Exelon Patch: A Comprehensive Discussion on Novel Drug Delivery System
VerifiedAdded on 2022/11/14
|18
|4166
|298
AI Summary
This essay provides a comprehensive discussion on Exelon Patch, a novel drug delivery system. It covers its ingredients, TDDS, pharmacy related information, manufacturers, countries where it is available, intellectual property, competition, sales, design/technology, limitations, advantages offered, excipients, the rationale for the novel delivery system and characteristics.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: EXELON PATCH 1
Exelon Patch
Name
Institutional Affiliation
Exelon Patch
Name
Institutional Affiliation
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

EXELON PATCH 2
Executive Summary
This essays is about Rivastigmine (generic name) or Exelon Patch (brand name)
which is a novel drug delivery system (NDDS). The discussion has focused on understanding
this NDDS in terms of various aspects. The main points discussed in this paper include the
active ingredients and quantity, , pharmacy related information, the countries where it is
available, competition, intellectual property, sales date in various countries,
design/technology, limitations, advantages offered, excipients, its manufacturers of Exelon
Patch, TDDS, the rationale for the novel delivery system and characteristics.
Executive Summary
This essays is about Rivastigmine (generic name) or Exelon Patch (brand name)
which is a novel drug delivery system (NDDS). The discussion has focused on understanding
this NDDS in terms of various aspects. The main points discussed in this paper include the
active ingredients and quantity, , pharmacy related information, the countries where it is
available, competition, intellectual property, sales date in various countries,
design/technology, limitations, advantages offered, excipients, its manufacturers of Exelon
Patch, TDDS, the rationale for the novel delivery system and characteristics.

EXELON PATCH 3
Table of Contents
Executive Summary...................................................................................................................2
Introduction................................................................................................................................4
Discussion..................................................................................................................................4
Name of the novel delivery system in Australian product.....................................................4
Manufactured or marketed by................................................................................................4
Excipients............................................................................................................................4
The rationale for novel delivery system, i.e. why...............................................................5
Relevant Drug(S) Characteristics; Physical, Chemical and Biological..............................6
Technology or Design Features, i.e. How..........................................................................7
Advantages Offered............................................................................................................9
Sales Data in Various Countries.......................................................................................10
Competition......................................................................................................................10
Limitations........................................................................................................................11
Pharmacy Related Information.........................................................................................11
Intellectual Property Position............................................................................................11
Conclusion................................................................................................................................12
References................................................................................................................................13
Table of Contents
Executive Summary...................................................................................................................2
Introduction................................................................................................................................4
Discussion..................................................................................................................................4
Name of the novel delivery system in Australian product.....................................................4
Manufactured or marketed by................................................................................................4
Excipients............................................................................................................................4
The rationale for novel delivery system, i.e. why...............................................................5
Relevant Drug(S) Characteristics; Physical, Chemical and Biological..............................6
Technology or Design Features, i.e. How..........................................................................7
Advantages Offered............................................................................................................9
Sales Data in Various Countries.......................................................................................10
Competition......................................................................................................................10
Limitations........................................................................................................................11
Pharmacy Related Information.........................................................................................11
Intellectual Property Position............................................................................................11
Conclusion................................................................................................................................12
References................................................................................................................................13

EXELON PATCH 4
Introduction
The main aim of this paper is to present a comprehensive discussion about Exelon
Patch. This discussion is presented in terms of its ingredients, TDDS, pharmacy related
information, its manufacturers, the countries where it is available, intellectual property,
competition, sales, design/technology, limitations, advantages offered, excipients, the
rationale for the novel delivery system and characteristics.
Discussion
Name of the novel delivery system in Australian product
TDDS (Transdermal Drug Delivery System)
Manufactured or marketed by
NOVARTIS Pharmaceuticals Australia Pty Limited ABN 18 004 244 160
Countries where available
It is available in over 80 countries including Germany, Australia, Kenya, The US,
India., China, Canada, Switzerland, England, South Africa and Pakistan,
Active ingredient(s) and quantity
The ingredients used in making the drug include capsules of about e (1.5–6 mg Q12H,
i.e., 3–12 mg/day) or patch (5–20 cm2) in ascending doses. Each of them is induced within
the ascending doses of 14 day period (Zhao et al., 2012). The patch is put with lower
percentages of 30% lower at 20 cm3cm2.c on the, 19.5 versus 29.3 ng/ml).
Excipients
Some of the excipients contained in the Exelon patch include; acrylic copolymer,
polybutylmethacrylate, silicon adhesive, silicon oil and vitamin E. polymethylmethacrylate is
also contained in the patch responsible for technology adoption. This type patch significantly
Introduction
The main aim of this paper is to present a comprehensive discussion about Exelon
Patch. This discussion is presented in terms of its ingredients, TDDS, pharmacy related
information, its manufacturers, the countries where it is available, intellectual property,
competition, sales, design/technology, limitations, advantages offered, excipients, the
rationale for the novel delivery system and characteristics.
Discussion
Name of the novel delivery system in Australian product
TDDS (Transdermal Drug Delivery System)
Manufactured or marketed by
NOVARTIS Pharmaceuticals Australia Pty Limited ABN 18 004 244 160
Countries where available
It is available in over 80 countries including Germany, Australia, Kenya, The US,
India., China, Canada, Switzerland, England, South Africa and Pakistan,
Active ingredient(s) and quantity
The ingredients used in making the drug include capsules of about e (1.5–6 mg Q12H,
i.e., 3–12 mg/day) or patch (5–20 cm2) in ascending doses. Each of them is induced within
the ascending doses of 14 day period (Zhao et al., 2012). The patch is put with lower
percentages of 30% lower at 20 cm3cm2.c on the, 19.5 versus 29.3 ng/ml).
Excipients
Some of the excipients contained in the Exelon patch include; acrylic copolymer,
polybutylmethacrylate, silicon adhesive, silicon oil and vitamin E. polymethylmethacrylate is
also contained in the patch responsible for technology adoption. This type patch significantly
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

EXELON PATCH 5
uses matrix technology. Matrix technology is used mostly because it leads to better skin
tolerability than older patch types. It is also significant when there is absence of a solvent
adjunct. The solvent adjunct is required for dissolving the active component. When it is not
dissolved matrix technology acts as Excipients and it is used.
The rationale for novel delivery system, i.e. why
For the treatment above, the Transdermal delivery system is used. The rationale for
picking this method of delivery was based on a lot of factors surrounding the disease and the
patient. Firstly, the transdermal delivery system provides an edge over the rest of the
injectable and the oral routes (Knopman, Donohue, & Gutterman, 2000). Since the disease
requires skin delivery the transdermal delivery system was the best method available.
It also provides for controlled system delivery. The drug can be delivered on the way
they are packaged. Since we are dealing with the skin the system also allows for short
delivery system (Morris et al., 2016). Short delivery system include; controlling of constant
administration of drugs and continuous slow input of drugs. Its advantage to the rest of the
delivery systems is that the rest of the delivery system do provide for undesirable drug side
effects. Some of these side effects include; pulse entry into the blood system and quick
release system (Larson, 2016). Other common side effects like vomiting, nausea and
headaches by the patient are also totally avoided.
Additionally, it limits hepatic first pass metabolism therefore enhancing efficiency
and maintenance of steady plasma on the level of drug used. In the case there is any levels of
drugs that might have been released into the blood it will be detected. Evidence of any
percutaneous drug levels released into the blood can easily be detected using measurable
amount of drugs. Apart from the blood very limited amounts of the same can also be traced
into urine and excretion. Using the transdermal delivery system has in recent times also
helped with the clinical response of the patient to the administered drug therapy, making it
uses matrix technology. Matrix technology is used mostly because it leads to better skin
tolerability than older patch types. It is also significant when there is absence of a solvent
adjunct. The solvent adjunct is required for dissolving the active component. When it is not
dissolved matrix technology acts as Excipients and it is used.
The rationale for novel delivery system, i.e. why
For the treatment above, the Transdermal delivery system is used. The rationale for
picking this method of delivery was based on a lot of factors surrounding the disease and the
patient. Firstly, the transdermal delivery system provides an edge over the rest of the
injectable and the oral routes (Knopman, Donohue, & Gutterman, 2000). Since the disease
requires skin delivery the transdermal delivery system was the best method available.
It also provides for controlled system delivery. The drug can be delivered on the way
they are packaged. Since we are dealing with the skin the system also allows for short
delivery system (Morris et al., 2016). Short delivery system include; controlling of constant
administration of drugs and continuous slow input of drugs. Its advantage to the rest of the
delivery systems is that the rest of the delivery system do provide for undesirable drug side
effects. Some of these side effects include; pulse entry into the blood system and quick
release system (Larson, 2016). Other common side effects like vomiting, nausea and
headaches by the patient are also totally avoided.
Additionally, it limits hepatic first pass metabolism therefore enhancing efficiency
and maintenance of steady plasma on the level of drug used. In the case there is any levels of
drugs that might have been released into the blood it will be detected. Evidence of any
percutaneous drug levels released into the blood can easily be detected using measurable
amount of drugs. Apart from the blood very limited amounts of the same can also be traced
into urine and excretion. Using the transdermal delivery system has in recent times also
helped with the clinical response of the patient to the administered drug therapy, making it

EXELON PATCH 6
even easier to monitor (Moga, Roberts, & Jicha, 2017). Topical administration of
therapeutic agents is often preferred. It is preferred since it offers many advantages over
conventional oral and invasive methods of drug delivery.
One of the most important advantages of transdermal drug delivery is that it limits
hepatic first pass metabolism, it also enhances therapeutic efficiencies to the targeted patients.
Additionally, the maintenance of steady plasma level of the drug is literally achieved through
TDDS. Another potentiality of NDDS used in terms of rationality is that it enhances patient
compliance since it is very easy to use. The ratios of patient inter and intra variability is at its
minimum. The patients and their caretakers have a very easy time of connecting the
therapeutic effects with the expected outcome. It becomes totally manageable to deal with
such events.
Relevant Drug(S) Characteristics; Physical, Chemical and Biological
To deliver a drug in the form of a TSSD, there are particular characteristics that the
drug should possess both chemically and physically. The drug has a low molecular mass of
about 0.45g which makes it easily absorbable into the streams. It also contains an
amphipathic structure which makes it adaptable within the skin. The potency of the drug is
also quite light and very small. The reason for making them such light is to allow for skin
tolerability. The drug also contains a patch; the patch has no important role except for patient
tolerability. Chemically composed riverstigmine contains parasympathomimetic or the
cholinergic agent. The agent is the one responsible for the treatment of mild dementia. It also
contains cholinesterase which inhibits both butyrylcholinesterase and acetylcholinesterase. It
contains acetylcholine as a result of selective loss of cholinergic neurons that is contained in
it. Tacrine is also postulated within the drug so that the cholinergic function is enhanced in
the drug. An additional tacrine contained in the postulated so that acetylcholine is reversible
in the inhibition of hydrolysis.
even easier to monitor (Moga, Roberts, & Jicha, 2017). Topical administration of
therapeutic agents is often preferred. It is preferred since it offers many advantages over
conventional oral and invasive methods of drug delivery.
One of the most important advantages of transdermal drug delivery is that it limits
hepatic first pass metabolism, it also enhances therapeutic efficiencies to the targeted patients.
Additionally, the maintenance of steady plasma level of the drug is literally achieved through
TDDS. Another potentiality of NDDS used in terms of rationality is that it enhances patient
compliance since it is very easy to use. The ratios of patient inter and intra variability is at its
minimum. The patients and their caretakers have a very easy time of connecting the
therapeutic effects with the expected outcome. It becomes totally manageable to deal with
such events.
Relevant Drug(S) Characteristics; Physical, Chemical and Biological
To deliver a drug in the form of a TSSD, there are particular characteristics that the
drug should possess both chemically and physically. The drug has a low molecular mass of
about 0.45g which makes it easily absorbable into the streams. It also contains an
amphipathic structure which makes it adaptable within the skin. The potency of the drug is
also quite light and very small. The reason for making them such light is to allow for skin
tolerability. The drug also contains a patch; the patch has no important role except for patient
tolerability. Chemically composed riverstigmine contains parasympathomimetic or the
cholinergic agent. The agent is the one responsible for the treatment of mild dementia. It also
contains cholinesterase which inhibits both butyrylcholinesterase and acetylcholinesterase. It
contains acetylcholine as a result of selective loss of cholinergic neurons that is contained in
it. Tacrine is also postulated within the drug so that the cholinergic function is enhanced in
the drug. An additional tacrine contained in the postulated so that acetylcholine is reversible
in the inhibition of hydrolysis.

EXELON PATCH 7
Carbamate is derivative and structurally related to physostigmine. Chemically the
drug doesn’t contain donepezil and tacrine within it. The drug binds fully with inactive
cholinesterase. It is the same combination with chlolinesterase that prevents further
hydrolysis. The drug has high concentration of acetylcholine which is very necessary for the
synapses. It is important for the peripheral tissues as it helps in the building of the tissues
slowly during usage by the patients (Sheth, 2019). Unlike oral rivastigmine, it has two
different peaks. The first cholinesterase inhibition correlates with two daily dosing regimens
(Pearce, 2016). The presence of the transdermal administration within the drug helps in
providing a smoother. The smoother is used continuous reduction of cholinesterase activity.
The drug changes from from the original cholinesterase-mediated hydrolysis to the
decarbamylated metabolite. All the process of changing the form is extensively metabolized.
Elimination of the metabolites is achieved through renal excretion. Around 1% of the dosage
given is later excreted through the faeces as contained in the drug.
The chemical structure:
Carbamate is derivative and structurally related to physostigmine. Chemically the
drug doesn’t contain donepezil and tacrine within it. The drug binds fully with inactive
cholinesterase. It is the same combination with chlolinesterase that prevents further
hydrolysis. The drug has high concentration of acetylcholine which is very necessary for the
synapses. It is important for the peripheral tissues as it helps in the building of the tissues
slowly during usage by the patients (Sheth, 2019). Unlike oral rivastigmine, it has two
different peaks. The first cholinesterase inhibition correlates with two daily dosing regimens
(Pearce, 2016). The presence of the transdermal administration within the drug helps in
providing a smoother. The smoother is used continuous reduction of cholinesterase activity.
The drug changes from from the original cholinesterase-mediated hydrolysis to the
decarbamylated metabolite. All the process of changing the form is extensively metabolized.
Elimination of the metabolites is achieved through renal excretion. Around 1% of the dosage
given is later excreted through the faeces as contained in the drug.
The chemical structure:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
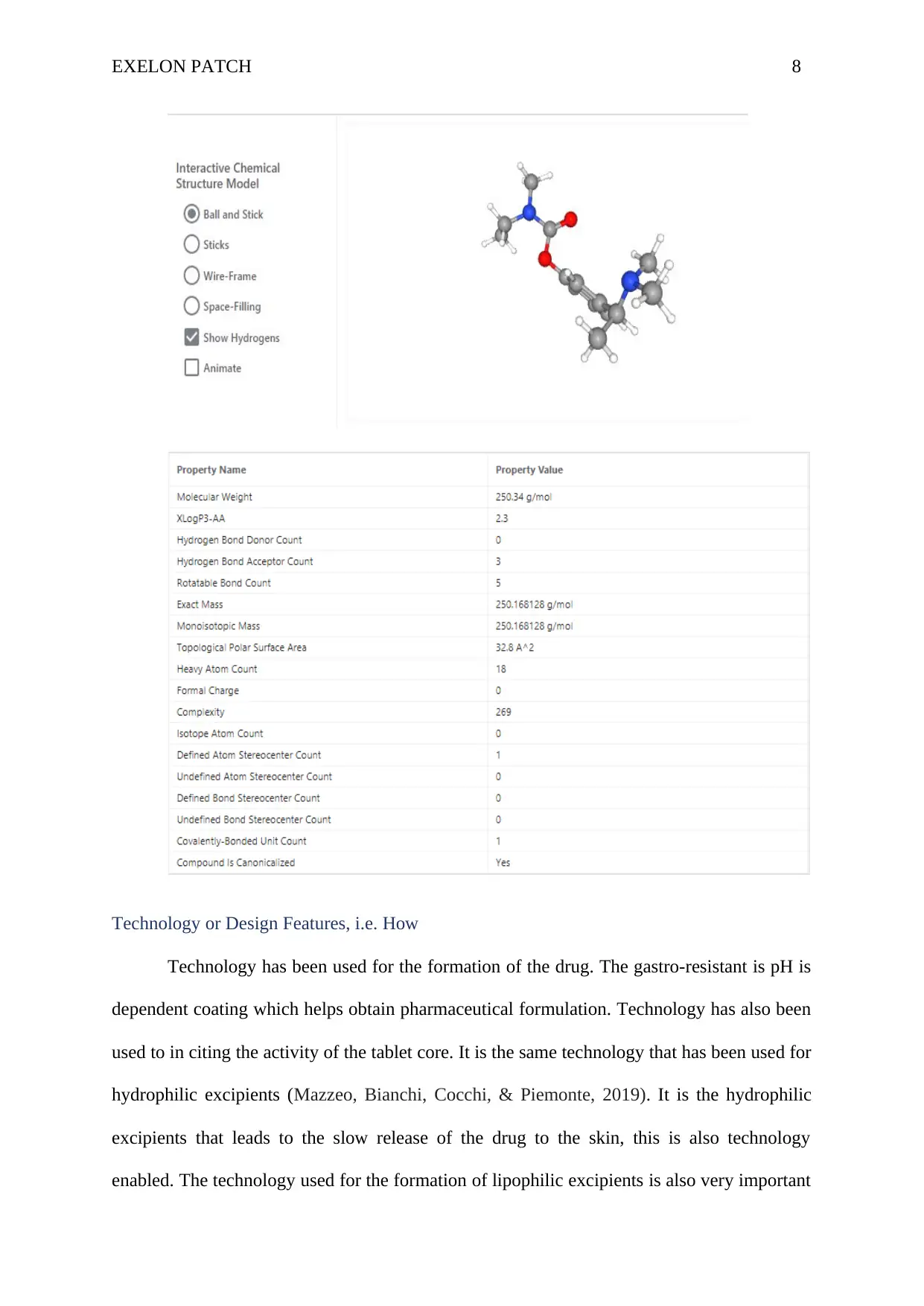
EXELON PATCH 8
Technology or Design Features, i.e. How
Technology has been used for the formation of the drug. The gastro-resistant is pH is
dependent coating which helps obtain pharmaceutical formulation. Technology has also been
used to in citing the activity of the tablet core. It is the same technology that has been used for
hydrophilic excipients (Mazzeo, Bianchi, Cocchi, & Piemonte, 2019). It is the hydrophilic
excipients that leads to the slow release of the drug to the skin, this is also technology
enabled. The technology used for the formation of lipophilic excipients is also very important
Technology or Design Features, i.e. How
Technology has been used for the formation of the drug. The gastro-resistant is pH is
dependent coating which helps obtain pharmaceutical formulation. Technology has also been
used to in citing the activity of the tablet core. It is the same technology that has been used for
hydrophilic excipients (Mazzeo, Bianchi, Cocchi, & Piemonte, 2019). It is the hydrophilic
excipients that leads to the slow release of the drug to the skin, this is also technology
enabled. The technology used for the formation of lipophilic excipients is also very important

EXELON PATCH 9
(Farlow, Grossberg, Sadowsky, Meng, & Somogyi, 2013). It is slows the penetration of the
fluid into the skin. Technology used in combination of the two substances has really helped in
ensuring that the skin has prolonged exposure to the drug.
The technology has also been used to enhance transdermal delivery. Within it the
technology there are physical enhancers. These physical enhances that are technological
include; ultrasound, iontophoresis, electroporation, magnetophoresis and microneedle.
Technology is also used for chemic al enhancers. Chemical enhancers are used to induce
chemicals into the drugs. Some of these chemicals include; sulphoxides, azones, glycols,
alkanols and terpenes.
There is a macroflux technology used in the making of the drug. The macroflux
technology system is used for the short period delivery that brings through the micro
projection (Lehmann et al., 2007). The technology designed for micro projection contains
medicament that adheres to an elastic adhesive backing. Another technology used is metered-
dose transdermal spray. The spray is used to make the drug non-volatile to the environment.
With that it remains on the skin. The volatility of the drug reduces its chances of being
absorbed to the skin. It, therefore, gives a very good advantage to the drug. Iontophoresis
involves the flow of electrode to any area of contact. The area of contact and drug is
enhanced so that it is also remains in contact with the administered formulation. The
ultrasound technology is used in the mixing of drugs substances. For the case of rivastigmine
the coupling agent normally crème or ointment is normally used (Marwah, Garg, Goyal, &
Rath, 2016). The coupling agent causes ultrasonic energy transfer from the system to the
skin.
Another technology used is the photo-mechanical wave (SHINGADE, 2012).
Photomechanical wave is significantly used when applied to the skin. The photomechanical
wave is the one used for permeabilisation due to the development of the transient channels.
(Farlow, Grossberg, Sadowsky, Meng, & Somogyi, 2013). It is slows the penetration of the
fluid into the skin. Technology used in combination of the two substances has really helped in
ensuring that the skin has prolonged exposure to the drug.
The technology has also been used to enhance transdermal delivery. Within it the
technology there are physical enhancers. These physical enhances that are technological
include; ultrasound, iontophoresis, electroporation, magnetophoresis and microneedle.
Technology is also used for chemic al enhancers. Chemical enhancers are used to induce
chemicals into the drugs. Some of these chemicals include; sulphoxides, azones, glycols,
alkanols and terpenes.
There is a macroflux technology used in the making of the drug. The macroflux
technology system is used for the short period delivery that brings through the micro
projection (Lehmann et al., 2007). The technology designed for micro projection contains
medicament that adheres to an elastic adhesive backing. Another technology used is metered-
dose transdermal spray. The spray is used to make the drug non-volatile to the environment.
With that it remains on the skin. The volatility of the drug reduces its chances of being
absorbed to the skin. It, therefore, gives a very good advantage to the drug. Iontophoresis
involves the flow of electrode to any area of contact. The area of contact and drug is
enhanced so that it is also remains in contact with the administered formulation. The
ultrasound technology is used in the mixing of drugs substances. For the case of rivastigmine
the coupling agent normally crème or ointment is normally used (Marwah, Garg, Goyal, &
Rath, 2016). The coupling agent causes ultrasonic energy transfer from the system to the
skin.
Another technology used is the photo-mechanical wave (SHINGADE, 2012).
Photomechanical wave is significantly used when applied to the skin. The photomechanical
wave is the one used for permeabilisation due to the development of the transient channels.

EXELON PATCH 10
Another technology mostly used is electroporation. It mostly involves electric
diffusion. The diffusion is always done to increase permeability. These electrical pulses are
always considered to form small pores within the stratum cornea. Another significance of this
technology is that it introduces closely spaced electrodes to the reserves within the skin. It
also helps in ensuring the small pores within skin remain painless. This same technology is
almost the same with electro-osmosis (Malvey, Rao, & Arumugam, 2019). Electro-osmosis is
done by a voltage difference. A voltage difference is giving charge to bulk fluid and volume
so that it takes place within the concentration gradients. Other forms of technology include
needle-free injections and powder-jet devices.
Advantages Offered
The advantages of the transdermal delivery system are so good and so are the drugs.
Firstly, the drugs are a source of avoidance of first pass metabolism of drugs. It is through the
first pass metabolism process that enhancement of therapeutic efficiency is achieved. A
reduced plasma concentration is also another advantage. The main advantage of the reduced
plasma is that it reduces all the decreased side effects (Govender et al., 2017). The NDDS is
also advantageous since it reduces fluctuations in plasma levels of drugs. This advantage
arises at the point the system will help in utilization of drug candidates. The candidates who
achieve this advantage are those with short half-life and low therapeutic index. The NDSS
system is also very advantageous in dealing with toxic effects that come from the drugs.
The system makes it easy to eliminate all the toxic substances (Bhoye, Shaikh, Shah,
& Patel, 2018). The system was also very easy in enhancing patient compliance. The patient
is made compliant through reducing cases of nausea and vomiting. Transdermal delivery
system and medication ensures a steady infusion of a drug over an extended period of time.
The effects of these drugs or failure that are associated with intermittent dosing are also quite
avoided (Avinash et al., 2016). The delivery also increases the therapeutic value of many
Another technology mostly used is electroporation. It mostly involves electric
diffusion. The diffusion is always done to increase permeability. These electrical pulses are
always considered to form small pores within the stratum cornea. Another significance of this
technology is that it introduces closely spaced electrodes to the reserves within the skin. It
also helps in ensuring the small pores within skin remain painless. This same technology is
almost the same with electro-osmosis (Malvey, Rao, & Arumugam, 2019). Electro-osmosis is
done by a voltage difference. A voltage difference is giving charge to bulk fluid and volume
so that it takes place within the concentration gradients. Other forms of technology include
needle-free injections and powder-jet devices.
Advantages Offered
The advantages of the transdermal delivery system are so good and so are the drugs.
Firstly, the drugs are a source of avoidance of first pass metabolism of drugs. It is through the
first pass metabolism process that enhancement of therapeutic efficiency is achieved. A
reduced plasma concentration is also another advantage. The main advantage of the reduced
plasma is that it reduces all the decreased side effects (Govender et al., 2017). The NDDS is
also advantageous since it reduces fluctuations in plasma levels of drugs. This advantage
arises at the point the system will help in utilization of drug candidates. The candidates who
achieve this advantage are those with short half-life and low therapeutic index. The NDSS
system is also very advantageous in dealing with toxic effects that come from the drugs.
The system makes it easy to eliminate all the toxic substances (Bhoye, Shaikh, Shah,
& Patel, 2018). The system was also very easy in enhancing patient compliance. The patient
is made compliant through reducing cases of nausea and vomiting. Transdermal delivery
system and medication ensures a steady infusion of a drug over an extended period of time.
The effects of these drugs or failure that are associated with intermittent dosing are also quite
avoided (Avinash et al., 2016). The delivery also increases the therapeutic value of many
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

EXELON PATCH 11
drugs through avoiding some side effects problems. Some of these effects include; lower
absorption, decomposition and all the hepatic first pass effects. Lastly, when the medication
is reduced the regimen leads to improved patient compliance. The compliance helps it
reduces inter- and the intra-patient variability.
Sales Data in Various Countries
The sales of the product were averagely done. In totality about 47% of the drugs
manufactured were sold. Most of the people in the hospitals reacted well to the sales since it
was a drug therapy. Drug therapies are usually well received compared to non-drug therapies.
The sales are also since there is reduction in concomitant medication. All these ensured that
40.4% of the drugs were totally sold out.
Competition
The TDS and rivastigmine has been used for several years for treatment. However it
receives a lot of competition from another approved treatment of the same skin controlled
disease (Govender et al., 2017). The capsule with gastrointestinal adverse events creates a
very stiff competition. The capsule is well tolerated with patients based on the following
assumptions. It is normally even and continuous which reduces fluctuation in the drug plasma
level. It also totally reduces the drug side effects (Forette, Anand, & Gharabawi, 1999). Once
in a day the application also enables patch and easy treatment of the schedule. It has ease of
handling, infrequent skin irritations and patient –caregiver acceptance mode. The drug all
results into slow rate of institutionalization and very low rates of healthcare and medical cost.
Because of the mentioned advantages the rivastigmine has received potential competition and
trans-delivery system in the treatment of the AD disease. It gives almost equal and excellent
option to other inhibitors making it almost preferred.
drugs through avoiding some side effects problems. Some of these effects include; lower
absorption, decomposition and all the hepatic first pass effects. Lastly, when the medication
is reduced the regimen leads to improved patient compliance. The compliance helps it
reduces inter- and the intra-patient variability.
Sales Data in Various Countries
The sales of the product were averagely done. In totality about 47% of the drugs
manufactured were sold. Most of the people in the hospitals reacted well to the sales since it
was a drug therapy. Drug therapies are usually well received compared to non-drug therapies.
The sales are also since there is reduction in concomitant medication. All these ensured that
40.4% of the drugs were totally sold out.
Competition
The TDS and rivastigmine has been used for several years for treatment. However it
receives a lot of competition from another approved treatment of the same skin controlled
disease (Govender et al., 2017). The capsule with gastrointestinal adverse events creates a
very stiff competition. The capsule is well tolerated with patients based on the following
assumptions. It is normally even and continuous which reduces fluctuation in the drug plasma
level. It also totally reduces the drug side effects (Forette, Anand, & Gharabawi, 1999). Once
in a day the application also enables patch and easy treatment of the schedule. It has ease of
handling, infrequent skin irritations and patient –caregiver acceptance mode. The drug all
results into slow rate of institutionalization and very low rates of healthcare and medical cost.
Because of the mentioned advantages the rivastigmine has received potential competition and
trans-delivery system in the treatment of the AD disease. It gives almost equal and excellent
option to other inhibitors making it almost preferred.

EXELON PATCH 12
Limitations
The delivery system and the drug contain little limitations. When the drug is in
contact with the skin it causes irritation. Additionally the excipients and the enhancers used
also because increased percutaneous. It is through the percutaneous caused that a second
limitation arises, making the delivery system not exactly comfortable to all the users. Another
disadvantage arises from the clinical need. The drug contains physiochemicals that penetrate
through stratum corneum entirely limiting the dosage and therapeutic value of the drug. The
physiochemical have some undesirable side effects that limits its adaptability to many users.
Pharmacy Related Information
Rivastigimine contains parasympathomimetic and cholinesterase inhibitor. The two
contained in the system are useful in the healing of the memory disease. The memory disease
associated with cognitive loss which creates a deficit is replaced by
acetylcholine. Acetylcholine is as a result of selective loss of cholinergic neurons situated in
the cerebral cortex. Part of it is also situated in the nucleus basalis and hippocampus
(Grossberg, Sadowsky & Olin, 2010).
The drug also contains tacrine. Tacrine is postulated so that it exerts its therapeutic
effect through enhancing cholinergic functions (Cummings, Farlow, Meng, Tekin & Olin,
2010). The indication is accomplished by increasing the concentration of aceytylcholine
through reversible inhibition. It is through the inhibition that hydrolysis by cholinesterase is
achieved. If the proposed mechanism is correct, the effect of ge drug and the delivery system
is reduced. The fewer the cholinergic neurons the function remains more functionally intact.
Intellectual Property Position
The intellectual position for the NDDS is not a borrowed substance from any other
company or other people (Lefevre et al., 2008). The spray drying NDDS method used to
prepare micro-particles on the drugs. The combination of the polymer adds to what makes it
Limitations
The delivery system and the drug contain little limitations. When the drug is in
contact with the skin it causes irritation. Additionally the excipients and the enhancers used
also because increased percutaneous. It is through the percutaneous caused that a second
limitation arises, making the delivery system not exactly comfortable to all the users. Another
disadvantage arises from the clinical need. The drug contains physiochemicals that penetrate
through stratum corneum entirely limiting the dosage and therapeutic value of the drug. The
physiochemical have some undesirable side effects that limits its adaptability to many users.
Pharmacy Related Information
Rivastigimine contains parasympathomimetic and cholinesterase inhibitor. The two
contained in the system are useful in the healing of the memory disease. The memory disease
associated with cognitive loss which creates a deficit is replaced by
acetylcholine. Acetylcholine is as a result of selective loss of cholinergic neurons situated in
the cerebral cortex. Part of it is also situated in the nucleus basalis and hippocampus
(Grossberg, Sadowsky & Olin, 2010).
The drug also contains tacrine. Tacrine is postulated so that it exerts its therapeutic
effect through enhancing cholinergic functions (Cummings, Farlow, Meng, Tekin & Olin,
2010). The indication is accomplished by increasing the concentration of aceytylcholine
through reversible inhibition. It is through the inhibition that hydrolysis by cholinesterase is
achieved. If the proposed mechanism is correct, the effect of ge drug and the delivery system
is reduced. The fewer the cholinergic neurons the function remains more functionally intact.
Intellectual Property Position
The intellectual position for the NDDS is not a borrowed substance from any other
company or other people (Lefevre et al., 2008). The spray drying NDDS method used to
prepare micro-particles on the drugs. The combination of the polymer adds to what makes it

EXELON PATCH 13
even more natural. The drug in its content and delivery contains natural polymer, chitosan
and transdermal delivery application. The original content is validated and determined by
High Performance Liquid Chromatography. All these information is found on the FDA
guidelines. FDA guidelines provide a clear procedure on how the formation of this drug and
the delivery system is achieved.
The legit measure of this information by the manufacturer comes through the level of
storage and packaging that the manufacturer will do. For example a drug can be stabilized at
the temperature of 7 degree Celsius. The drug can be 42% higher if the solution is meant to
be injected (Farlow, Grossberg, Sadowsky, Meng, & Somogyi, 2013). Also unique to
particular manufacturers is making the TDDS with micro-particles.
At times the micro particles are distorted in different ratios of 5, 10 to 15%. The
different permeation leads to micro particles patch or might lead to crystallization. The 6 day
prepared TDDS can be considered an alternative for one week application of 6 Exelon
patches (SHINGADE, 2012). All this information is proof that the intellectual property
position is all printed in the FDA but the different manufacturers dictate how much they
package and how they deliver them to the public.
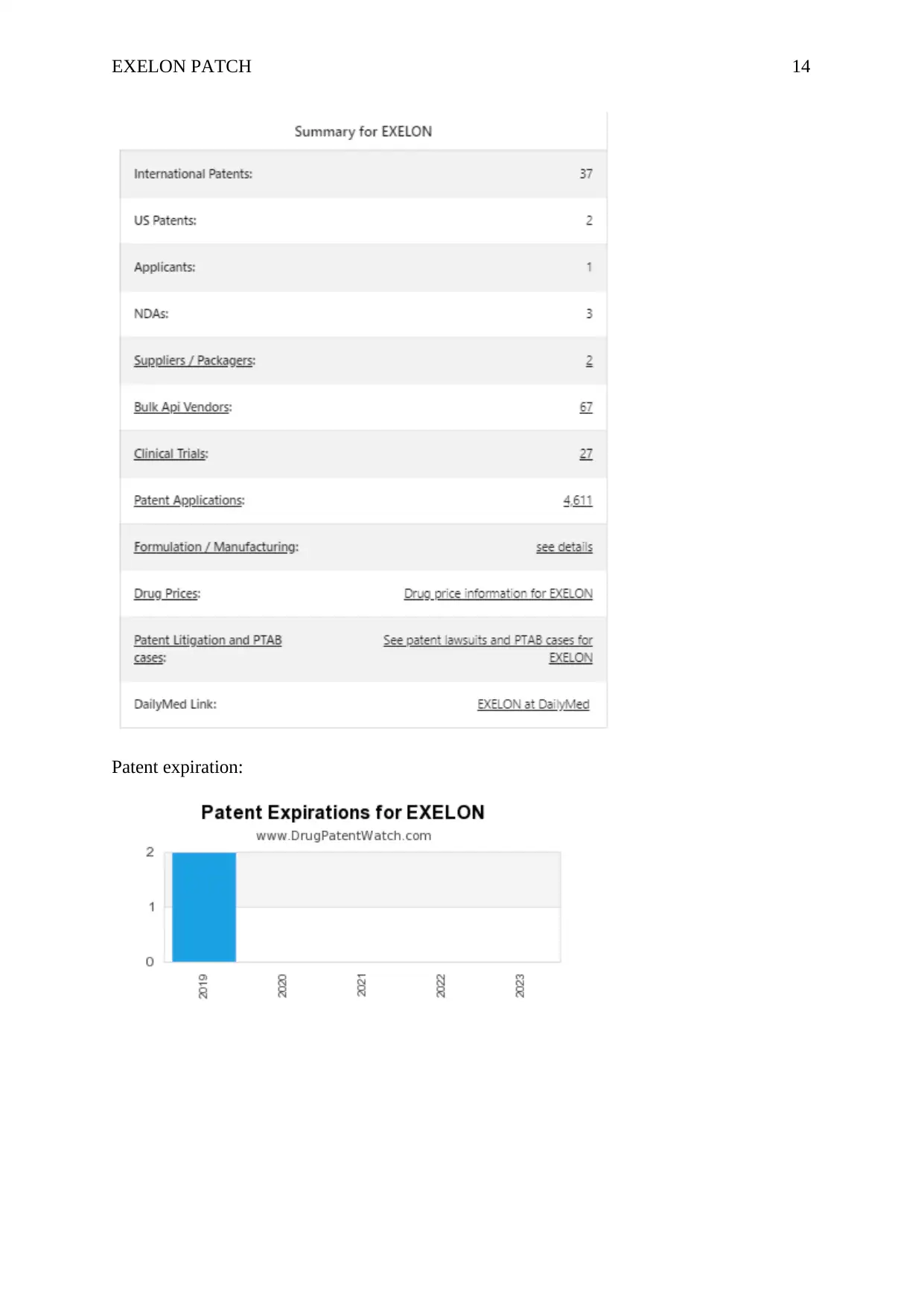
Exelon Patch is a drug which is marketed by Novartis and it is encompassed in 3
NDAs. Two patents protect Exelon patch and four paragraph IV challenges. Exelon Patch has
37 patent family members in 20 countries. The generic of it is called rivastigmine. There are
32 drug master file entries for Exelon patch. The seven suppliers are listed for this drug.
even more natural. The drug in its content and delivery contains natural polymer, chitosan
and transdermal delivery application. The original content is validated and determined by
High Performance Liquid Chromatography. All these information is found on the FDA
guidelines. FDA guidelines provide a clear procedure on how the formation of this drug and
the delivery system is achieved.
The legit measure of this information by the manufacturer comes through the level of
storage and packaging that the manufacturer will do. For example a drug can be stabilized at
the temperature of 7 degree Celsius. The drug can be 42% higher if the solution is meant to
be injected (Farlow, Grossberg, Sadowsky, Meng, & Somogyi, 2013). Also unique to
particular manufacturers is making the TDDS with micro-particles.
At times the micro particles are distorted in different ratios of 5, 10 to 15%. The
different permeation leads to micro particles patch or might lead to crystallization. The 6 day
prepared TDDS can be considered an alternative for one week application of 6 Exelon
patches (SHINGADE, 2012). All this information is proof that the intellectual property
position is all printed in the FDA but the different manufacturers dictate how much they
package and how they deliver them to the public.
Exelon Patch is a drug which is marketed by Novartis and it is encompassed in 3
NDAs. Two patents protect Exelon patch and four paragraph IV challenges. Exelon Patch has
37 patent family members in 20 countries. The generic of it is called rivastigmine. There are
32 drug master file entries for Exelon patch. The seven suppliers are listed for this drug.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
EXELON PATCH 14
Patent expiration:
Patent expiration:

EXELON PATCH 15
Conclusion
This paper has presented a discussion about Exelon Patch in terms of its ingredients,
TDDS, pharmacy related information, its manufacturers, the countries where it is available,
intellectual property, competition, sales, design/technology, limitations, advantages offered,
excipients, the rationale for the novel delivery system and characteristics.
Conclusion
This paper has presented a discussion about Exelon Patch in terms of its ingredients,
TDDS, pharmacy related information, its manufacturers, the countries where it is available,
intellectual property, competition, sales, design/technology, limitations, advantages offered,
excipients, the rationale for the novel delivery system and characteristics.

EXELON PATCH 16
References
Avinash, A., Mounica, M., Raj, C. P., Mounika, B., Kumar, B. M., & Sreenivasulu, M.
(2016). A Comprehensive Review On Transdermal Drug Delivery System.
Bhoye, A. H., Shaikh, A. A., Shah, D. P., & Patel, T. J. (2018). Transdermal Drug Delivery
System: A Review.
Cummings, J. L., Farlow, M. R., Meng, X., Tekin, S., & Olin, J. T. (2010). Rivastigmine
Transdermal Patch Skin Tolerability. Clinical drug investigation, 30(1), 41-49.
Farlow, M. R., Grossberg, G. T., Sadowsky, C. H., Meng, X., & Somogyi, M. (2013). A 24‐
week, randomized, controlled trial of rivastigmine patch 13.3 mg/24 h versus 4.6
mg/24 h in severe Alzheimer's dementia. CNS neuroscience & therapeutics, 19(10),
745-752.
Forette, F., Anand, R., & Gharabawi, G. (1999). A phase II study in patients with Alzheimer's
disease to assess the preliminary efficacy and maximum tolerated dose of
rivastigmine (Exelon®). European journal of neurology, 6(4), 423-429.
Govender, T., Choonara, Y. E., Kumar, P., Bijukumar, D., du Toit, L. C., Modi, G., ... &
Pillay, V. (2017). Implantable and transdermal polymeric drug delivery technologies
for the treatment of central nervous system disorders. Pharmaceutical development
and technology, 22(4), 476-486.
Grossberg, G. T., Sadowsky, C., & Olin, J. T. (2010). Rivastigmine transdermal system for
the treatment of mild to moderate Alzheimer’s disease. International journal of
clinical practice, 64(5), 651-660.
Knopman, D., Donohue, J. A., & Gutterman, E. M. (2000). Patterns of care in the early stages
of Alzheimer's disease: impediments to timely diagnosis. Journal of the American
Geriatrics Society, 48(3), 300-304.
References
Avinash, A., Mounica, M., Raj, C. P., Mounika, B., Kumar, B. M., & Sreenivasulu, M.
(2016). A Comprehensive Review On Transdermal Drug Delivery System.
Bhoye, A. H., Shaikh, A. A., Shah, D. P., & Patel, T. J. (2018). Transdermal Drug Delivery
System: A Review.
Cummings, J. L., Farlow, M. R., Meng, X., Tekin, S., & Olin, J. T. (2010). Rivastigmine
Transdermal Patch Skin Tolerability. Clinical drug investigation, 30(1), 41-49.
Farlow, M. R., Grossberg, G. T., Sadowsky, C. H., Meng, X., & Somogyi, M. (2013). A 24‐
week, randomized, controlled trial of rivastigmine patch 13.3 mg/24 h versus 4.6
mg/24 h in severe Alzheimer's dementia. CNS neuroscience & therapeutics, 19(10),
745-752.
Forette, F., Anand, R., & Gharabawi, G. (1999). A phase II study in patients with Alzheimer's
disease to assess the preliminary efficacy and maximum tolerated dose of
rivastigmine (Exelon®). European journal of neurology, 6(4), 423-429.
Govender, T., Choonara, Y. E., Kumar, P., Bijukumar, D., du Toit, L. C., Modi, G., ... &
Pillay, V. (2017). Implantable and transdermal polymeric drug delivery technologies
for the treatment of central nervous system disorders. Pharmaceutical development
and technology, 22(4), 476-486.
Grossberg, G. T., Sadowsky, C., & Olin, J. T. (2010). Rivastigmine transdermal system for
the treatment of mild to moderate Alzheimer’s disease. International journal of
clinical practice, 64(5), 651-660.
Knopman, D., Donohue, J. A., & Gutterman, E. M. (2000). Patterns of care in the early stages
of Alzheimer's disease: impediments to timely diagnosis. Journal of the American
Geriatrics Society, 48(3), 300-304.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

EXELON PATCH 17
Larson, E. B. (2016). Evaluation of cognitive impairment and dementia. UpToDate.
Waltham, MA: UpToDate.
Lefevre, G., Sędek, G., Jhee, S. S., Leibowitz, M. T., Huang, H. L., Enz, A., ... & Appel‐
Dingemanse, S. (2008). Pharmacokinetics and pharmacodynamics of the novel daily
rivastigmine transdermal patch compared with twice‐daily capsules in Alzheimer's
disease patients. Clinical Pharmacology & Therapeutics, 83(1), 106-114.
Lehmann, C., Koenig, T., Jelic, V., Prichep, L., John, R. E., Wahlund, L. O., ... & Dierks, T.
(2007). Application and comparison of classification algorithms for recognition of
Alzheimer's disease in electrical brain activity (EEG). Journal of neuroscience
methods, 161(2), 342-350.
Malvey, S., Rao, J. V., & Arumugam, K. M. (2019). Transdermal drug delivery system: A
mini review.
Marwah, H., Garg, T., Goyal, A. K., & Rath, G. (2016). Permeation enhancer strategies in
transdermal drug delivery. Drug delivery, 23(2), 564-578.
Mazzeo, L., Bianchi, M., Cocchi, M., & Piemonte, V. (2019). Drug Delivery With
Membranes Systems. In Current Trends and Future Developments on (Bio-)
Membranes (pp. 291-309). Elsevier.
Moga, D. C., Roberts, M., & Jicha, G. (2017). Dementia for the primary care
provider. Primary Care: Clinics in Office Practice, 44(3), 439-456.
Morris, E., Chalkidou, A., Hammers, A., Peacock, J., Summers, J., & Keevil, S. (2016).
Diagnostic accuracy of 18 F amyloid PET tracers for the diagnosis of Alzheimer’s
disease: a systematic review and meta-analysis. European journal of nuclear
medicine and molecular imaging, 43(2), 374-385.
Pearce, E. N. (2016). Subclinical Hyperthyroidism, but not Subclinical Hypothyroidism, Is
Associated with Increased Dementia Risk. Clinical Thyroidology, 28(11), 340-342.
Larson, E. B. (2016). Evaluation of cognitive impairment and dementia. UpToDate.
Waltham, MA: UpToDate.
Lefevre, G., Sędek, G., Jhee, S. S., Leibowitz, M. T., Huang, H. L., Enz, A., ... & Appel‐
Dingemanse, S. (2008). Pharmacokinetics and pharmacodynamics of the novel daily
rivastigmine transdermal patch compared with twice‐daily capsules in Alzheimer's
disease patients. Clinical Pharmacology & Therapeutics, 83(1), 106-114.
Lehmann, C., Koenig, T., Jelic, V., Prichep, L., John, R. E., Wahlund, L. O., ... & Dierks, T.
(2007). Application and comparison of classification algorithms for recognition of
Alzheimer's disease in electrical brain activity (EEG). Journal of neuroscience
methods, 161(2), 342-350.
Malvey, S., Rao, J. V., & Arumugam, K. M. (2019). Transdermal drug delivery system: A
mini review.
Marwah, H., Garg, T., Goyal, A. K., & Rath, G. (2016). Permeation enhancer strategies in
transdermal drug delivery. Drug delivery, 23(2), 564-578.
Mazzeo, L., Bianchi, M., Cocchi, M., & Piemonte, V. (2019). Drug Delivery With
Membranes Systems. In Current Trends and Future Developments on (Bio-)
Membranes (pp. 291-309). Elsevier.
Moga, D. C., Roberts, M., & Jicha, G. (2017). Dementia for the primary care
provider. Primary Care: Clinics in Office Practice, 44(3), 439-456.
Morris, E., Chalkidou, A., Hammers, A., Peacock, J., Summers, J., & Keevil, S. (2016).
Diagnostic accuracy of 18 F amyloid PET tracers for the diagnosis of Alzheimer’s
disease: a systematic review and meta-analysis. European journal of nuclear
medicine and molecular imaging, 43(2), 374-385.
Pearce, E. N. (2016). Subclinical Hyperthyroidism, but not Subclinical Hypothyroidism, Is
Associated with Increased Dementia Risk. Clinical Thyroidology, 28(11), 340-342.

EXELON PATCH 18
Sheth, K. (2019). Cientific Drug Delivery Research (CDDR)
SHINGADE, G. M. (2012). Review on: recent trend on transdermal drug delivery
system. Journal of drug delivery and therapeutics, 2(1
Zhao, X., Liu, Y., Wang, X., Liu, B., Xi, Q., Guo, Q., ... & Wang, P. (2012). Disrupted small-
world brain networks in moderate Alzheimer's disease: a resting-state FMRI
study. PloS one, 7(3), e33540.
Sheth, K. (2019). Cientific Drug Delivery Research (CDDR)
SHINGADE, G. M. (2012). Review on: recent trend on transdermal drug delivery
system. Journal of drug delivery and therapeutics, 2(1
Zhao, X., Liu, Y., Wang, X., Liu, B., Xi, Q., Guo, Q., ... & Wang, P. (2012). Disrupted small-
world brain networks in moderate Alzheimer's disease: a resting-state FMRI
study. PloS one, 7(3), e33540.
1 out of 18
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
