Proteins, Enzymes, and Metabolism
VerifiedAdded on 2019/09/26
|5
|954
|412
Practical Assignment
AI Summary
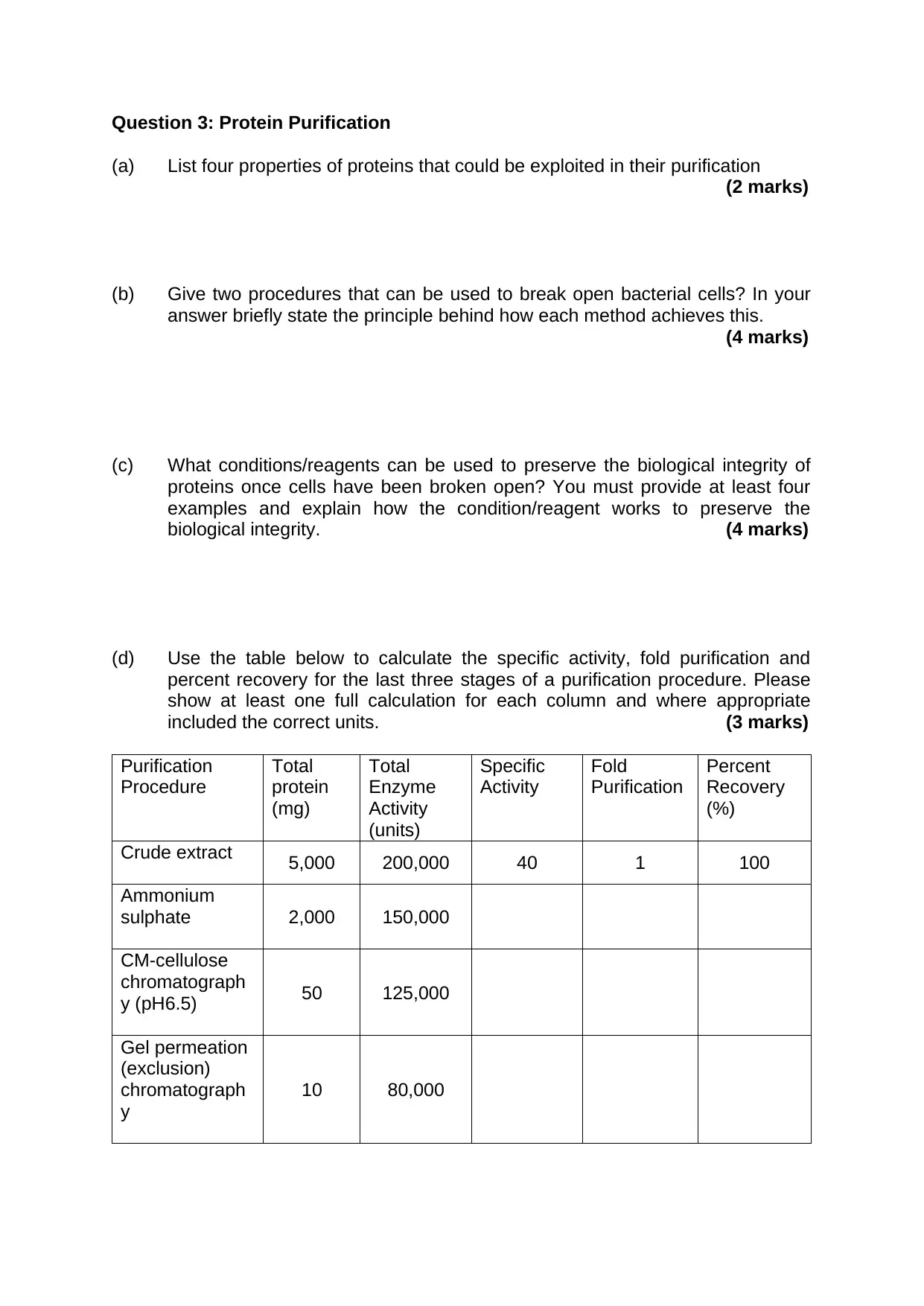
This assignment is for students in the Faculty of Science, Engineering and Computing, Department of Biomolecular Sciences and Applied & Human Sciences. The assignment is divided into three questions: Enzymes, Protein Structure, and Protein Purification. The first question focuses on enzymes, discussing their importance, properties, and regulation. The second question covers protein structure, including topics such as hydrogen bonding in alpha-helix and beta-sheets, tertiary structure formation, and the interaction between FMN and azoreductase. The third question is about protein purification, covering topics such as exploiting protein properties for purification, breaking open bacterial cells, preserving biological integrity, calculating specific activity, fold purification, and percent recovery, and explaining the use of SDS electrophoresis to deduce purity.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.
1 out of 5






![[object Object]](/_next/static/media/star-bottom.7253800d.svg)