Factors Affecting Reaction | Report
Added on 2022-08-21
11 Pages1747 Words35 Views
Reaction kinetics 1
FACTORS AFFECTING REACTION
by (Name)
Course
Instructor
University
City
Date
FACTORS AFFECTING REACTION
by (Name)
Course
Instructor
University
City
Date

Reaction kinetics 2
ABSTRACT
Chemical kinetics, which is also called reaction kinetics is the study of chemical processes.
These are not limited to the investigation of how various experimental situations can affect the
speed of a chemical reaction and it products. Chemical reactions differ greatly in quickness at
which they take place. Some are instantaneous while others take ages to come to attain
equilibrium position. The experiment is conducted to confirm the factors affecting reaction rate.
ABSTRACT
Chemical kinetics, which is also called reaction kinetics is the study of chemical processes.
These are not limited to the investigation of how various experimental situations can affect the
speed of a chemical reaction and it products. Chemical reactions differ greatly in quickness at
which they take place. Some are instantaneous while others take ages to come to attain
equilibrium position. The experiment is conducted to confirm the factors affecting reaction rate.

Reaction kinetics 3
FACTORS AFFECTING REACTION RATE
Introduction
Rate of a reaction is a measure of how fast or slow a reaction occurs. To be able to
determine how a reaction progresses, time taken for reaction reactants to be completely
consumed over a duration of time.
Reaction rate= quantity of substance change / time for change to occur
Hence, reaction rate is a rate of change of an amount or concentration of a particular reactant per
unit time. A number of aspects which affect reaction rate; reacting species concentration ,
temperature, reactants surface area and the catalyst.
EXPERIMENT TO INVESTIGATE FACTORS AFFECTING REACTION OF MAGNESIUM
CARBONATE AND DILUTE NITRIC ACID.
Results and discussion
Results
Reactants
With a catalyst
Time for gas to be collected (s)
At 50 °C At 60 °C
Powdered metal carbonate, 2 moldm-3 acid 40 30
Lumps of metal carbonate, 2 moldm-3 acid 80 60
Powdered metal carbonate, 1 moldm-3 acid 60 50
Lumps of metal carbonate, 1 moldm-3 acid 120 100
Without a catalyst
FACTORS AFFECTING REACTION RATE
Introduction
Rate of a reaction is a measure of how fast or slow a reaction occurs. To be able to
determine how a reaction progresses, time taken for reaction reactants to be completely
consumed over a duration of time.
Reaction rate= quantity of substance change / time for change to occur
Hence, reaction rate is a rate of change of an amount or concentration of a particular reactant per
unit time. A number of aspects which affect reaction rate; reacting species concentration ,
temperature, reactants surface area and the catalyst.
EXPERIMENT TO INVESTIGATE FACTORS AFFECTING REACTION OF MAGNESIUM
CARBONATE AND DILUTE NITRIC ACID.
Results and discussion
Results
Reactants
With a catalyst
Time for gas to be collected (s)
At 50 °C At 60 °C
Powdered metal carbonate, 2 moldm-3 acid 40 30
Lumps of metal carbonate, 2 moldm-3 acid 80 60
Powdered metal carbonate, 1 moldm-3 acid 60 50
Lumps of metal carbonate, 1 moldm-3 acid 120 100
Without a catalyst
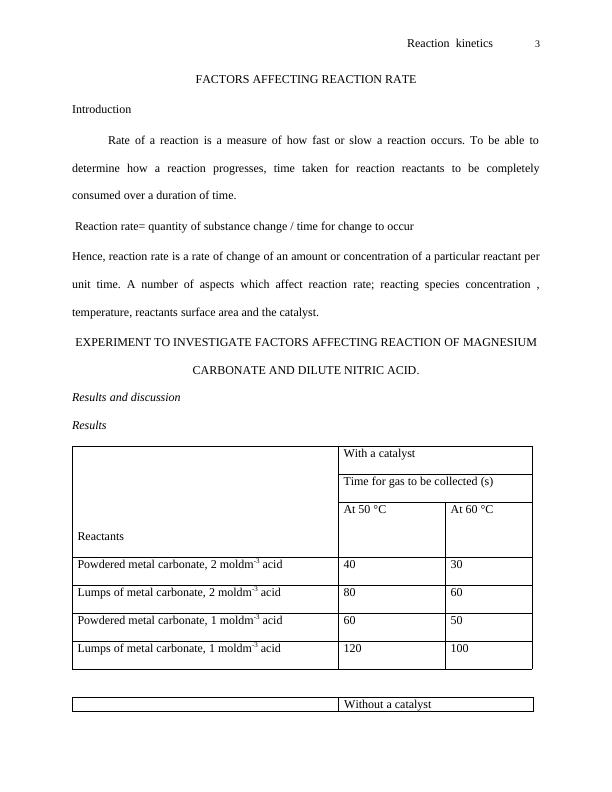
Reaction kinetics 4
Reactants
Time for gas to be collected (s)
At 50 °C At 60 °C
Powdered metal carbonate, 2 moldm-3 acid 80 60
Lumps of metal carbonate, 2 moldm-3 acid 160 120
Powdered metal carbonate, 1 moldm-3 acid 120 100
Lumps of metal carbonate, 1 moldm-3 acid 240 200
Discussions
Influence of temperature on reaction rate
When equal powdered magnesium carbonate was introduced in heated nitric acid with a
temperature of 60 °C, it took relatively shorter time for carbon IV oxide gas to be collected than
when it was introduced in heated nitric acid with a temperature of 50 °C.
Illustration of a chemical reaction
MgCO3 (s) + HNO3 (aq.) → Mg(NO3)2 (aq.) + H2O (l) + CO2 (aq.)
According to collision theory, temperature rise escalates the kinetic energy of the reacting
species (MgCO3 (s) and HNO3 (aq.)) which in turn increases the incidence of collision of the
reacting species. The escalation in kinetic energy of the colliding particles provides the needed
activation energy necessary for the reaction to occur. A rise in temperature therefore escalates
the rate of reaction, making it to proceed faster (Laidler, J. 2016).
Figure 1: showing the influence of temperature on reaction rate
Reactants
Time for gas to be collected (s)
At 50 °C At 60 °C
Powdered metal carbonate, 2 moldm-3 acid 80 60
Lumps of metal carbonate, 2 moldm-3 acid 160 120
Powdered metal carbonate, 1 moldm-3 acid 120 100
Lumps of metal carbonate, 1 moldm-3 acid 240 200
Discussions
Influence of temperature on reaction rate
When equal powdered magnesium carbonate was introduced in heated nitric acid with a
temperature of 60 °C, it took relatively shorter time for carbon IV oxide gas to be collected than
when it was introduced in heated nitric acid with a temperature of 50 °C.
Illustration of a chemical reaction
MgCO3 (s) + HNO3 (aq.) → Mg(NO3)2 (aq.) + H2O (l) + CO2 (aq.)
According to collision theory, temperature rise escalates the kinetic energy of the reacting
species (MgCO3 (s) and HNO3 (aq.)) which in turn increases the incidence of collision of the
reacting species. The escalation in kinetic energy of the colliding particles provides the needed
activation energy necessary for the reaction to occur. A rise in temperature therefore escalates
the rate of reaction, making it to proceed faster (Laidler, J. 2016).
Figure 1: showing the influence of temperature on reaction rate
End of preview
Want to access all the pages? Upload your documents or become a member.
Related Documents
Comparison of SN1 and SN2 Mechanismslg...
|5
|1232
|75
