodic Protection of Offshore Structures – History and Current Status.
Corrosion, 120711075009003. doi:10.5006/0718
Knudsen, O. Ø., & Forsgren, A. (2017). Corrosion control through organic coatings. CRC Press.
Leygraf, C., Wallinder, I. O., Tidblad, J., & Graedel, T. (2016). Atmospheric corrosion. John
Wiley & Sons.
Morcillo, M., Chico, B., Díaz, I., Cano, H., & De la Fuente, D. (2013). Atmospheric corrosion
data of weathering steels. A review. Corrosion Science, 77, 6-24.
Saito, Y., Önay, B., & Maruyama, T. (Eds.). (2012). High temperature corrosion of advanced
materials and protective coatings. Elsevier.
Sanderson, R. (2012). Chemical bonds and bonds energy (Vol. 21). Elsevier.
Singh, J. K., & Singh, D. D. N. (2012). The nature of rusts and corrosion characteristics of low
alloy and plain carbon steels in three kinds of concrete pore solution with salinity and
different pH. Corrosion Science, 56, 129-142.
Singh, R. (2014). Corrosion control for offshore structures: cathodic protection and high-
efficiency Coating. Gulf Professional Publishing.

Monitoring Corrosion 33
Speight, J. G. (2015). Handbook of offshore oil and gas operations. Amsterdam, Netherlands:
Elsevier.
Talbot, D. E., & Talbot, J. D. (2018). Corrosion science and technology. CRC press.
Venzlaff, H., Enning, D., Srinivasan, J., Mayrhofer, K. J., Hassel, A. W., Widdel, F., &
Stratmann, M. (2013). Accelerated cathodic reaction in microbial corrosion of iron due to
direct electron uptake by sulfate-reducing bacteria. Corrosion Science, 66, 88-96.
Speight, J. G. (2015). Handbook of offshore oil and gas operations. Amsterdam, Netherlands:
Elsevier.
Talbot, D. E., & Talbot, J. D. (2018). Corrosion science and technology. CRC press.
Venzlaff, H., Enning, D., Srinivasan, J., Mayrhofer, K. J., Hassel, A. W., Widdel, F., &
Stratmann, M. (2013). Accelerated cathodic reaction in microbial corrosion of iron due to
direct electron uptake by sulfate-reducing bacteria. Corrosion Science, 66, 88-96.
1 out of 33
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.

