Factors Affecting Boiling Points of Organic Compounds
VerifiedAdded on 2023/06/04
|8
|1485
|248
AI Summary
This article discusses the factors that affect the boiling points of organic compounds, including intermolecular forces, molecular weight, and functional groups. It also explains the differences in boiling points between alcohols and ketones, and how branching affects boiling points. The article includes a table and a diagram showing the boiling points of different organic compounds.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

The phase at which the attractive forces in liquids are completely broken for the liquid to
become gaseous is known as the boiling point. Boiling points differ based on the strength of
the attractive forces in the molecules. Four types of intermolecular forces hold most of the
organic substances and these are: Ionic forces, hydrogen bonding, dipole-dipole moments and
Van der Waals dispersion forces. According to Mumford and Phillips (1950 p.75-84), an
increase in polarization of the molecule consequently leads to an increase in boiling points
resulting from stronger intermolecular associations. The highest boiling points are in
compounds held by strong ionic bonds, followed by molecules held by relatively strong
hydrogen bonds, followed by those held by dipole-dipole interactions and lastly the
molecules head by weak Van der Waals dispersion forces have the least boiling points.
The loss or gain of electrons results in the formation of charged particles held together by
strong ionic bonds. The positively charged ions which are mostly metals like magnesium
(Mg+2) and aluminium (Al+3) are called cations whereas, the negatively charged ions which
are mostly non-metals like oxygen (O-2) and fluoride (F-) are called anions. Coulomb, in his
law describes the attraction between opposing forces which increases with an increase in the
charge density and decreases when the distance separating the ions is increased. According to
Mumford and Phillips (1950 p.75-84 ), ionic bonded molecules have very high boiling
points as a result of their very high polarization. In addition to this, these molecules are very
soluble in water.
According to Jim Clark (2013), the measure of the tendency of an atom to attract a bonding
pair of electrons is what is known as electronegativity. Various elements like fluorine,
oxygen, nitrogen and chlorine are very electronegative and hence end up drawing electrons
become gaseous is known as the boiling point. Boiling points differ based on the strength of
the attractive forces in the molecules. Four types of intermolecular forces hold most of the
organic substances and these are: Ionic forces, hydrogen bonding, dipole-dipole moments and
Van der Waals dispersion forces. According to Mumford and Phillips (1950 p.75-84), an
increase in polarization of the molecule consequently leads to an increase in boiling points
resulting from stronger intermolecular associations. The highest boiling points are in
compounds held by strong ionic bonds, followed by molecules held by relatively strong
hydrogen bonds, followed by those held by dipole-dipole interactions and lastly the
molecules head by weak Van der Waals dispersion forces have the least boiling points.
The loss or gain of electrons results in the formation of charged particles held together by
strong ionic bonds. The positively charged ions which are mostly metals like magnesium
(Mg+2) and aluminium (Al+3) are called cations whereas, the negatively charged ions which
are mostly non-metals like oxygen (O-2) and fluoride (F-) are called anions. Coulomb, in his
law describes the attraction between opposing forces which increases with an increase in the
charge density and decreases when the distance separating the ions is increased. According to
Mumford and Phillips (1950 p.75-84 ), ionic bonded molecules have very high boiling
points as a result of their very high polarization. In addition to this, these molecules are very
soluble in water.
According to Jim Clark (2013), the measure of the tendency of an atom to attract a bonding
pair of electrons is what is known as electronegativity. Various elements like fluorine,
oxygen, nitrogen and chlorine are very electronegative and hence end up drawing electrons
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

from the bonded atoms near them, creating temporary charges on these atoms. The most
electronegative atom acquires a temporary negative charge, while the lesser electronegative
atom acquires a temporary positive charge. The hydrogen proton which is positively charged
therefore gets attracted to these negatively charged atoms in solution, forming the hydrogen
bond. These bonds are transient and hence, are short-lived as a result of the rapid molecular
motion in the solution. Their strength is relatively high, about 9 kJ/mol, and hence, they need
huge amounts of energies to break. Hydrogen bonds become stronger with an increase in
electronegativity of the bonded atoms.
Besides the attraction of unlike charges, dipoles too can rearrange themselves in an attractive
manner creating the Van der Waal dipole-dipole interaction. These bonds are weaker than
hydrogen bonds as they are carbon-hydrogen bonds. The carbon has lower electronegativity
as compared to the halogens and nitrogen and hence, polarity of the molecule is reduced.
Since dipole-dipole interactions are weaker than hydrogen bonds, they will require less
energy to break therefore, have a lower boiling point.
According to Mumford and Phillips (1950 p.75-84), dispersion forces are adjacent dipole
attractions in a molecule that are very easy to break on application of energy. This is
attributed to the fact that dipoles are weak and transient and are dependent on the interaction
with molecules directly. Therefore, dispersion forces increase with surface area.
Consequently, an increase in molecular weight leads to an increase with the dispersion forces
and hence increase in boiling points in molecules in the same family.
The functionality of an organic compound influences boiling points. For instance,
compounds with hydroxyl groups like the alcohols that contain hydrogen bonds have greater
boiling points than the ketones that possess the carbonyl group that contains dipole-dipole
moments.
electronegative atom acquires a temporary negative charge, while the lesser electronegative
atom acquires a temporary positive charge. The hydrogen proton which is positively charged
therefore gets attracted to these negatively charged atoms in solution, forming the hydrogen
bond. These bonds are transient and hence, are short-lived as a result of the rapid molecular
motion in the solution. Their strength is relatively high, about 9 kJ/mol, and hence, they need
huge amounts of energies to break. Hydrogen bonds become stronger with an increase in
electronegativity of the bonded atoms.
Besides the attraction of unlike charges, dipoles too can rearrange themselves in an attractive
manner creating the Van der Waal dipole-dipole interaction. These bonds are weaker than
hydrogen bonds as they are carbon-hydrogen bonds. The carbon has lower electronegativity
as compared to the halogens and nitrogen and hence, polarity of the molecule is reduced.
Since dipole-dipole interactions are weaker than hydrogen bonds, they will require less
energy to break therefore, have a lower boiling point.
According to Mumford and Phillips (1950 p.75-84), dispersion forces are adjacent dipole
attractions in a molecule that are very easy to break on application of energy. This is
attributed to the fact that dipoles are weak and transient and are dependent on the interaction
with molecules directly. Therefore, dispersion forces increase with surface area.
Consequently, an increase in molecular weight leads to an increase with the dispersion forces
and hence increase in boiling points in molecules in the same family.
The functionality of an organic compound influences boiling points. For instance,
compounds with hydroxyl groups like the alcohols that contain hydrogen bonds have greater
boiling points than the ketones that possess the carbonyl group that contains dipole-dipole
moments.
Molecular structural isotopes differ in their molecular surface area that sequentially
influences the strength of the Van der Waals dispersion forces holding them and thus, the
boiling points of the molecules are affected Burger et al (2011 p.121). Surface area of a
molecule is directly proportional to the molecular weight of the molecule and therefore, long
chain molecules with greater surface area have higher molecular weights, consequently, have
higher boiling points due to the increase in the strength of the bonds. In the process of
increasing surface area, the attraction ability of the molecules is also increased.
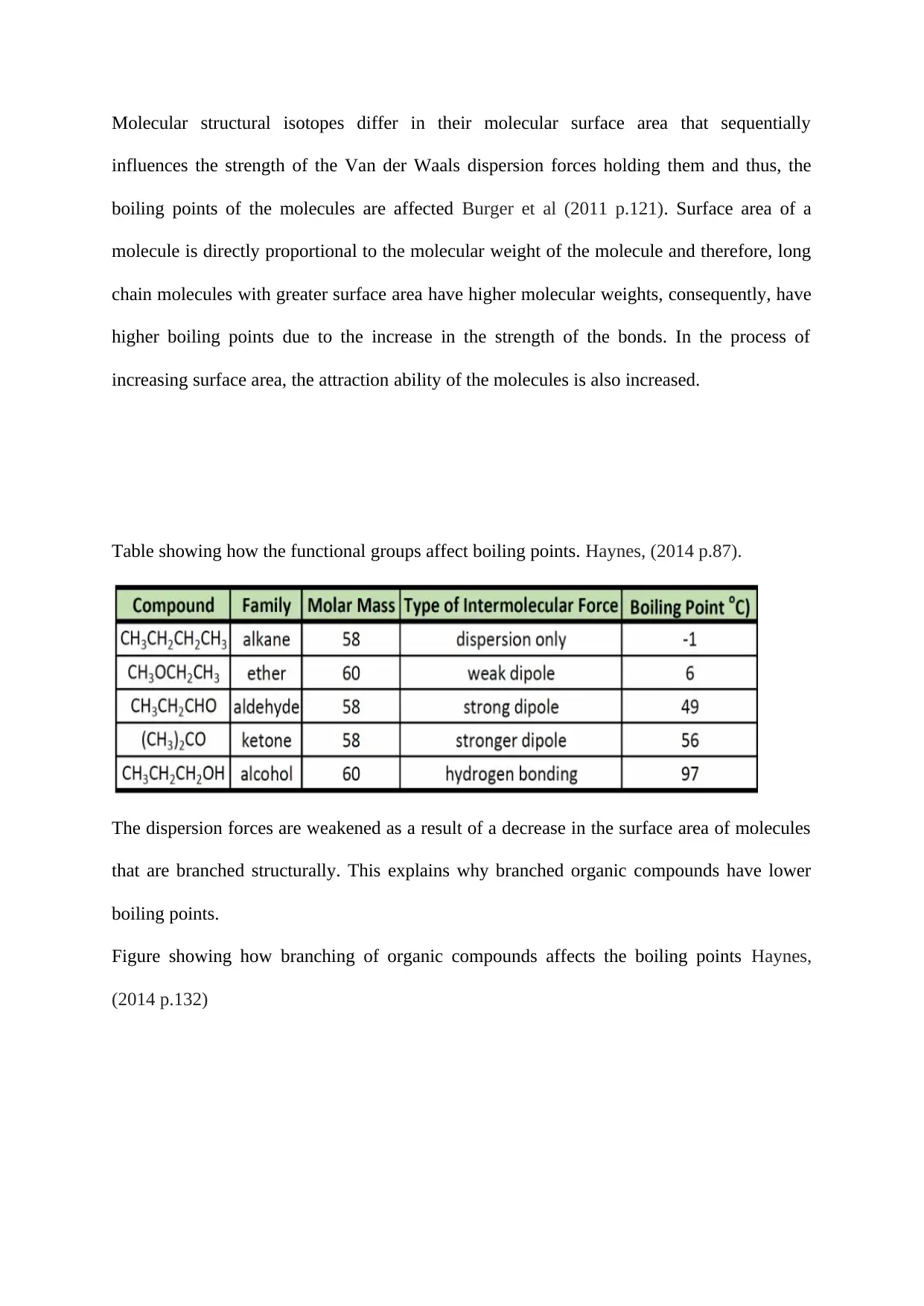
Table showing how the functional groups affect boiling points. Haynes, (2014 p.87).
The dispersion forces are weakened as a result of a decrease in the surface area of molecules
that are branched structurally. This explains why branched organic compounds have lower
boiling points.
Figure showing how branching of organic compounds affects the boiling points Haynes,
(2014 p.132)
influences the strength of the Van der Waals dispersion forces holding them and thus, the
boiling points of the molecules are affected Burger et al (2011 p.121). Surface area of a
molecule is directly proportional to the molecular weight of the molecule and therefore, long
chain molecules with greater surface area have higher molecular weights, consequently, have
higher boiling points due to the increase in the strength of the bonds. In the process of
increasing surface area, the attraction ability of the molecules is also increased.
Table showing how the functional groups affect boiling points. Haynes, (2014 p.87).
The dispersion forces are weakened as a result of a decrease in the surface area of molecules
that are branched structurally. This explains why branched organic compounds have lower
boiling points.
Figure showing how branching of organic compounds affects the boiling points Haynes,
(2014 p.132)
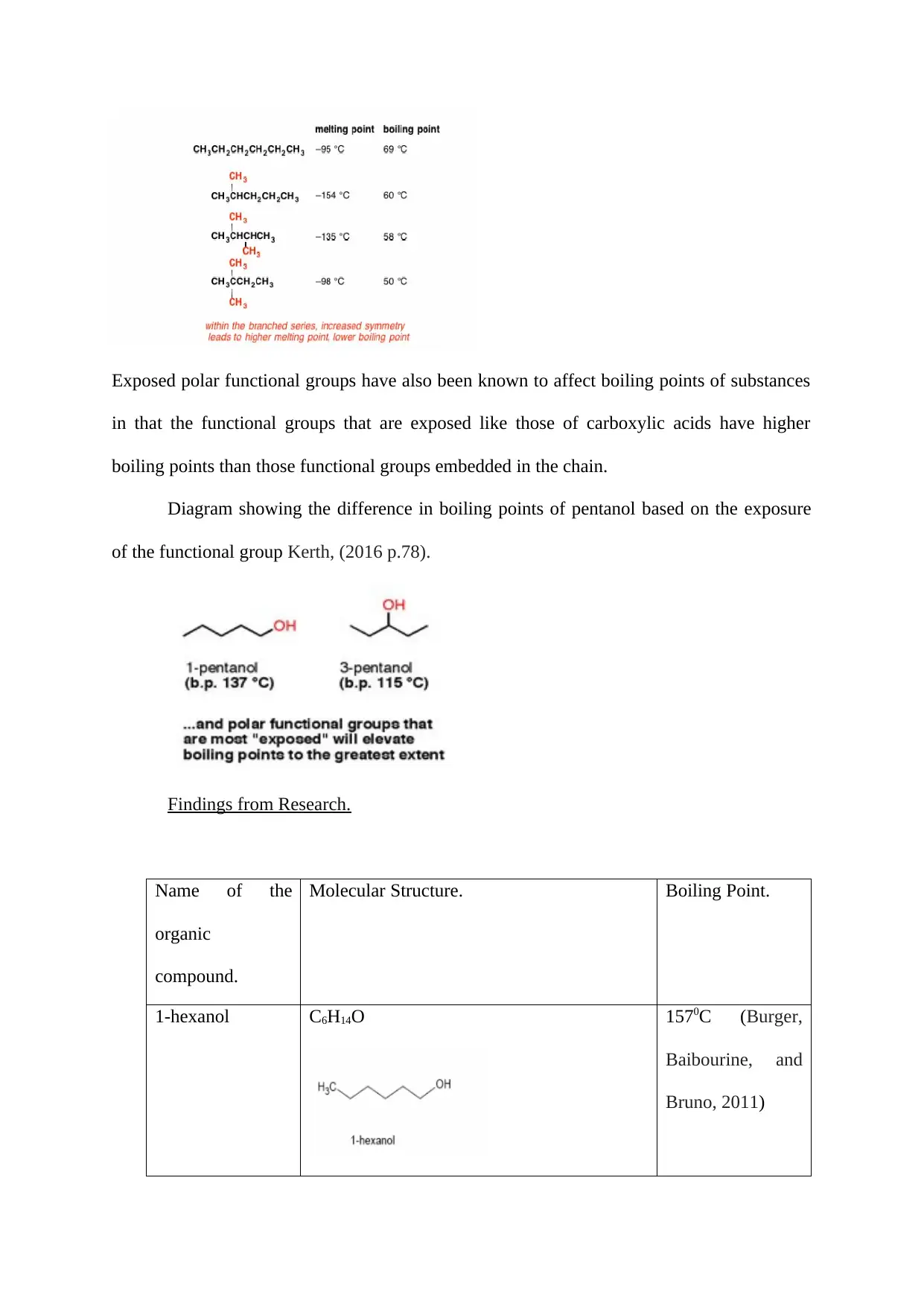
Exposed polar functional groups have also been known to affect boiling points of substances
in that the functional groups that are exposed like those of carboxylic acids have higher
boiling points than those functional groups embedded in the chain.
Diagram showing the difference in boiling points of pentanol based on the exposure
of the functional group Kerth, (2016 p.78).
Findings from Research.
Name of the
organic
compound.
Molecular Structure. Boiling Point.
1-hexanol C6H14O 1570C (Burger,
Baibourine, and
Bruno, 2011)
in that the functional groups that are exposed like those of carboxylic acids have higher
boiling points than those functional groups embedded in the chain.
Diagram showing the difference in boiling points of pentanol based on the exposure
of the functional group Kerth, (2016 p.78).
Findings from Research.
Name of the
organic
compound.
Molecular Structure. Boiling Point.
1-hexanol C6H14O 1570C (Burger,
Baibourine, and
Bruno, 2011)
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
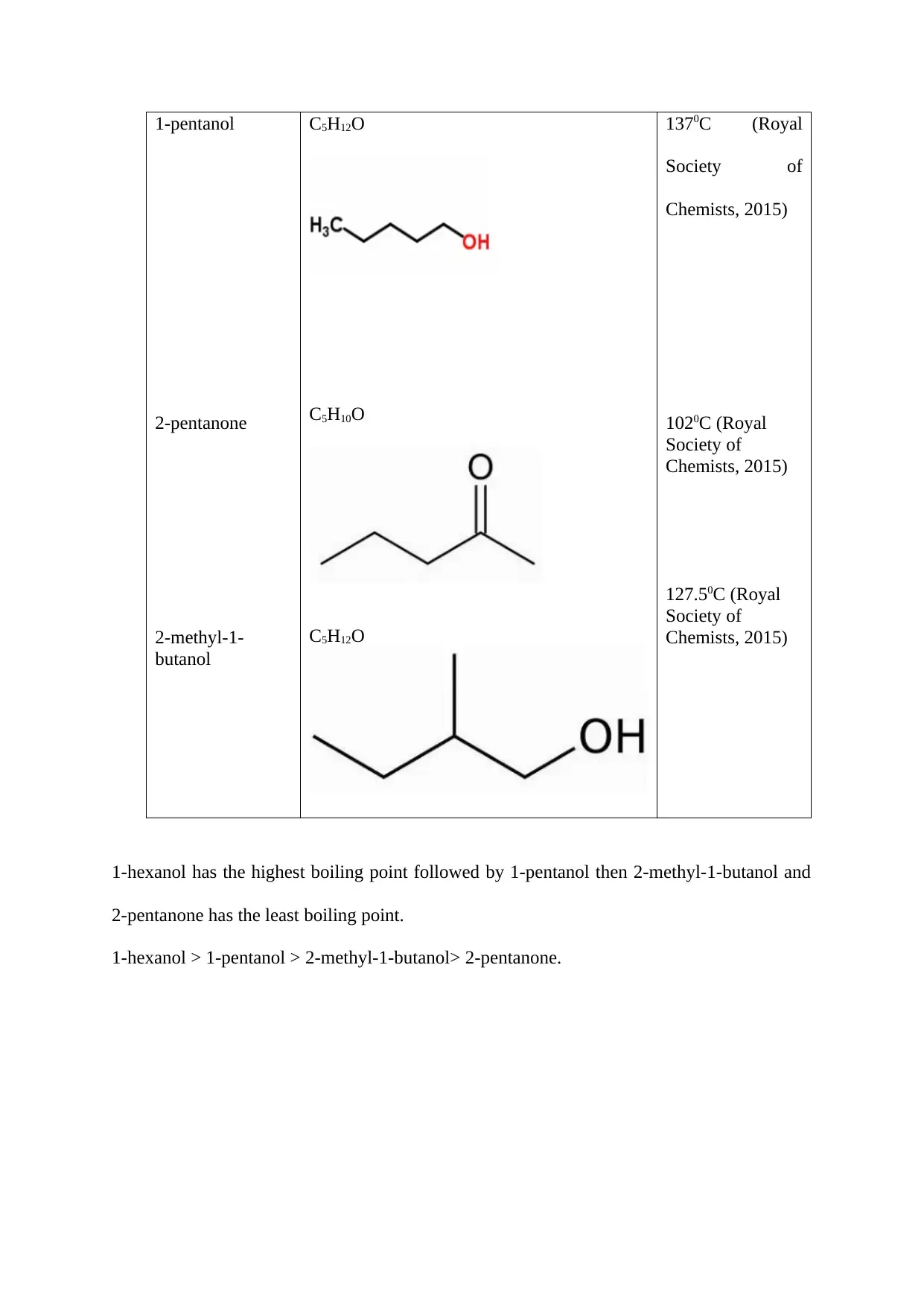
1-pentanol
2-pentanone
2-methyl-1-
butanol
C5H12O
C5H10O
C5H12O
1370C (Royal
Society of
Chemists, 2015)
1020C (Royal
Society of
Chemists, 2015)
127.50C (Royal
Society of
Chemists, 2015)
1-hexanol has the highest boiling point followed by 1-pentanol then 2-methyl-1-butanol and
2-pentanone has the least boiling point.
1-hexanol > 1-pentanol > 2-methyl-1-butanol> 2-pentanone.
2-pentanone
2-methyl-1-
butanol
C5H12O
C5H10O
C5H12O
1370C (Royal
Society of
Chemists, 2015)
1020C (Royal
Society of
Chemists, 2015)
127.50C (Royal
Society of
Chemists, 2015)
1-hexanol has the highest boiling point followed by 1-pentanol then 2-methyl-1-butanol and
2-pentanone has the least boiling point.
1-hexanol > 1-pentanol > 2-methyl-1-butanol> 2-pentanone.

Discussion.
1-hexanol, 1-pentanol and 2-methyl-1-butanol are all alcohols held by strong hydrogen bonds
in their structure that attribute to their high boiling points as compared to the 2-pentanone
which is a ketone held by dipole-dipole moments which are weaker than the hydrogen bonds
and hence its lower boiling points. However, despite the alcohols possessing the hydrogen
bonds, their boiling points differ from each other with 1-hexanol having the highest boiling
point. This difference is a result of the 1-hexanol molecule possessing a sic carbon chain
molecule that increases its surface area as well as its molecular weight and hence, it has
stronger hydrogen bonds than the other molecules and hence, the higher boiling point. 1-
pentanol and 2-methyl-1-butanol are molecular structural isomers. This means that the
difference in their structure causes the difference in their boiling points. Branched
compounds are known to have a lesser boiling point as a result of reduction in surface area
which in turn reduces the strength of the binds in the molecule. For this reason, 2-methyl-1-
butanol has a lower boiling point than the 1-pentanol.
1-hexanol, 1-pentanol and 2-methyl-1-butanol are all alcohols held by strong hydrogen bonds
in their structure that attribute to their high boiling points as compared to the 2-pentanone
which is a ketone held by dipole-dipole moments which are weaker than the hydrogen bonds
and hence its lower boiling points. However, despite the alcohols possessing the hydrogen
bonds, their boiling points differ from each other with 1-hexanol having the highest boiling
point. This difference is a result of the 1-hexanol molecule possessing a sic carbon chain
molecule that increases its surface area as well as its molecular weight and hence, it has
stronger hydrogen bonds than the other molecules and hence, the higher boiling point. 1-
pentanol and 2-methyl-1-butanol are molecular structural isomers. This means that the
difference in their structure causes the difference in their boiling points. Branched
compounds are known to have a lesser boiling point as a result of reduction in surface area
which in turn reduces the strength of the binds in the molecule. For this reason, 2-methyl-1-
butanol has a lower boiling point than the 1-pentanol.

Conclusion.
In conclusion, the hydrogen bonding in the alcohol molecules are the cause of the observed
difference in the boiling points of the alcohols and the ketone since the hydrogen bond need
more energy to overcome them as compared to the dipole-dipole bonds found in the ketone
molecule. Also, branching in molecules and the molecular weight of the molecule have been
observed to affect the boiling points of the molecules, where branched molecules and low
molecular weight molecules have lower boiling points.
In conclusion, the hydrogen bonding in the alcohol molecules are the cause of the observed
difference in the boiling points of the alcohols and the ketone since the hydrogen bond need
more energy to overcome them as compared to the dipole-dipole bonds found in the ketone
molecule. Also, branching in molecules and the molecular weight of the molecule have been
observed to affect the boiling points of the molecules, where branched molecules and low
molecular weight molecules have lower boiling points.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References.
Burger, J.L., Baibourine, E. and Bruno, T.J., 2011. Comparison of diesel fuel oxygenate
additives to the composition-explicit distillation curve method. Part 4: alcohols, aldehydes,
hydroxy ethers, and esters of butanoic acid. Energy & Fuels, 26(2), pp.1114-1123.
Haynes, W.M., 2014. CRC handbook of chemistry and physics. CRC press.
Kerth, C., 2016. Determination of volatile aroma compounds in beef using differences in
steak thickness and cook surface temperature. Meat science, 117, pp.27-35.
Vogel, A.I., 2013. A text-book of practical organic chemistry including qualitative organic
analysis. Longmans Green And Co; London; New York; Toronto.
Mumford, S. A. and Phillip, J.W. (1950). The Physical Propertis of Some Aliphatic
Compounds. J. Chem.Soc, 19, pp 75-84
Clark, J. (2013). Electronegativity. ChemGuide Journal. Available from,
https://chemguide.co.uk/atoms/bonding/electroneg.html Retrieved [10th October, 2018].
Royal Society of Chemists. (2015). Organic Compounds. ChemSpider Data Base. Available
from, http://www.chemspider.com/Chemical-Structure.7012.html Retrieved [10th October,
2018].
Burger, J.L., Baibourine, E. and Bruno, T.J., 2011. Comparison of diesel fuel oxygenate
additives to the composition-explicit distillation curve method. Part 4: alcohols, aldehydes,
hydroxy ethers, and esters of butanoic acid. Energy & Fuels, 26(2), pp.1114-1123.
Haynes, W.M., 2014. CRC handbook of chemistry and physics. CRC press.
Kerth, C., 2016. Determination of volatile aroma compounds in beef using differences in
steak thickness and cook surface temperature. Meat science, 117, pp.27-35.
Vogel, A.I., 2013. A text-book of practical organic chemistry including qualitative organic
analysis. Longmans Green And Co; London; New York; Toronto.
Mumford, S. A. and Phillip, J.W. (1950). The Physical Propertis of Some Aliphatic
Compounds. J. Chem.Soc, 19, pp 75-84
Clark, J. (2013). Electronegativity. ChemGuide Journal. Available from,
https://chemguide.co.uk/atoms/bonding/electroneg.html Retrieved [10th October, 2018].
Royal Society of Chemists. (2015). Organic Compounds. ChemSpider Data Base. Available
from, http://www.chemspider.com/Chemical-Structure.7012.html Retrieved [10th October,
2018].
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.





