Galantamine Treatment Effects on 5XFAD Mouse Model of Alzheimer's
VerifiedAdded on 2023/04/21
|12
|11185
|141
Report
AI Summary
This study investigates the impact of chronic galantamine treatment on the 5XFAD mouse model of Alzheimer's disease, focusing on behavioral changes and amyloid beta (Aβ) plaque deposition. The research reveals a gender-specific phenotype in untreated mice, with females showing higher plaque density than males. Galantamine treatment improved performance in behavioral tests and significantly reduced plaque density in the entorhinal cortex and hippocampus. Higher doses of galantamine resulted in more pronounced positive effects on plaque density and behavior. The findings suggest that galantamine may possess disease-modifying and neuroprotective properties beyond symptomatic relief, as indicated by delayed Aβ plaque formation and reduced gliosis. Desklib offers a variety of study tools, including similar reports and solved assignments, to aid students in their academic pursuits.

Galantamine Slows Down Plaque Formation and
Behavioral Decline in the 5XFAD Mouse Model of
Alzheimer’s Disease
Soumee Bhattacharya1, Christin Haertel1, Alfred Maelicke2, Dirk Montag 1*
1 Neurogenetics SpecialLaboratory,Leibniz Institute for Neurobiology,Magdeburg,Germany,2 Galantos Pharma GmbH,Nieder-Olm,Germany
Abstract
The plantalkaloid galantamine is an established symptomatic drug treatmentfor Alzheimer’s disease (AD),providing
temporary cognitive and globalrelief in human patients.In this study,the 5X FamilialAlzheimer’s Disease (5XFAD) mouse
modelwas used to investigate the effectof chronic galantamine treatmenton behaviorand amyloid b (Ab)plaque
deposition in the mouse brain. Quantification of plaques in untreated 5XFAD mice showed a gender specific phenotype
plaque density increased steadily reaching saturation in males after 10 months ofage,whereas in females the density
furtherincreased untilafter14 months ofage.Moreover,females consistently displayed a higherplaque density in
comparison to males ofthe same age.Chronic oraltreatmentwith galantamine resulted in improved performance in
behavioraltests,such as open field and light-dark avoidance,already at mildly affected stages compared to untreated
controls. Treated animals of both sexes showed significantly lower plaque density in the brain, i.e., the entorhinal corte
hippocampus,gliosis being always positively correlated to plaque load.A high dose treatment with a daily uptake of
26 mg/kg body weight was tolerated welland produced significantly larger positive effects than a lower dose treatment
(14 mg/kg body weight)in terms ofplaque density and behavior.These results strongly support that galantamine,in
addition to improving cognitive and behavioralsymptomsin AD, may have disease-modifying and neuroprotective
properties,as is indicated by delayed Ab plaque formation and reduced gliosis.
Citation: Bhattacharya S, Haertel C, Maelicke A, Montag D (2014) Galantamine Slows Down Plaque Formation and Behavioral Decline in the 5XFAD Mouse Mo
of Alzheimer’s Disease.PLoS ONE 9(2):e89454.doi:10.1371/journal.pone.0089454
Editor: Thomas Arendt,University of Leipzig,Germany
Received November 1,2013;Accepted January 21,2014;Published February 21,2014
Copyright: ß 2014 Bhattacharya et al.This is an open-access article distributed under the terms of the Creative Commons Attribution License,which permits
unrestricted use,distribution,and reproduction in any medium,provided the originalauthor and source are credited.
Funding: This study was supported by the German Ministry for Education and Research (BMBF) special network program KMU-Innovativ-2(http://www.bmbf.d
en/986.php).The funders had no role in study design,data collection and analysis,decision to publish,or preparation of the manuscript.
Competing Interests: Dr.A. Maelicke is CEO of Galantos Pharma GmbH and Managing Director Europe of Neurodyn Inc.The authors SB,CH,and DM have
declared that no competing interests exist.This does not alter the authors’adherence to allthe PLOS ONE policies on sharing data and materials.
* E-mail:montag@LIN-magdeburg.de
Introduction
Alzheimer’sdisease (AD)is a progressive neurodegenerative
disorderand the mostcommon causeof old-agedementia.
Neuritic plaquescontaining amyloid b (Ab)and neurofibrillary
tangles composed of hyperphosphorylated Tau protein constitute
major neuropathologicalhallmarks ofAD. The amyloid cascade
theoryprovidesa rationalefor manyfeaturesof the disease
including the pathologicalmarkers,the phenotypescaused by
autosomaldominant disease genes,and the risk conferred by the
APOE gene status [1]. Increased production of certain Ab species,
their aggregation,and deposition as insoluble plaques is regarded
as an early and key pathology in the development of AD [2].Ab
plaques may serve as reservoirs ofsoluble Ab oligomers injuring
surrounding neuritesand synapses[3,4].At a systemiclevel,
therapeutic strategies to reverse or prevent Ab deposits could lead
to partialfunctionalrestoration ofneuralcircuits [5].Therefore,
mostAD treatmentapproaches aim atprevention or reversalof
Ab plaque deposition [6,7].
The acetylcholinesterase inhibitors donepezil, galantamine, and
rivastigmine serve as first-line symptomatic drug treatment in mild
to moderate Alzheimer’sdementia [8].Whereasdonepeziland
rivastigmine are designed acetylcholinesterase inhibitors, the plant
alkaloid galantamine additionally acts as an allosterically poten-
tiating ligand ofnicotinic receptors,increasing their sensitivity to
the neurotransmitter acetylcholine [9].Chronic low-levelstimu-
lation ofnicotinic receptorsmightup-regulate theirexpression
[10],slow down neurodegeneration [11],and confer protection
against b-amyloid toxicity [12].Furthermore,galantamine exerts
in cell systemsneuroprotective effectsby anti-apoptotic action
[13],by modulating amyloid precursor processing [14],and by
inhibiting b-amyloid aggregation and cytotoxicity [15].Galanta-
mine activates microglia resulting in enhanced Ab clearance [16].
Long-term galantamine treatmentof AD patientsslowsdown
cognitiveand global decline[17] and reducesbehavioral
symptoms,moststrongly in patients with moderate or advanced
forms of the disease [18]. Similar long-term positive effects are al
reflected in PET measurements [19].
The 5X FamilialAlzheimer’s Disease (5XFAD)mouse line co-
overexpressesAPP with three FAD mutations (K670N/M671L,
I716V, and V717I) and PS1 with two FAD mutations (M146L and
L286V)under the controlof the neuron-specific thy1 promoter
[20]. This modelrecapitulates a variety of AD features, including
working memory impairment,reduced anxiety,extensive extra-
cellularplaque formation beginning at2 monthsof age,and
selective neuron loss, making it a suitable research model for earl
onset AD [20–24].
PLOS ONE |www.plosone.org 1 February 2014 |Volume 9 |Issue 2 | e89454
Behavioral Decline in the 5XFAD Mouse Model of
Alzheimer’s Disease
Soumee Bhattacharya1, Christin Haertel1, Alfred Maelicke2, Dirk Montag 1*
1 Neurogenetics SpecialLaboratory,Leibniz Institute for Neurobiology,Magdeburg,Germany,2 Galantos Pharma GmbH,Nieder-Olm,Germany
Abstract
The plantalkaloid galantamine is an established symptomatic drug treatmentfor Alzheimer’s disease (AD),providing
temporary cognitive and globalrelief in human patients.In this study,the 5X FamilialAlzheimer’s Disease (5XFAD) mouse
modelwas used to investigate the effectof chronic galantamine treatmenton behaviorand amyloid b (Ab)plaque
deposition in the mouse brain. Quantification of plaques in untreated 5XFAD mice showed a gender specific phenotype
plaque density increased steadily reaching saturation in males after 10 months ofage,whereas in females the density
furtherincreased untilafter14 months ofage.Moreover,females consistently displayed a higherplaque density in
comparison to males ofthe same age.Chronic oraltreatmentwith galantamine resulted in improved performance in
behavioraltests,such as open field and light-dark avoidance,already at mildly affected stages compared to untreated
controls. Treated animals of both sexes showed significantly lower plaque density in the brain, i.e., the entorhinal corte
hippocampus,gliosis being always positively correlated to plaque load.A high dose treatment with a daily uptake of
26 mg/kg body weight was tolerated welland produced significantly larger positive effects than a lower dose treatment
(14 mg/kg body weight)in terms ofplaque density and behavior.These results strongly support that galantamine,in
addition to improving cognitive and behavioralsymptomsin AD, may have disease-modifying and neuroprotective
properties,as is indicated by delayed Ab plaque formation and reduced gliosis.
Citation: Bhattacharya S, Haertel C, Maelicke A, Montag D (2014) Galantamine Slows Down Plaque Formation and Behavioral Decline in the 5XFAD Mouse Mo
of Alzheimer’s Disease.PLoS ONE 9(2):e89454.doi:10.1371/journal.pone.0089454
Editor: Thomas Arendt,University of Leipzig,Germany
Received November 1,2013;Accepted January 21,2014;Published February 21,2014
Copyright: ß 2014 Bhattacharya et al.This is an open-access article distributed under the terms of the Creative Commons Attribution License,which permits
unrestricted use,distribution,and reproduction in any medium,provided the originalauthor and source are credited.
Funding: This study was supported by the German Ministry for Education and Research (BMBF) special network program KMU-Innovativ-2(http://www.bmbf.d
en/986.php).The funders had no role in study design,data collection and analysis,decision to publish,or preparation of the manuscript.
Competing Interests: Dr.A. Maelicke is CEO of Galantos Pharma GmbH and Managing Director Europe of Neurodyn Inc.The authors SB,CH,and DM have
declared that no competing interests exist.This does not alter the authors’adherence to allthe PLOS ONE policies on sharing data and materials.
* E-mail:montag@LIN-magdeburg.de
Introduction
Alzheimer’sdisease (AD)is a progressive neurodegenerative
disorderand the mostcommon causeof old-agedementia.
Neuritic plaquescontaining amyloid b (Ab)and neurofibrillary
tangles composed of hyperphosphorylated Tau protein constitute
major neuropathologicalhallmarks ofAD. The amyloid cascade
theoryprovidesa rationalefor manyfeaturesof the disease
including the pathologicalmarkers,the phenotypescaused by
autosomaldominant disease genes,and the risk conferred by the
APOE gene status [1]. Increased production of certain Ab species,
their aggregation,and deposition as insoluble plaques is regarded
as an early and key pathology in the development of AD [2].Ab
plaques may serve as reservoirs ofsoluble Ab oligomers injuring
surrounding neuritesand synapses[3,4].At a systemiclevel,
therapeutic strategies to reverse or prevent Ab deposits could lead
to partialfunctionalrestoration ofneuralcircuits [5].Therefore,
mostAD treatmentapproaches aim atprevention or reversalof
Ab plaque deposition [6,7].
The acetylcholinesterase inhibitors donepezil, galantamine, and
rivastigmine serve as first-line symptomatic drug treatment in mild
to moderate Alzheimer’sdementia [8].Whereasdonepeziland
rivastigmine are designed acetylcholinesterase inhibitors, the plant
alkaloid galantamine additionally acts as an allosterically poten-
tiating ligand ofnicotinic receptors,increasing their sensitivity to
the neurotransmitter acetylcholine [9].Chronic low-levelstimu-
lation ofnicotinic receptorsmightup-regulate theirexpression
[10],slow down neurodegeneration [11],and confer protection
against b-amyloid toxicity [12].Furthermore,galantamine exerts
in cell systemsneuroprotective effectsby anti-apoptotic action
[13],by modulating amyloid precursor processing [14],and by
inhibiting b-amyloid aggregation and cytotoxicity [15].Galanta-
mine activates microglia resulting in enhanced Ab clearance [16].
Long-term galantamine treatmentof AD patientsslowsdown
cognitiveand global decline[17] and reducesbehavioral
symptoms,moststrongly in patients with moderate or advanced
forms of the disease [18]. Similar long-term positive effects are al
reflected in PET measurements [19].
The 5X FamilialAlzheimer’s Disease (5XFAD)mouse line co-
overexpressesAPP with three FAD mutations (K670N/M671L,
I716V, and V717I) and PS1 with two FAD mutations (M146L and
L286V)under the controlof the neuron-specific thy1 promoter
[20]. This modelrecapitulates a variety of AD features, including
working memory impairment,reduced anxiety,extensive extra-
cellularplaque formation beginning at2 monthsof age,and
selective neuron loss, making it a suitable research model for earl
onset AD [20–24].
PLOS ONE |www.plosone.org 1 February 2014 |Volume 9 |Issue 2 | e89454
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
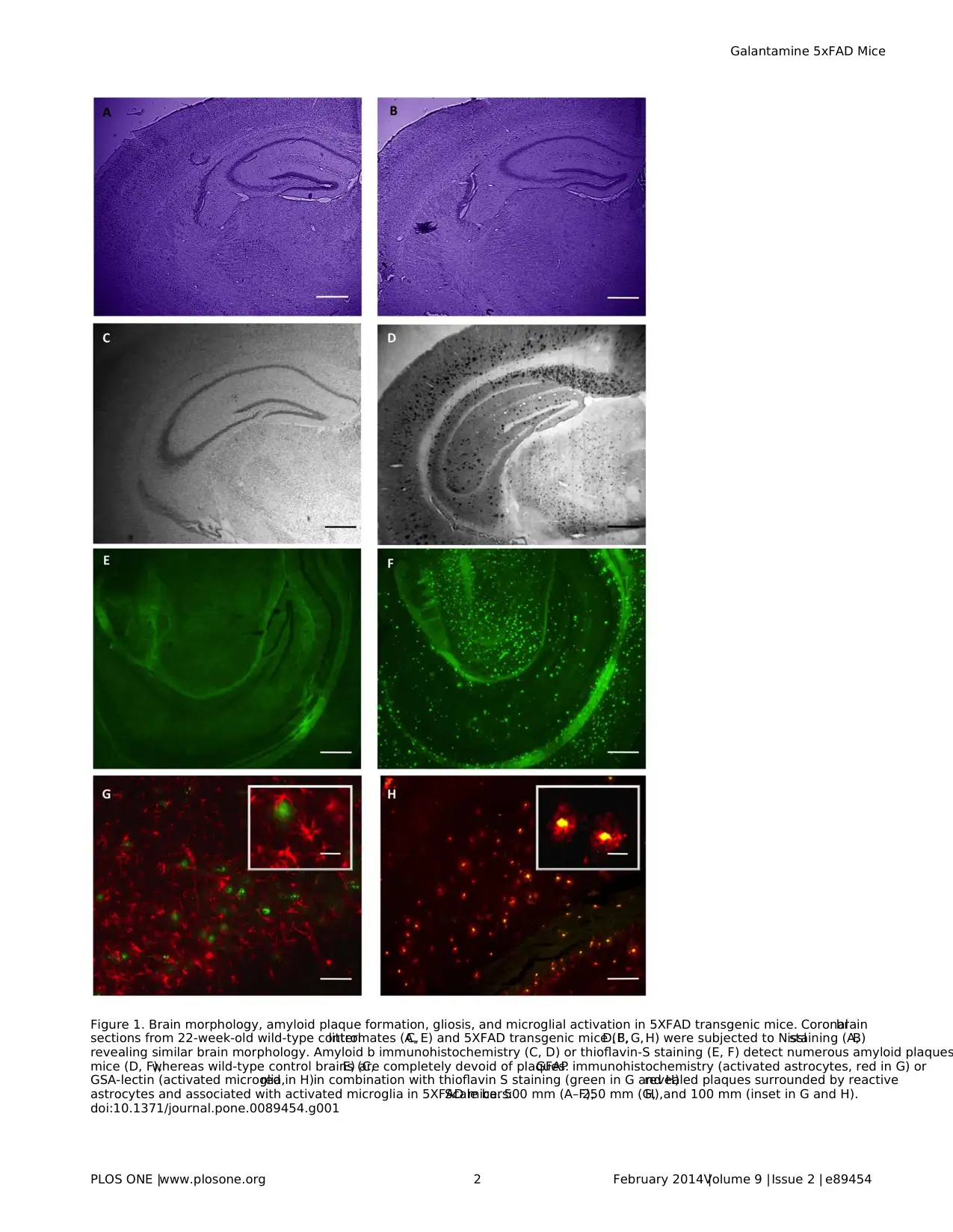
Figure 1. Brain morphology, amyloid plaque formation, gliosis, and microglial activation in 5XFAD transgenic mice. Coronalbrain
sections from 22-week-old wild-type controllittermates (A,C, E) and 5XFAD transgenic mice (B,D, F, G, H) were subjected to Nisslstaining (A,B)
revealing similar brain morphology. Amyloid b immunohistochemistry (C, D) or thioflavin-S staining (E, F) detect numerous amyloid plaques
mice (D, F),whereas wild-type control brains (C,E) are completely devoid of plaques.GFAP immunohistochemistry (activated astrocytes, red in G) or
GSA-lectin (activated microglia,red in H)in combination with thioflavin S staining (green in G and H)revealed plaques surrounded by reactive
astrocytes and associated with activated microglia in 5XFAD mice.Scale bars:500 mm (A–F),250 mm (G,H),and 100 mm (inset in G and H).
doi:10.1371/journal.pone.0089454.g001
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 2 February 2014 |Volume 9 |Issue 2 | e89454
sections from 22-week-old wild-type controllittermates (A,C, E) and 5XFAD transgenic mice (B,D, F, G, H) were subjected to Nisslstaining (A,B)
revealing similar brain morphology. Amyloid b immunohistochemistry (C, D) or thioflavin-S staining (E, F) detect numerous amyloid plaques
mice (D, F),whereas wild-type control brains (C,E) are completely devoid of plaques.GFAP immunohistochemistry (activated astrocytes, red in G) or
GSA-lectin (activated microglia,red in H)in combination with thioflavin S staining (green in G and H)revealed plaques surrounded by reactive
astrocytes and associated with activated microglia in 5XFAD mice.Scale bars:500 mm (A–F),250 mm (G,H),and 100 mm (inset in G and H).
doi:10.1371/journal.pone.0089454.g001
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 2 February 2014 |Volume 9 |Issue 2 | e89454

In this study,we used the 5XFAD modelto investigate the
effectsof chronic galantaminetreatmenton behaviorand
cognition,formation ofb amyloid plaques,and gliosis.Our data
show that galantamineslowsdown plaquedepositionand
improves behavioralperformance.
Materials and Methods
Ethics Statement
Animalexperimentswere in line with the guidelinesfor the
welfareof experimentalanimalsand approved bythe local
authoritiesof Sachsen-Anhalt/Germany (numbers42502/2-382
and -945)and carried outin accordancewith theEuropean
Communities Council Directive of 24th November 1986 (86/609/
EEC).
Mice
5XFAD (B6SJL-Tg(APPSwFlLon,P-
SEN1*M146L*L286V)6799 Vas/J mice were described by Oakley
et al.[20] and were obtained from The Jackson Laboratory (Bar
Harbor, stock number 006554). These ‘‘5XFAD’’ transgenic mice
overexpressboth mutanthuman APP(695)with the Swedish
(K670N,M671L),Florida (I716V),and London (V717I)Familial
Alzheimer’s Disease (FAD)mutations and human PS1 harboring
two FAD mutations,M146L and L286V.Expression ofboth
transgenes is regulated by neural-specific elements ofthe mouse
Thy1 promoterto drive overexpression in the brain.5XFAD
transgenic male mice were crossed with B6SJLF1/J female mice
(Jackson Laboratory,stock number100012).The resulting F2-
offspring were used in allexperiments.Transgenic mice were
identified by PCR according to the supplier’s protocol.
Galantamine
Galantaminehydrobromidewas obtainedfrom Macfarlan
Smith (Edinburgh,UK). The naturally occurring alkaloid was
extracted from daffodilbulbs (Narcissus pseudonarcissus)and was
isolated and purified as the hydrobromide salt, as described in the
related drug master file. Purity was .99%. The molecular formula
is C17H22NO3Br and the molecular weight is 368.28.
Galantamine Treatment
10 to 12-week-old mice received galantamineHBr dissolved in
drinking water ata concentration ofeither 12 mg/lduring four
weeks followed by three weeks with 60 mg/l (low dose), or 36 mg/
l during four weeks followed by three weeks with 120 mg/l(high
dose).Thereafter,the mice were water deprived overnightand
receivedin the morningdrinkingwatercontaining120 mg
galantamineHBr/luntilthe behavioralexperiments were termi-
nated and animalswere sacrificed for histologicalexamination.
Behavioralexperimentswereconducted aftereightweeksof
treatmentand waterdeprivation wasterminated 30 to 60 min
before the behavioraltest to ensurea high galantamine
concentration during the experiment.Waterconsumption and
body weight were monitored during the application period.
Behavior
For the behavioralanalysis,sex-and age-matched littermate
wild-type mice were used as controls. During the light phase (12h/
12h light-dark cycle), mice were subjected to a series of behavioral
tests [25,26] by an experimenter not aware of the genotype. First,
generalparameters indicative of the health and neurologicalstate
were addressedfollowingthe neurobehavioralexamination
described by Whishaw and colleagues[27] and the testsof the
primary screen ofthe SHIRPA protocolexceptstartle response
[28]. Then, the following behavioral paradigms were conducted in
sequential order: Grip strength. Strength was measured with a hig
precision force sensor to evaluate neuromuscular functioning (TSE
SystemsGmbH, Bad Homburg,Germany).Rota-rod performance.
Animals received two training sessions (3 h interval) on a rota-rod
apparatus(TSE) with increasing speed from 4 to 40 rpm for
5 min.After 4 days,mice were tested at 16,24,32,and 40 rpm
constant speed. The latency to fall off the rod was measured. Ope
field.Exploration was assessed by placing mice in the middle of a
50650 cm arena for 15 min. Using the VideoMot 2 system (TSE),
trackswere analyzed for path length,visits,walking speed,and
relative time spent in the central area (infield), in the area close to
the walls(,10 cm, outfield),and in the corners.O-Maze.Mice
were placed in the center ofan open area ofan O-maze (San
Diego Instruments). Their behavior during 5 min was recorded on
videotape.Number ofentries into the closed or open areas was
counted and the time spent in these compartments was determin
using the VideoMot 2 system (TSE).Light-dark Avoidance.Anxiety-
related behaviorwastested by placing mice in a brightly lit
compartment(250 lux,25625 cm)adjacentto a dark compart-
ment(12.5625 cm).The numberof transitionsbetween the
compartmentsand the time spentwithin each were analyzed
during 10 min. As a test for long-term memory [29], animals were
placed at the last day of testing again in the light-dark avoidance
box.The latency to enter the dark compartmentwas measured
and compared to the latency at the first time in the box.Acoustic
startle response and prepulse inhibition (PPI).A startle stimulus (50 ms,
120 dB)was delivered to the mice in a startle-box system (TSE)
with or without preceding prepulse stimulus (30 ms, 100 ms befor
the startle stimulus)at eight different intensities (73–94 dB,3 dB
increments) on a 70 dB white noise background. After habituation
to the box (3 min), 2 startle trials were followed in pseudo-random
orderby 10 startle trialsand 5 trialsat each ofthe prepulse
intensitieswith stochastically varied intertrialintervals(5–30 s).
The maximalstartleamplitudewas measuredby a sensor
platform.
For conditioned fear testing of 5XFAD mice,the experimental
protocol used for the study of Tg2576 mice by Comery etal. [30],
Jacobsen etal.[31],and Schilling et al.[6] was followed closely.
Mice were trained and tested on 2 consecutive days.Training
consisted of placing the subject in an operant chamber (San Diego
Instruments)and allowing exploration for 2 min.Afterwards,an
auditory cue was presented for 15 sec followed by a footshock for
2 sec (1.5 mA un-pulsed). This procedure was repeated, and mice
were returned to the home cage 30 sec later.24 hoursafter
training,micewerereturned to thesame chamberin which
training had occurred (context), and freezing behavior (immobility
wasrecorded.At the end ofthe 5 min contexttest,mice were
returned to their home cage. One hour later, mice were placed in
a novelenvironmentand freezingbehavior(immobility)was
recorded for 3 min. The auditory cue (CS) was then presented for
3 min and freezing behavior (immobility)was recorded.Freezing
scores are expressed as percentage for each portion of the test.
Histology
Animals were anesthetized with CO2 and perfused intracardi-
ally with PBS (10ml/min,pH7.4,10 min)followed by freshly
prepared 4% PFA in PBS (10ml/min,10 minutes).Brains were
post fixed in the same fixative at 4uC overnight, serially infiltrated
with 0.5M and 1M saccharose for 24–48 hrs each and frozen in
methylbutane at around 270uC.60 consecutive coronalsections
(40 mm wide)from approximately bregma 0 to bregma 23.5mm
were obtained per animal.In order to normalize regionalbias in
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 3 February 2014 |Volume 9 |Issue 2 | e89454
effectsof chronic galantaminetreatmenton behaviorand
cognition,formation ofb amyloid plaques,and gliosis.Our data
show that galantamineslowsdown plaquedepositionand
improves behavioralperformance.
Materials and Methods
Ethics Statement
Animalexperimentswere in line with the guidelinesfor the
welfareof experimentalanimalsand approved bythe local
authoritiesof Sachsen-Anhalt/Germany (numbers42502/2-382
and -945)and carried outin accordancewith theEuropean
Communities Council Directive of 24th November 1986 (86/609/
EEC).
Mice
5XFAD (B6SJL-Tg(APPSwFlLon,P-
SEN1*M146L*L286V)6799 Vas/J mice were described by Oakley
et al.[20] and were obtained from The Jackson Laboratory (Bar
Harbor, stock number 006554). These ‘‘5XFAD’’ transgenic mice
overexpressboth mutanthuman APP(695)with the Swedish
(K670N,M671L),Florida (I716V),and London (V717I)Familial
Alzheimer’s Disease (FAD)mutations and human PS1 harboring
two FAD mutations,M146L and L286V.Expression ofboth
transgenes is regulated by neural-specific elements ofthe mouse
Thy1 promoterto drive overexpression in the brain.5XFAD
transgenic male mice were crossed with B6SJLF1/J female mice
(Jackson Laboratory,stock number100012).The resulting F2-
offspring were used in allexperiments.Transgenic mice were
identified by PCR according to the supplier’s protocol.
Galantamine
Galantaminehydrobromidewas obtainedfrom Macfarlan
Smith (Edinburgh,UK). The naturally occurring alkaloid was
extracted from daffodilbulbs (Narcissus pseudonarcissus)and was
isolated and purified as the hydrobromide salt, as described in the
related drug master file. Purity was .99%. The molecular formula
is C17H22NO3Br and the molecular weight is 368.28.
Galantamine Treatment
10 to 12-week-old mice received galantamineHBr dissolved in
drinking water ata concentration ofeither 12 mg/lduring four
weeks followed by three weeks with 60 mg/l (low dose), or 36 mg/
l during four weeks followed by three weeks with 120 mg/l(high
dose).Thereafter,the mice were water deprived overnightand
receivedin the morningdrinkingwatercontaining120 mg
galantamineHBr/luntilthe behavioralexperiments were termi-
nated and animalswere sacrificed for histologicalexamination.
Behavioralexperimentswereconducted aftereightweeksof
treatmentand waterdeprivation wasterminated 30 to 60 min
before the behavioraltest to ensurea high galantamine
concentration during the experiment.Waterconsumption and
body weight were monitored during the application period.
Behavior
For the behavioralanalysis,sex-and age-matched littermate
wild-type mice were used as controls. During the light phase (12h/
12h light-dark cycle), mice were subjected to a series of behavioral
tests [25,26] by an experimenter not aware of the genotype. First,
generalparameters indicative of the health and neurologicalstate
were addressedfollowingthe neurobehavioralexamination
described by Whishaw and colleagues[27] and the testsof the
primary screen ofthe SHIRPA protocolexceptstartle response
[28]. Then, the following behavioral paradigms were conducted in
sequential order: Grip strength. Strength was measured with a hig
precision force sensor to evaluate neuromuscular functioning (TSE
SystemsGmbH, Bad Homburg,Germany).Rota-rod performance.
Animals received two training sessions (3 h interval) on a rota-rod
apparatus(TSE) with increasing speed from 4 to 40 rpm for
5 min.After 4 days,mice were tested at 16,24,32,and 40 rpm
constant speed. The latency to fall off the rod was measured. Ope
field.Exploration was assessed by placing mice in the middle of a
50650 cm arena for 15 min. Using the VideoMot 2 system (TSE),
trackswere analyzed for path length,visits,walking speed,and
relative time spent in the central area (infield), in the area close to
the walls(,10 cm, outfield),and in the corners.O-Maze.Mice
were placed in the center ofan open area ofan O-maze (San
Diego Instruments). Their behavior during 5 min was recorded on
videotape.Number ofentries into the closed or open areas was
counted and the time spent in these compartments was determin
using the VideoMot 2 system (TSE).Light-dark Avoidance.Anxiety-
related behaviorwastested by placing mice in a brightly lit
compartment(250 lux,25625 cm)adjacentto a dark compart-
ment(12.5625 cm).The numberof transitionsbetween the
compartmentsand the time spentwithin each were analyzed
during 10 min. As a test for long-term memory [29], animals were
placed at the last day of testing again in the light-dark avoidance
box.The latency to enter the dark compartmentwas measured
and compared to the latency at the first time in the box.Acoustic
startle response and prepulse inhibition (PPI).A startle stimulus (50 ms,
120 dB)was delivered to the mice in a startle-box system (TSE)
with or without preceding prepulse stimulus (30 ms, 100 ms befor
the startle stimulus)at eight different intensities (73–94 dB,3 dB
increments) on a 70 dB white noise background. After habituation
to the box (3 min), 2 startle trials were followed in pseudo-random
orderby 10 startle trialsand 5 trialsat each ofthe prepulse
intensitieswith stochastically varied intertrialintervals(5–30 s).
The maximalstartleamplitudewas measuredby a sensor
platform.
For conditioned fear testing of 5XFAD mice,the experimental
protocol used for the study of Tg2576 mice by Comery etal. [30],
Jacobsen etal.[31],and Schilling et al.[6] was followed closely.
Mice were trained and tested on 2 consecutive days.Training
consisted of placing the subject in an operant chamber (San Diego
Instruments)and allowing exploration for 2 min.Afterwards,an
auditory cue was presented for 15 sec followed by a footshock for
2 sec (1.5 mA un-pulsed). This procedure was repeated, and mice
were returned to the home cage 30 sec later.24 hoursafter
training,micewerereturned to thesame chamberin which
training had occurred (context), and freezing behavior (immobility
wasrecorded.At the end ofthe 5 min contexttest,mice were
returned to their home cage. One hour later, mice were placed in
a novelenvironmentand freezingbehavior(immobility)was
recorded for 3 min. The auditory cue (CS) was then presented for
3 min and freezing behavior (immobility)was recorded.Freezing
scores are expressed as percentage for each portion of the test.
Histology
Animals were anesthetized with CO2 and perfused intracardi-
ally with PBS (10ml/min,pH7.4,10 min)followed by freshly
prepared 4% PFA in PBS (10ml/min,10 minutes).Brains were
post fixed in the same fixative at 4uC overnight, serially infiltrated
with 0.5M and 1M saccharose for 24–48 hrs each and frozen in
methylbutane at around 270uC.60 consecutive coronalsections
(40 mm wide)from approximately bregma 0 to bregma 23.5mm
were obtained per animal.In order to normalize regionalbias in
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 3 February 2014 |Volume 9 |Issue 2 | e89454
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
plaque load,12 sections200 mm apartfrom each otherwere
selected from allanimals in every experiment.
Nissl staining. Cryosections were subjected to the standard
Nisslstaining protocolusing cresylvioletacetate (Sigma)and
viewed using an Axioplan 2 microscope(Zeiss).Imageswere
capturedwith PhotometrixcoolSNAP EZ (Visitron systems
GmbH)and analyzed with ImageJ software (NIH).
Immunofluorescence. Slide-mounted cryostat sections were
blocked with BSA (5% in phosphate-buffered saline)for 1 h and
then incubated with primary antibody singly or in combination
overnight at 4uC in a humidified chamber. Different combinations
of primary antibodies used were;4G8 (Covance,1/10000)alone
for detection ofb amyloid,4G8 togetherwith GFAP (Sigma
Aldrich,1/10000)for detection ofreactive astrocytes,and 4G8
with biotinylated Isolectin GSA (Sigma Aldrich,10 mg/ml)for
detection of microglia.Post incubation,the sections were washed
with PBS and probed with the secondary antibody/reagentsas
required,in a sequentialmanner.Fluorochromated secondary
reagentsused were StreptavidinAlexaFluor488 (Molecular
Probes,1/200),Cy3GoatAnti Rabbit (Abcam,1/200) and
Streptavidin Cy3 (Jackson Laboratories,1 mg/ml).Sections were
examined by Axioplan2 (Carl Zeiss) and images captured by Spot
RT-KE (Diagnostic Instruments).
Immunoperoxidase. Free floating sectionswere collected,
treated with a 1:1 solution of PBS and methanolwith 1% H2O 2,
blockedwith BSA (5% in phosphate-bufferedsaline)and
incubated overnightat 4uC with 4G8 antibody (Covance,1/
10000)in a humidified chamber.The sections were then treated
with Vectashield ABC kit(Vector laboratories)followed by the
chromogenic substrate DAB (Vector laboratories).Sections were
viewed using Axioplan 2 microscope (CarlZeiss).Imageswere
capturedwith PhotometrixcoolSNAP EZ (Visitron systems
GmbH)and analyzed with ImageJ software (NIH).
Thioflavin-S staining. An improved thioflavin-S staining
protocol[32] wasused to ensure reduced photobleaching and
tissue damage. Briefly, sections were treated with 0.25% potassiu
permanganate solution (quenching)at room temperature for4
minutesfollowed by 1% sodium borohydride solution for2–3
minutes. This was followed by incubation with 0.05% thioflavin-S
(SigmaAldrich T1892) solutionin 50% ethanolat room
temperaturefor 8 minutesin the dark. Sectionswerethen
subjected to 2 washesof 10 secondseach with 80% ethanol,3
washes of 30 seconds each with water, and post treatment with 5
phosphate buffered saline (pH 7.4)at 4uC in the dark.Sections
were mounted in Entellan (Merck)after a briefwash with water
and viewed under the FITC filter set of Axioplan2 (CarlZeiss).
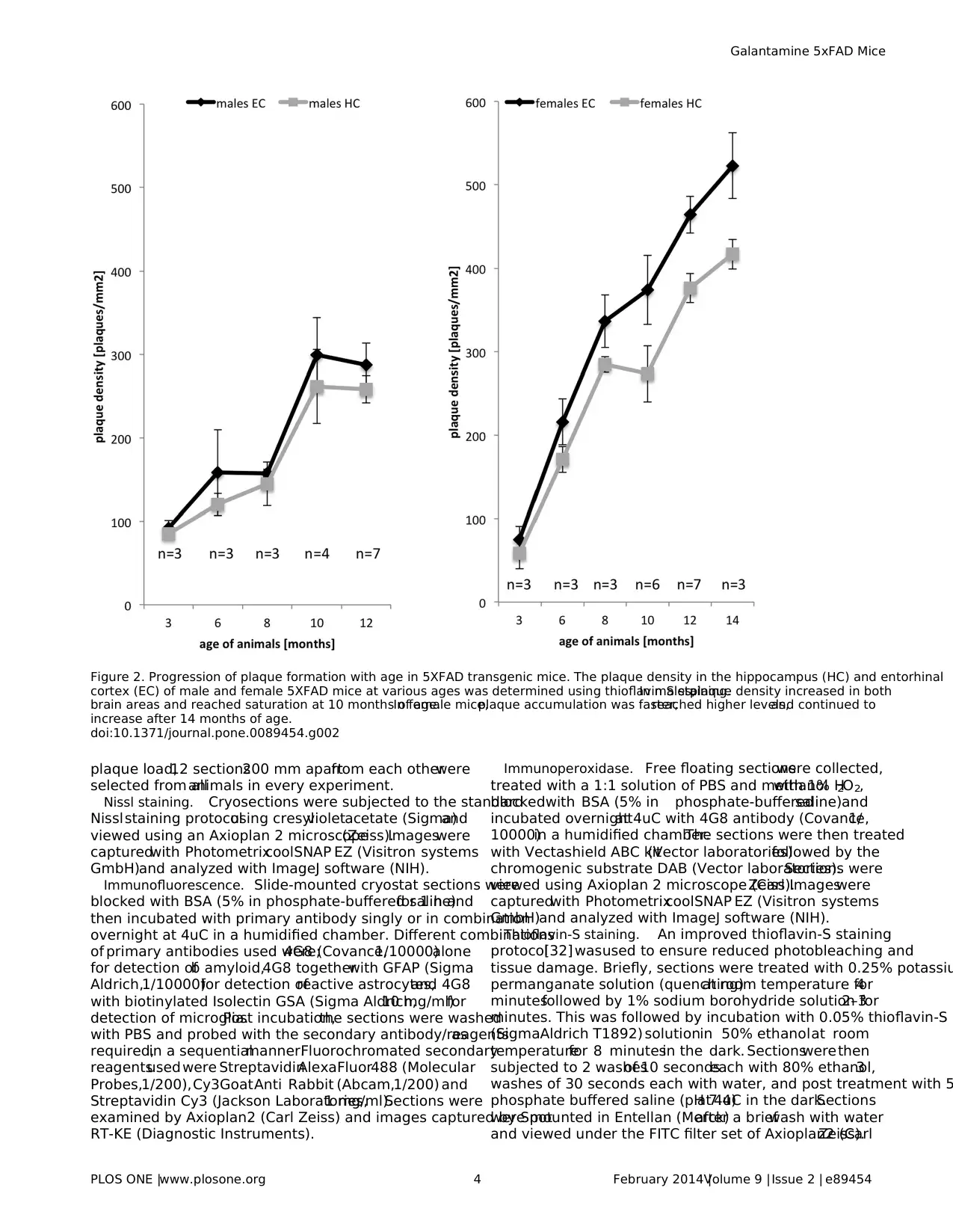
Figure 2. Progression of plaque formation with age in 5XFAD transgenic mice. The plaque density in the hippocampus (HC) and entorhinal
cortex (EC) of male and female 5XFAD mice at various ages was determined using thioflavin S staining.In males,plaque density increased in both
brain areas and reached saturation at 10 months of age.In female mice,plaque accumulation was faster,reached higher levels,and continued to
increase after 14 months of age.
doi:10.1371/journal.pone.0089454.g002
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 4 February 2014 |Volume 9 |Issue 2 | e89454
selected from allanimals in every experiment.
Nissl staining. Cryosections were subjected to the standard
Nisslstaining protocolusing cresylvioletacetate (Sigma)and
viewed using an Axioplan 2 microscope(Zeiss).Imageswere
capturedwith PhotometrixcoolSNAP EZ (Visitron systems
GmbH)and analyzed with ImageJ software (NIH).
Immunofluorescence. Slide-mounted cryostat sections were
blocked with BSA (5% in phosphate-buffered saline)for 1 h and
then incubated with primary antibody singly or in combination
overnight at 4uC in a humidified chamber. Different combinations
of primary antibodies used were;4G8 (Covance,1/10000)alone
for detection ofb amyloid,4G8 togetherwith GFAP (Sigma
Aldrich,1/10000)for detection ofreactive astrocytes,and 4G8
with biotinylated Isolectin GSA (Sigma Aldrich,10 mg/ml)for
detection of microglia.Post incubation,the sections were washed
with PBS and probed with the secondary antibody/reagentsas
required,in a sequentialmanner.Fluorochromated secondary
reagentsused were StreptavidinAlexaFluor488 (Molecular
Probes,1/200),Cy3GoatAnti Rabbit (Abcam,1/200) and
Streptavidin Cy3 (Jackson Laboratories,1 mg/ml).Sections were
examined by Axioplan2 (Carl Zeiss) and images captured by Spot
RT-KE (Diagnostic Instruments).
Immunoperoxidase. Free floating sectionswere collected,
treated with a 1:1 solution of PBS and methanolwith 1% H2O 2,
blockedwith BSA (5% in phosphate-bufferedsaline)and
incubated overnightat 4uC with 4G8 antibody (Covance,1/
10000)in a humidified chamber.The sections were then treated
with Vectashield ABC kit(Vector laboratories)followed by the
chromogenic substrate DAB (Vector laboratories).Sections were
viewed using Axioplan 2 microscope (CarlZeiss).Imageswere
capturedwith PhotometrixcoolSNAP EZ (Visitron systems
GmbH)and analyzed with ImageJ software (NIH).
Thioflavin-S staining. An improved thioflavin-S staining
protocol[32] wasused to ensure reduced photobleaching and
tissue damage. Briefly, sections were treated with 0.25% potassiu
permanganate solution (quenching)at room temperature for4
minutesfollowed by 1% sodium borohydride solution for2–3
minutes. This was followed by incubation with 0.05% thioflavin-S
(SigmaAldrich T1892) solutionin 50% ethanolat room
temperaturefor 8 minutesin the dark. Sectionswerethen
subjected to 2 washesof 10 secondseach with 80% ethanol,3
washes of 30 seconds each with water, and post treatment with 5
phosphate buffered saline (pH 7.4)at 4uC in the dark.Sections
were mounted in Entellan (Merck)after a briefwash with water
and viewed under the FITC filter set of Axioplan2 (CarlZeiss).
Figure 2. Progression of plaque formation with age in 5XFAD transgenic mice. The plaque density in the hippocampus (HC) and entorhinal
cortex (EC) of male and female 5XFAD mice at various ages was determined using thioflavin S staining.In males,plaque density increased in both
brain areas and reached saturation at 10 months of age.In female mice,plaque accumulation was faster,reached higher levels,and continued to
increase after 14 months of age.
doi:10.1371/journal.pone.0089454.g002
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 4 February 2014 |Volume 9 |Issue 2 | e89454
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Quantitative analysis of plaques and astrocytes. For
quantification of plaque load, images were captured by a Spot RT-
KE camera (Diagnostic Instruments) at a magnification of 2.5X so
as to include the entire hippocampus/entorhinal cortex in a single
frame.Plaqueload wasdetermined by counting thioflavin-S-
positive plaques using ImageJ software (NIH)and Adobe Photo-
shop (CS3 version).Reactive astrocytes in the hippocampus and
the entorhinalcortex were identified using GFAP staining and
images were captured by a Spot RT-KE camera at a magnifica-
tion of 10X. Five to seven sectionsfrom each animalwere
analyzedusing ImageJ software(NIH) and GFAP positive
astrocytes were counted.
StatisticalAnalysis
Behavioraland imaging data were analyzed using analysis of
variance (ANOVA with genotype,sex,and treatmentas factors)
and posthoc analysis using Scheffe’s test (STATVIEW Program,SAS
Institute Inc.,Cary,NC) to determine statisticalsignificance.For
the rota-rod,open field,and startle/PPI experiments,statistical
analysiswas additionallyperformed usingrepeated measures
ANOVA (with between-subject factor genotype and within-subject
factor session).A P-value smallerthan 0.05 (p,0.05) was
considered significant.Regression analysis(STATVIEW Program)
wasused forthe correlation ofplaque countand numberof
reactive astrocytes.
Results
Quantitative Analysis of Plaque Deposition in 5XFAD
Mice
Early deposition of amyloid plaques is a characteristic feature o
the 5XFAD mouse model [20]. To exploit this model for the study
of drugs for the treatment ofAD, we analyzed quantitatively the
progression ofplaque deposition with age and characterized the
behavior at ages with moderate disease progression.In 22-week-
old mice,the generalbrain morphology of5XFAD transgenic
mice isnot affected by plaque deposition asexemplified in the
hippocampusand cortexby Nissl staining(Figure1A, B).
However,Ab deposits are easily identified by immunohistochem-
istry (Figure 1D)or by thioflavin-S staining (Figure 1F).Further-
more,b amyloid depositsare usually associated with reactive
astrocytes identified by immunohistochemicalstaining for GFAP
(Figure 1G)and microglia identified by isolectin GSA staining
(Figure 1H).Using thioflavin-S as a marker,we investigated the
progression of amyloid b deposition with age in two representativ
brain areas, the hippocampal formation and the entorhinal cortex
(Figure2). Plaqueswere detectablealreadyin 2-month-old
animals.The quantification revealed a strong increase in plaque
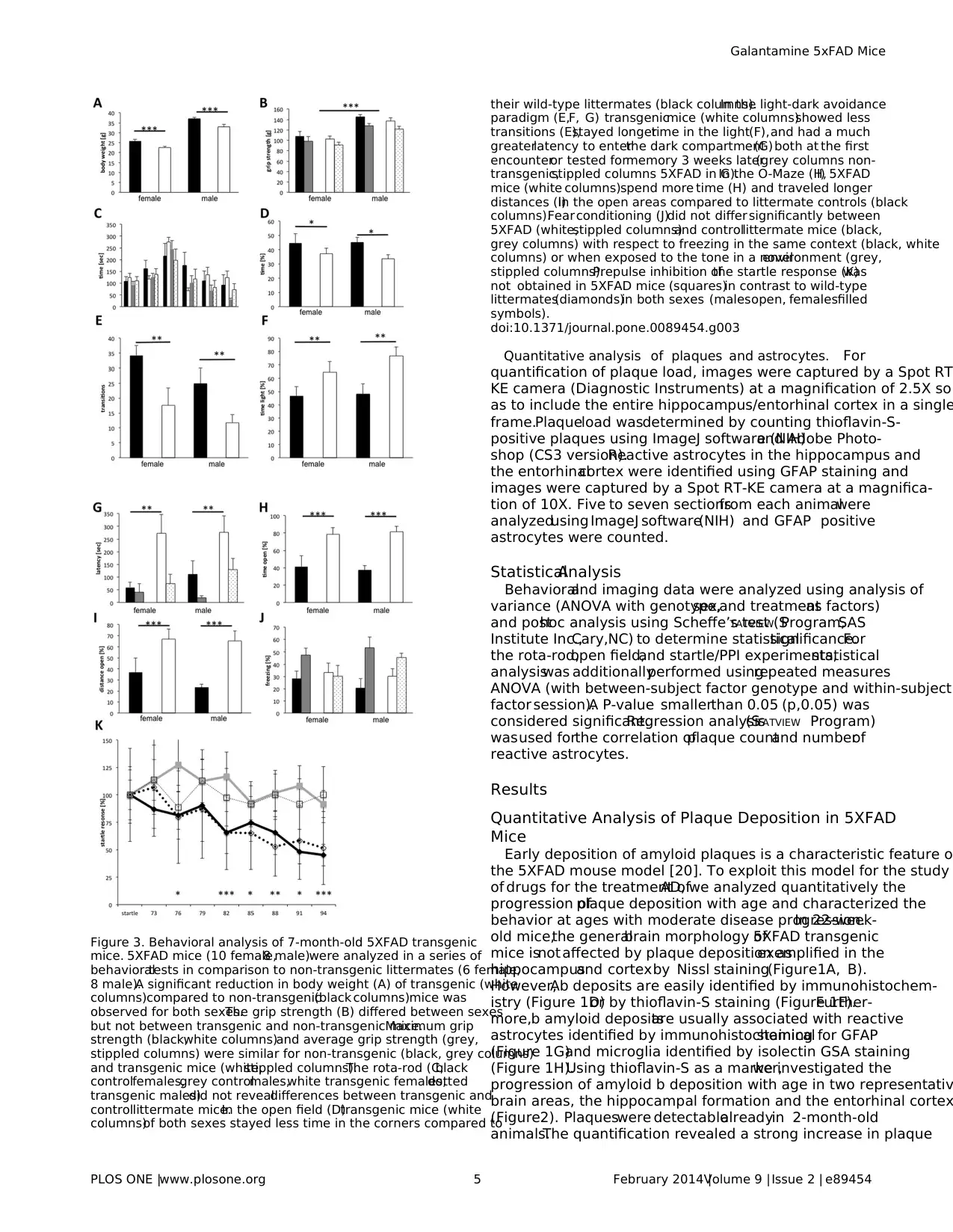
Figure 3. Behavioral analysis of 7-month-old 5XFAD transgenic
mice. 5XFAD mice (10 female,8 male)were analyzed in a series of
behavioraltests in comparison to non-transgenic littermates (6 female,
8 male).A significant reduction in body weight (A) of transgenic (white
columns)compared to non-transgenic(black columns)mice was
observed for both sexes.The grip strength (B) differed between sexes
but not between transgenic and non-transgenic mice.Maximum grip
strength (black,white columns)and average grip strength (grey,
stippled columns) were similar for non-transgenic (black, grey columns)
and transgenic mice (white,stippled columns).The rota-rod (C;black
controlfemales,grey controlmales,white transgenic females,dotted
transgenic males)did not revealdifferences between transgenic and
controllittermate mice.In the open field (D)transgenic mice (white
columns)of both sexes stayed less time in the corners compared to
their wild-type littermates (black columns).In the light-dark avoidance
paradigm (E,F, G) transgenicmice (white columns)showed less
transitions (E),stayed longertime in the light(F),and had a much
greaterlatency to enterthe dark compartment(G) both at the first
encounteror tested formemory 3 weeks later(grey columns non-
transgenic,stippled columns 5XFAD in G).In the O-Maze (H,I) 5XFAD
mice (white columns)spend more time (H) and traveled longer
distances (I)in the open areas compared to littermate controls (black
columns).Fear conditioning (J)did not differ significantly between
5XFAD (white,stippled columns)and controllittermate mice (black,
grey columns) with respect to freezing in the same context (black, white
columns) or when exposed to the tone in a novelenvironment (grey,
stippled columns).Prepulse inhibition ofthe startle response (K)was
not obtained in 5XFAD mice (squares)in contrast to wild-type
littermates(diamonds)in both sexes (malesopen, femalesfilled
symbols).
doi:10.1371/journal.pone.0089454.g003
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 5 February 2014 |Volume 9 |Issue 2 | e89454
quantification of plaque load, images were captured by a Spot RT-
KE camera (Diagnostic Instruments) at a magnification of 2.5X so
as to include the entire hippocampus/entorhinal cortex in a single
frame.Plaqueload wasdetermined by counting thioflavin-S-
positive plaques using ImageJ software (NIH)and Adobe Photo-
shop (CS3 version).Reactive astrocytes in the hippocampus and
the entorhinalcortex were identified using GFAP staining and
images were captured by a Spot RT-KE camera at a magnifica-
tion of 10X. Five to seven sectionsfrom each animalwere
analyzedusing ImageJ software(NIH) and GFAP positive
astrocytes were counted.
StatisticalAnalysis
Behavioraland imaging data were analyzed using analysis of
variance (ANOVA with genotype,sex,and treatmentas factors)
and posthoc analysis using Scheffe’s test (STATVIEW Program,SAS
Institute Inc.,Cary,NC) to determine statisticalsignificance.For
the rota-rod,open field,and startle/PPI experiments,statistical
analysiswas additionallyperformed usingrepeated measures
ANOVA (with between-subject factor genotype and within-subject
factor session).A P-value smallerthan 0.05 (p,0.05) was
considered significant.Regression analysis(STATVIEW Program)
wasused forthe correlation ofplaque countand numberof
reactive astrocytes.
Results
Quantitative Analysis of Plaque Deposition in 5XFAD
Mice
Early deposition of amyloid plaques is a characteristic feature o
the 5XFAD mouse model [20]. To exploit this model for the study
of drugs for the treatment ofAD, we analyzed quantitatively the
progression ofplaque deposition with age and characterized the
behavior at ages with moderate disease progression.In 22-week-
old mice,the generalbrain morphology of5XFAD transgenic
mice isnot affected by plaque deposition asexemplified in the
hippocampusand cortexby Nissl staining(Figure1A, B).
However,Ab deposits are easily identified by immunohistochem-
istry (Figure 1D)or by thioflavin-S staining (Figure 1F).Further-
more,b amyloid depositsare usually associated with reactive
astrocytes identified by immunohistochemicalstaining for GFAP
(Figure 1G)and microglia identified by isolectin GSA staining
(Figure 1H).Using thioflavin-S as a marker,we investigated the
progression of amyloid b deposition with age in two representativ
brain areas, the hippocampal formation and the entorhinal cortex
(Figure2). Plaqueswere detectablealreadyin 2-month-old
animals.The quantification revealed a strong increase in plaque
Figure 3. Behavioral analysis of 7-month-old 5XFAD transgenic
mice. 5XFAD mice (10 female,8 male)were analyzed in a series of
behavioraltests in comparison to non-transgenic littermates (6 female,
8 male).A significant reduction in body weight (A) of transgenic (white
columns)compared to non-transgenic(black columns)mice was
observed for both sexes.The grip strength (B) differed between sexes
but not between transgenic and non-transgenic mice.Maximum grip
strength (black,white columns)and average grip strength (grey,
stippled columns) were similar for non-transgenic (black, grey columns)
and transgenic mice (white,stippled columns).The rota-rod (C;black
controlfemales,grey controlmales,white transgenic females,dotted
transgenic males)did not revealdifferences between transgenic and
controllittermate mice.In the open field (D)transgenic mice (white
columns)of both sexes stayed less time in the corners compared to
their wild-type littermates (black columns).In the light-dark avoidance
paradigm (E,F, G) transgenicmice (white columns)showed less
transitions (E),stayed longertime in the light(F),and had a much
greaterlatency to enterthe dark compartment(G) both at the first
encounteror tested formemory 3 weeks later(grey columns non-
transgenic,stippled columns 5XFAD in G).In the O-Maze (H,I) 5XFAD
mice (white columns)spend more time (H) and traveled longer
distances (I)in the open areas compared to littermate controls (black
columns).Fear conditioning (J)did not differ significantly between
5XFAD (white,stippled columns)and controllittermate mice (black,
grey columns) with respect to freezing in the same context (black, white
columns) or when exposed to the tone in a novelenvironment (grey,
stippled columns).Prepulse inhibition ofthe startle response (K)was
not obtained in 5XFAD mice (squares)in contrast to wild-type
littermates(diamonds)in both sexes (malesopen, femalesfilled
symbols).
doi:10.1371/journal.pone.0089454.g003
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 5 February 2014 |Volume 9 |Issue 2 | e89454
number and density with age.Maximaldensity was not reached
even at 14 months of age in females, whereas in males no increas
in plaquedensitywas observedafter 10 monthsof age.
Noteworthy,as also observed forothertransgenic Alzheimer’s
disease models [33–35],we found a strikingly higher number of
thioflavin-stained plaques in female compared to male mice.For
both sexes, the plaque density was always higher in the entorhina
cortexthan in the hippocampus.In controlnon-transgenic
littermates, plaques were never observed at any age. In conclusio
the age before saturation at 10 months appeared to be best suite
for the analysis of plaque deposition after a chronic treatment.
BehavioralAnalysis of Untreated Medium Age 5XFAD
Mice
Therefore,we analyzed whether behavioraldeficits are detect-
able in mice with a moderate plaque load. 7-month-old transgenic
(10 female,8 male)and non-transgenic(6 female,8 male)
littermate mice were subjected to a seriesof behavioraltests.
During the neurologicalexamination,transgenic mice did not
displayobviousabnormalitieswith respectto body posture,
reflexes(uprighting,eye-blink),or generalsensoryperception
(vision,hearing,touch,pain).In contrast,the body weightof
transgenic mice wasapproximately 10% lessthan thatof their
controllittermates,both,before and afterthe behavioraltests
(Figure 3A)(2-way ANOVA and post hoc analysis,weight before
tests:factor genotypeF(1,28)= 15.912,p = 0.0004,factor sex
F(1,28)= 136,797,p,0.0001; Fisher’sPLSD ,0.0001 for each
factor;weightaftertestsfactorgenotypeF(1,28)= 24.426,p,
0.0001,factor sex F(1,28)= 147.182,p,0.0001;Fisher’s PLSD ,
0.0001 for each factor).As expected,the maximum and average
grip strength were significantly higherfor malescompared to
females(F(1,28)= 32.038,p,0.0001;F(1,28)= 25.969,p,0.0001,
respectively),but did not differbetween transgenicand non-
transgenicmice (Figure3B). Furthermore,motoricabilities
examined on the Rota-Rod were similar for controland 5XFAD
mice (Figure 3C), indicating that motor coordination and motoric
capabilities are not generally impaired in 5XFAD transgenic mice
of this age.
In the open field, transgenic mice spend significantly less time i
the corners(F(1,28)= 5.245,p = 0.0297,posthoc Fisher’sPLSD
p = 0.0311)compared to controllittermates(Figure 3D).In the
light-dark avoidance paradigm,5XFAD transgenic mice made
fewer transitions between compartments(Figure 3E)
(F(1,28)= 9.077,p = 0.0054;posthoc Fisher’sPLSD p = 0.0084)
and spendmore time in the illuminatedpart (Figure3F)
(F(1,28)= 9.026,p = 0.0056;posthoc Fisher’sPLSD p = 0.0067).
In addition,transgenic mice entered the dark compartment after
longerlatency (Figure 3G,F(1,28)= 8.635,p = 0.0065;posthoc
Fisher’sPLSD p = 0.0071).The latency with which transgenic
mice entered the dark compartment when tested for memory was
stilllonger asof their non-transgenic littermates(F(1,28)= 4.345,
p = 0.0464;posthoc Fisher’sPLSD p = 0.0491).The reduced
latency in comparison to the first exposure to the box,however,
indicates formation of long-term memories by 5XFAD transgenic
mice.In the O-Maze,5XFAD transgenic mice spent longer time
(Figure 3H)and moved longer distances (Figure 3I)in the open
areas compared to their controllittermates (time F(1,24)= 23,679,
p,0.0001; distanceF(1,24)= 15.319,p = 0.0007).In summary,
5XFAD transgenic mice spend in these mazes more time in the
open illuminated areas.
When analyzedfor the startleresponseand its prepulse
inhibition,5XFAD transgenicmice displayeda significantly
reduced startle response at120 dB (F(1,28)= 4.578,p = 0.0412;
posthoc Fisher’s PLSD p = 0.0265),which was notinhibited by
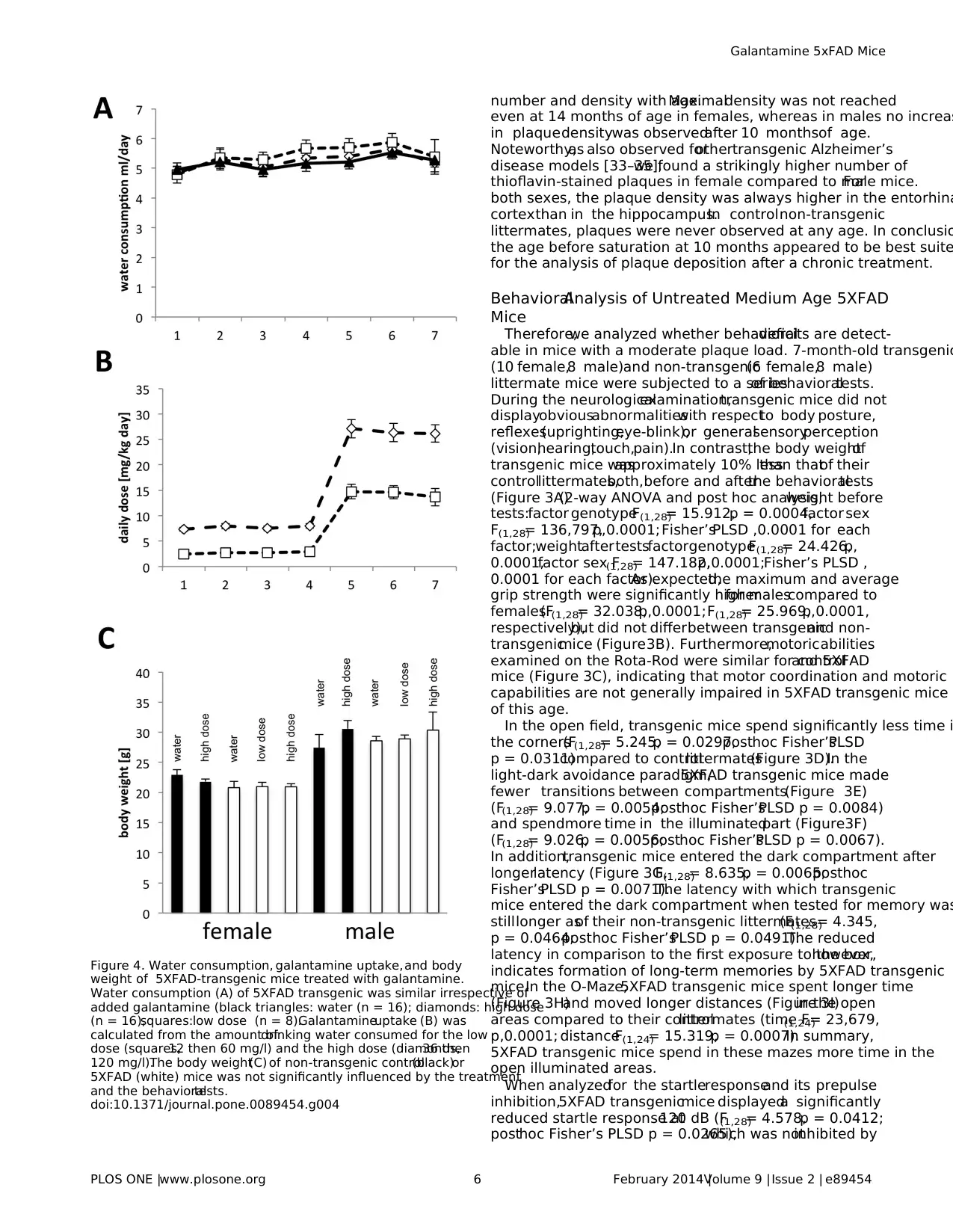
Figure 4. Water consumption, galantamine uptake,and body
weight of 5XFAD-transgenic mice treated with galantamine.
Water consumption (A) of 5XFAD transgenic was similar irrespective of
added galantamine (black triangles: water (n = 16); diamonds: high dose
(n = 16),squares:low dose (n = 8).Galantamineuptake (B) was
calculated from the amount ofdrinking water consumed for the low
dose (squares,12 then 60 mg/l) and the high dose (diamonds,36 then
120 mg/l).The body weight(C) of non-transgenic control(black)or
5XFAD (white) mice was not significantly influenced by the treatment
and the behavioraltests.
doi:10.1371/journal.pone.0089454.g004
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 6 February 2014 |Volume 9 |Issue 2 | e89454
even at 14 months of age in females, whereas in males no increas
in plaquedensitywas observedafter 10 monthsof age.
Noteworthy,as also observed forothertransgenic Alzheimer’s
disease models [33–35],we found a strikingly higher number of
thioflavin-stained plaques in female compared to male mice.For
both sexes, the plaque density was always higher in the entorhina
cortexthan in the hippocampus.In controlnon-transgenic
littermates, plaques were never observed at any age. In conclusio
the age before saturation at 10 months appeared to be best suite
for the analysis of plaque deposition after a chronic treatment.
BehavioralAnalysis of Untreated Medium Age 5XFAD
Mice
Therefore,we analyzed whether behavioraldeficits are detect-
able in mice with a moderate plaque load. 7-month-old transgenic
(10 female,8 male)and non-transgenic(6 female,8 male)
littermate mice were subjected to a seriesof behavioraltests.
During the neurologicalexamination,transgenic mice did not
displayobviousabnormalitieswith respectto body posture,
reflexes(uprighting,eye-blink),or generalsensoryperception
(vision,hearing,touch,pain).In contrast,the body weightof
transgenic mice wasapproximately 10% lessthan thatof their
controllittermates,both,before and afterthe behavioraltests
(Figure 3A)(2-way ANOVA and post hoc analysis,weight before
tests:factor genotypeF(1,28)= 15.912,p = 0.0004,factor sex
F(1,28)= 136,797,p,0.0001; Fisher’sPLSD ,0.0001 for each
factor;weightaftertestsfactorgenotypeF(1,28)= 24.426,p,
0.0001,factor sex F(1,28)= 147.182,p,0.0001;Fisher’s PLSD ,
0.0001 for each factor).As expected,the maximum and average
grip strength were significantly higherfor malescompared to
females(F(1,28)= 32.038,p,0.0001;F(1,28)= 25.969,p,0.0001,
respectively),but did not differbetween transgenicand non-
transgenicmice (Figure3B). Furthermore,motoricabilities
examined on the Rota-Rod were similar for controland 5XFAD
mice (Figure 3C), indicating that motor coordination and motoric
capabilities are not generally impaired in 5XFAD transgenic mice
of this age.
In the open field, transgenic mice spend significantly less time i
the corners(F(1,28)= 5.245,p = 0.0297,posthoc Fisher’sPLSD
p = 0.0311)compared to controllittermates(Figure 3D).In the
light-dark avoidance paradigm,5XFAD transgenic mice made
fewer transitions between compartments(Figure 3E)
(F(1,28)= 9.077,p = 0.0054;posthoc Fisher’sPLSD p = 0.0084)
and spendmore time in the illuminatedpart (Figure3F)
(F(1,28)= 9.026,p = 0.0056;posthoc Fisher’sPLSD p = 0.0067).
In addition,transgenic mice entered the dark compartment after
longerlatency (Figure 3G,F(1,28)= 8.635,p = 0.0065;posthoc
Fisher’sPLSD p = 0.0071).The latency with which transgenic
mice entered the dark compartment when tested for memory was
stilllonger asof their non-transgenic littermates(F(1,28)= 4.345,
p = 0.0464;posthoc Fisher’sPLSD p = 0.0491).The reduced
latency in comparison to the first exposure to the box,however,
indicates formation of long-term memories by 5XFAD transgenic
mice.In the O-Maze,5XFAD transgenic mice spent longer time
(Figure 3H)and moved longer distances (Figure 3I)in the open
areas compared to their controllittermates (time F(1,24)= 23,679,
p,0.0001; distanceF(1,24)= 15.319,p = 0.0007).In summary,
5XFAD transgenic mice spend in these mazes more time in the
open illuminated areas.
When analyzedfor the startleresponseand its prepulse
inhibition,5XFAD transgenicmice displayeda significantly
reduced startle response at120 dB (F(1,28)= 4.578,p = 0.0412;
posthoc Fisher’s PLSD p = 0.0265),which was notinhibited by
Figure 4. Water consumption, galantamine uptake,and body
weight of 5XFAD-transgenic mice treated with galantamine.
Water consumption (A) of 5XFAD transgenic was similar irrespective of
added galantamine (black triangles: water (n = 16); diamonds: high dose
(n = 16),squares:low dose (n = 8).Galantamineuptake (B) was
calculated from the amount ofdrinking water consumed for the low
dose (squares,12 then 60 mg/l) and the high dose (diamonds,36 then
120 mg/l).The body weight(C) of non-transgenic control(black)or
5XFAD (white) mice was not significantly influenced by the treatment
and the behavioraltests.
doi:10.1371/journal.pone.0089454.g004
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 6 February 2014 |Volume 9 |Issue 2 | e89454
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
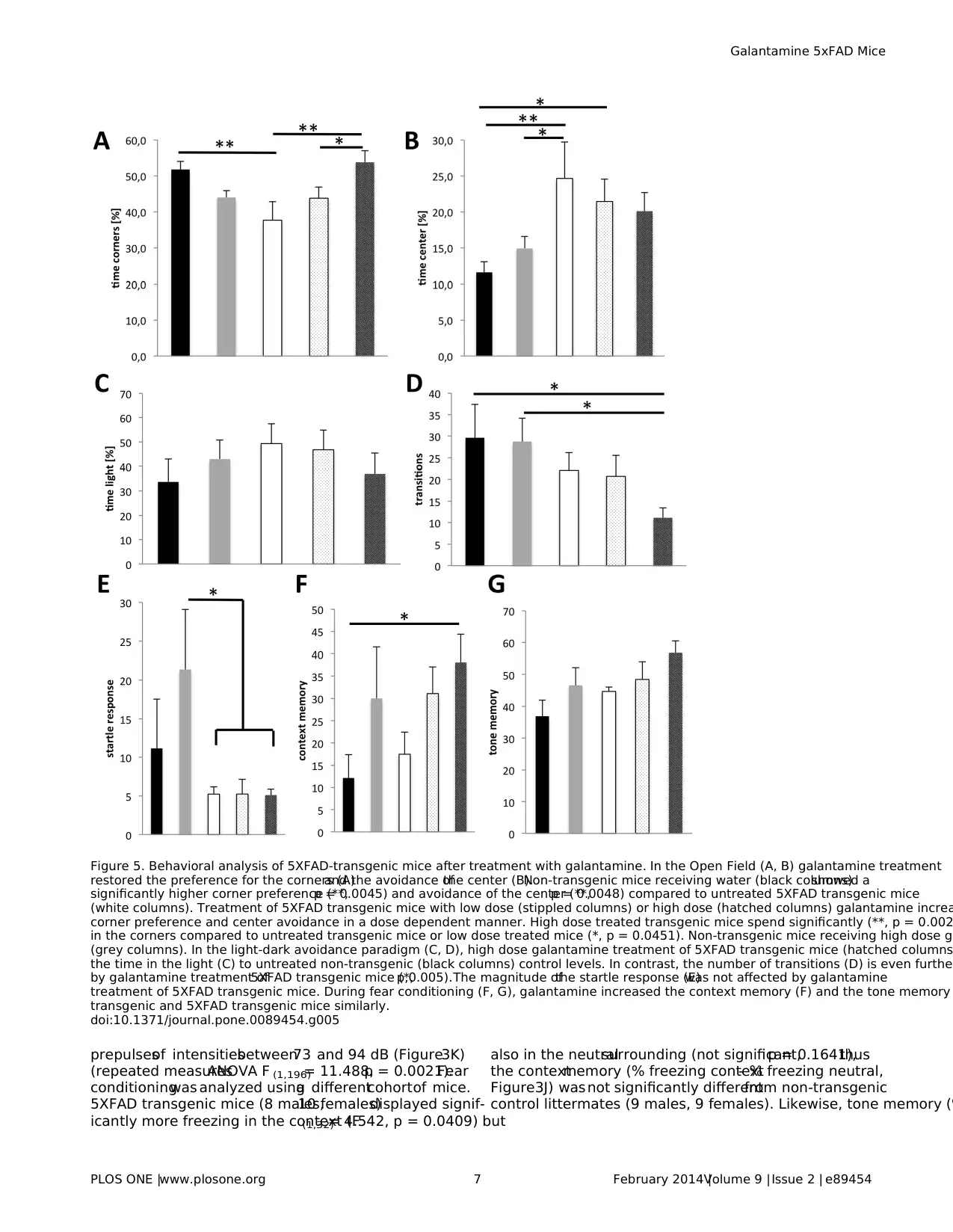
prepulsesof intensitiesbetween73 and 94 dB (Figure3K)
(repeated measuresANOVA F (1,196)= 11.488,p = 0.0021).Fear
conditioningwas analyzed usinga differentcohortof mice.
5XFAD transgenic mice (8 males,10 females)displayed signif-
icantly more freezing in the context (F(1,32)= 4.542, p = 0.0409) but
also in the neutralsurrounding (not significant,p = 0.1641),thus
the contextmemory (% freezing context- % freezing neutral,
Figure3J) was not significantly differentfrom non-transgenic
control littermates (9 males, 9 females). Likewise, tone memory (%
Figure 5. Behavioral analysis of 5XFAD-transgenic mice after treatment with galantamine. In the Open Field (A, B) galantamine treatment
restored the preference for the corners (A)and the avoidance ofthe center (B).Non-transgenic mice receiving water (black columns)showed a
significantly higher corner preference (**,p = 0.0045) and avoidance of the center (**,p = 0.0048) compared to untreated 5XFAD transgenic mice
(white columns). Treatment of 5XFAD transgenic mice with low dose (stippled columns) or high dose (hatched columns) galantamine increa
corner preference and center avoidance in a dose dependent manner. High dose treated transgenic mice spend significantly (**, p = 0.002
in the corners compared to untreated transgenic mice or low dose treated mice (*, p = 0.0451). Non-transgenic mice receiving high dose ga
(grey columns). In the light-dark avoidance paradigm (C, D), high dose galantamine treatment of 5XFAD transgenic mice (hatched columns
the time in the light (C) to untreated non-transgenic (black columns) control levels. In contrast, the number of transitions (D) is even further
by galantamine treatment of5XFAD transgenic mice (*,p,0.005).The magnitude ofthe startle response (E)was not affected by galantamine
treatment of 5XFAD transgenic mice. During fear conditioning (F, G), galantamine increased the context memory (F) and the tone memory
transgenic and 5XFAD transgenic mice similarly.
doi:10.1371/journal.pone.0089454.g005
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 7 February 2014 |Volume 9 |Issue 2 | e89454
(repeated measuresANOVA F (1,196)= 11.488,p = 0.0021).Fear
conditioningwas analyzed usinga differentcohortof mice.
5XFAD transgenic mice (8 males,10 females)displayed signif-
icantly more freezing in the context (F(1,32)= 4.542, p = 0.0409) but
also in the neutralsurrounding (not significant,p = 0.1641),thus
the contextmemory (% freezing context- % freezing neutral,
Figure3J) was not significantly differentfrom non-transgenic
control littermates (9 males, 9 females). Likewise, tone memory (%
Figure 5. Behavioral analysis of 5XFAD-transgenic mice after treatment with galantamine. In the Open Field (A, B) galantamine treatment
restored the preference for the corners (A)and the avoidance ofthe center (B).Non-transgenic mice receiving water (black columns)showed a
significantly higher corner preference (**,p = 0.0045) and avoidance of the center (**,p = 0.0048) compared to untreated 5XFAD transgenic mice
(white columns). Treatment of 5XFAD transgenic mice with low dose (stippled columns) or high dose (hatched columns) galantamine increa
corner preference and center avoidance in a dose dependent manner. High dose treated transgenic mice spend significantly (**, p = 0.002
in the corners compared to untreated transgenic mice or low dose treated mice (*, p = 0.0451). Non-transgenic mice receiving high dose ga
(grey columns). In the light-dark avoidance paradigm (C, D), high dose galantamine treatment of 5XFAD transgenic mice (hatched columns
the time in the light (C) to untreated non-transgenic (black columns) control levels. In contrast, the number of transitions (D) is even further
by galantamine treatment of5XFAD transgenic mice (*,p,0.005).The magnitude ofthe startle response (E)was not affected by galantamine
treatment of 5XFAD transgenic mice. During fear conditioning (F, G), galantamine increased the context memory (F) and the tone memory
transgenic and 5XFAD transgenic mice similarly.
doi:10.1371/journal.pone.0089454.g005
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 7 February 2014 |Volume 9 |Issue 2 | e89454
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
freezing with tone in neutral - % freezing neutral without tone) was
slightly but not significantly (p = 0.09)less in transgenic mice.
In conclusion,we identified severalsignificantAD related
behavioraldifferences between 5XFAD and controlmice already
at the age ofseven months.Therefore,the 5XFAD transgenic
modelappearsto be a suitable system to study the effectsof
pharmacologically active substances both on behavior and plaque
load.
Chronic Galantamine Treatment of 5XFAD Mice
In order to further explore this model for assessing symptomatic
and disease-modifying properties of AD drugs, or drug candidates
in development,we investigated the effects ofchronic treatment
with galantamineon behaviorand plaqueload.Becausethis
model is characterized by early-onset plaque deposition, we chose
to treat 10–12-week-old5XFAD transgenicmice and non-
transgenic littermates with galantamine for 2 months and during
the following behavioral tests, which were characterized above for
the untreated animals.To reduceany potentialadverseside
effects,we administered during the first 4 weeks a lower dose of
galantamine that thereafter was followed by a much higher dose.
During the first 4 weeks,one group ofanimals received 36 mg/l
(high dose) and a second group 12 mg/l (low dose) galantamine in
the drinking water.Thereafter,the dose in the firstgroup of
animalswasincreased to 120 mg/l(high dose)and to 60 mg/l
(low dose)in the second group.7 weeks after onset oftreatment,
the animals were water deprived during the night to ensure a high
uptakeof drinking waterin the morning.During behavioral
experiments,animalsreceived 30–60 min priorto testing the
drinking solution,so as to ensure a high drug dosage during the
experiment. Control animals received water without drug. During
the treatmentperiod the generalcondition,water uptake
(Figure 4A),and body weight(Figure 4C)were monitored and
did not revealany difference between treated and controlor
transgenic and non-transgenic mice indicating that the treatment
was welltolerated.This application scheme resulted in the daily
uptake of14 mg/kg body weight (low dose)and 26 mg/kg body
weightduringthe last phaseof the treatment(Figure4B).
Consumptionof similar amountsof water indicatesthat
galantamineat the concentrationuseddid not induceany
preferenceor avoidanceof the drug. Behavioraltestswere
conducted with 4–5-month-old animals(130–150 days).After
the behavioraltests,brainswere sectioned forthe analysisof
plaque density using thioflavin S staining [32,36].
In the open field,mock-treated transgenic and non-transgenic
mice showed similardifferencesas described above forthe 7-
month-old animals.Transgenic mice spend significantly less time
in the cornersof the maze,but treatmentwith galantamine
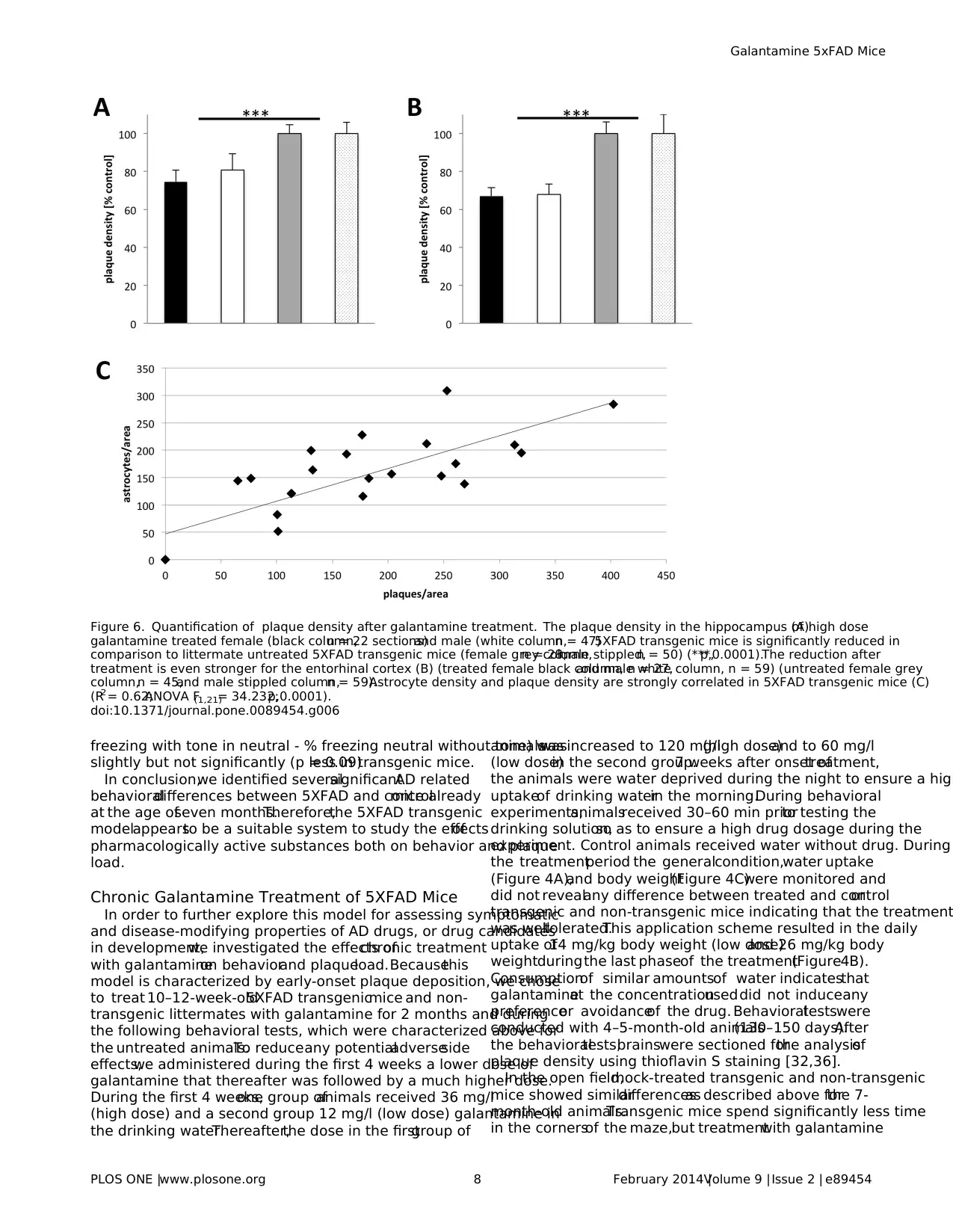
Figure 6. Quantification of plaque density after galantamine treatment. The plaque density in the hippocampus (A)of high dose
galantamine treated female (black column,n = 22 sections)and male (white column,n = 47)5XFAD transgenic mice is significantly reduced in
comparison to littermate untreated 5XFAD transgenic mice (female grey column,n = 28;male stippled,n = 50) (***,p,0.0001).The reduction after
treatment is even stronger for the entorhinal cortex (B) (treated female black column, n = 27,and male white column, n = 59) (untreated female grey
column,n = 45,and male stippled column,n = 59).Astrocyte density and plaque density are strongly correlated in 5XFAD transgenic mice (C)
(R2= 0.62;ANOVA F(1,21)= 34.232;p,0.0001).
doi:10.1371/journal.pone.0089454.g006
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 8 February 2014 |Volume 9 |Issue 2 | e89454
slightly but not significantly (p = 0.09)less in transgenic mice.
In conclusion,we identified severalsignificantAD related
behavioraldifferences between 5XFAD and controlmice already
at the age ofseven months.Therefore,the 5XFAD transgenic
modelappearsto be a suitable system to study the effectsof
pharmacologically active substances both on behavior and plaque
load.
Chronic Galantamine Treatment of 5XFAD Mice
In order to further explore this model for assessing symptomatic
and disease-modifying properties of AD drugs, or drug candidates
in development,we investigated the effects ofchronic treatment
with galantamineon behaviorand plaqueload.Becausethis
model is characterized by early-onset plaque deposition, we chose
to treat 10–12-week-old5XFAD transgenicmice and non-
transgenic littermates with galantamine for 2 months and during
the following behavioral tests, which were characterized above for
the untreated animals.To reduceany potentialadverseside
effects,we administered during the first 4 weeks a lower dose of
galantamine that thereafter was followed by a much higher dose.
During the first 4 weeks,one group ofanimals received 36 mg/l
(high dose) and a second group 12 mg/l (low dose) galantamine in
the drinking water.Thereafter,the dose in the firstgroup of
animalswasincreased to 120 mg/l(high dose)and to 60 mg/l
(low dose)in the second group.7 weeks after onset oftreatment,
the animals were water deprived during the night to ensure a high
uptakeof drinking waterin the morning.During behavioral
experiments,animalsreceived 30–60 min priorto testing the
drinking solution,so as to ensure a high drug dosage during the
experiment. Control animals received water without drug. During
the treatmentperiod the generalcondition,water uptake
(Figure 4A),and body weight(Figure 4C)were monitored and
did not revealany difference between treated and controlor
transgenic and non-transgenic mice indicating that the treatment
was welltolerated.This application scheme resulted in the daily
uptake of14 mg/kg body weight (low dose)and 26 mg/kg body
weightduringthe last phaseof the treatment(Figure4B).
Consumptionof similar amountsof water indicatesthat
galantamineat the concentrationuseddid not induceany
preferenceor avoidanceof the drug. Behavioraltestswere
conducted with 4–5-month-old animals(130–150 days).After
the behavioraltests,brainswere sectioned forthe analysisof
plaque density using thioflavin S staining [32,36].
In the open field,mock-treated transgenic and non-transgenic
mice showed similardifferencesas described above forthe 7-
month-old animals.Transgenic mice spend significantly less time
in the cornersof the maze,but treatmentwith galantamine
Figure 6. Quantification of plaque density after galantamine treatment. The plaque density in the hippocampus (A)of high dose
galantamine treated female (black column,n = 22 sections)and male (white column,n = 47)5XFAD transgenic mice is significantly reduced in
comparison to littermate untreated 5XFAD transgenic mice (female grey column,n = 28;male stippled,n = 50) (***,p,0.0001).The reduction after
treatment is even stronger for the entorhinal cortex (B) (treated female black column, n = 27,and male white column, n = 59) (untreated female grey
column,n = 45,and male stippled column,n = 59).Astrocyte density and plaque density are strongly correlated in 5XFAD transgenic mice (C)
(R2= 0.62;ANOVA F(1,21)= 34.232;p,0.0001).
doi:10.1371/journal.pone.0089454.g006
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 8 February 2014 |Volume 9 |Issue 2 | e89454
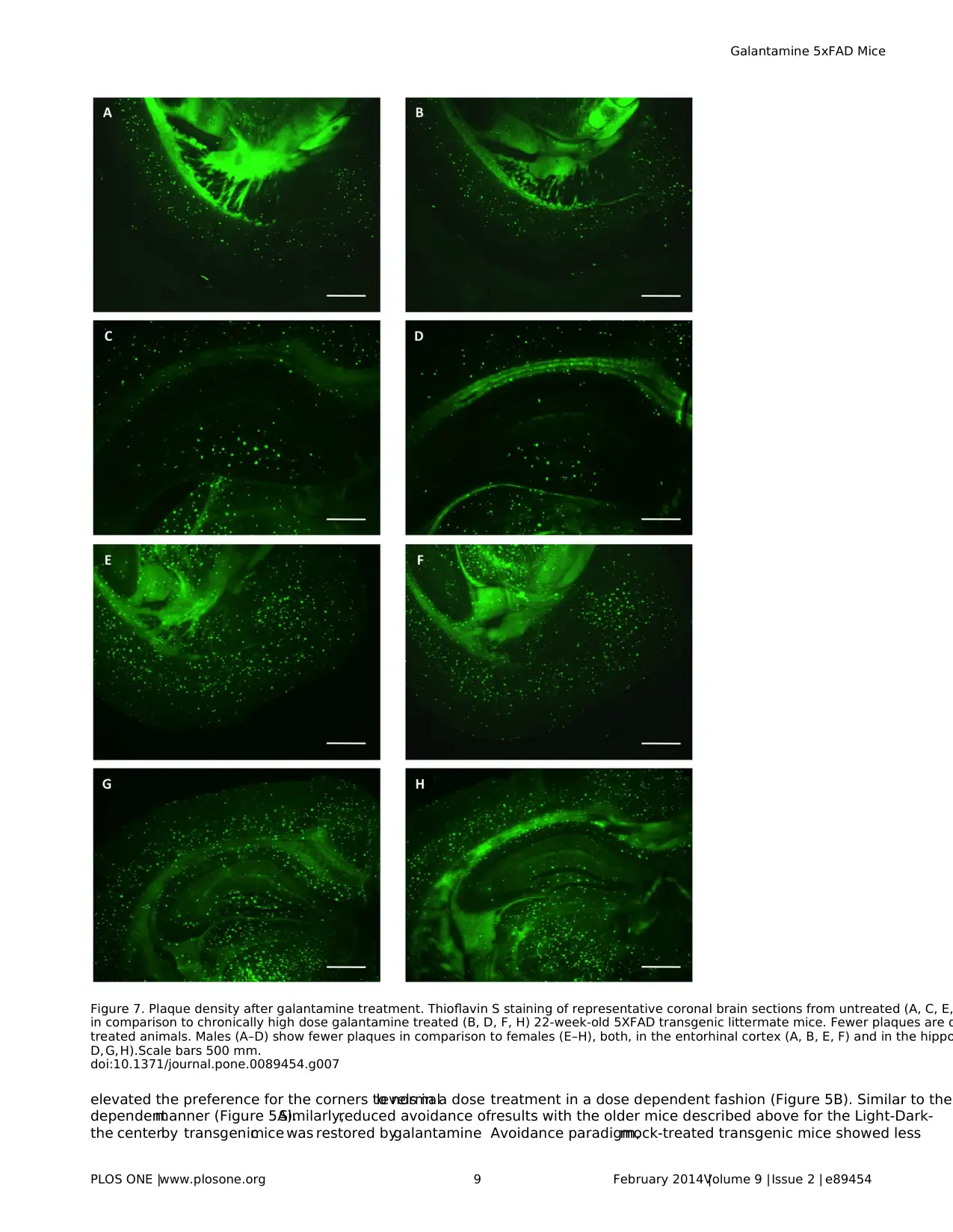
elevated the preference for the corners to normallevels in a dose
dependentmanner (Figure 5A).Similarly,reduced avoidance of
the centerby transgenicmice was restored bygalantamine
treatment in a dose dependent fashion (Figure 5B). Similar to the
results with the older mice described above for the Light-Dark-
Avoidance paradigm,mock-treated transgenic mice showed less
Figure 7. Plaque density after galantamine treatment. Thioflavin S staining of representative coronal brain sections from untreated (A, C, E,
in comparison to chronically high dose galantamine treated (B, D, F, H) 22-week-old 5XFAD transgenic littermate mice. Fewer plaques are d
treated animals. Males (A–D) show fewer plaques in comparison to females (E–H), both, in the entorhinal cortex (A, B, E, F) and in the hippo
D, G,H).Scale bars 500 mm.
doi:10.1371/journal.pone.0089454.g007
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 9 February 2014 |Volume 9 |Issue 2 | e89454
dependentmanner (Figure 5A).Similarly,reduced avoidance of
the centerby transgenicmice was restored bygalantamine
treatment in a dose dependent fashion (Figure 5B). Similar to the
results with the older mice described above for the Light-Dark-
Avoidance paradigm,mock-treated transgenic mice showed less
Figure 7. Plaque density after galantamine treatment. Thioflavin S staining of representative coronal brain sections from untreated (A, C, E,
in comparison to chronically high dose galantamine treated (B, D, F, H) 22-week-old 5XFAD transgenic littermate mice. Fewer plaques are d
treated animals. Males (A–D) show fewer plaques in comparison to females (E–H), both, in the entorhinal cortex (A, B, E, F) and in the hippo
D, G,H).Scale bars 500 mm.
doi:10.1371/journal.pone.0089454.g007
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 9 February 2014 |Volume 9 |Issue 2 | e89454
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

transitions and longer presence in the illuminated compartment.
Treatment with galantamine reduced the time spent in the light to
normallevels (Figure 5C),but reduced the number of transitions
even more (Figure 5D). The latency of transgenic mice compared
to non-transgenic mice was higher on the first exposure and not
significantly altered by galantamine treatment(data notshown).
The latency at the second encounter with the test box was reduced
irrespectiveof genotypeor treatmentindicatinglong-term
memory formation.The lower magnitude ofthe startle response
was reproduced with these younger transgenic mice (Figure 5E),
but the treatmentwith galantaminehad no effecton the
magnitude ofthe startle response,or itsinhibition by prepulses
(data not shown).During fear conditioning,freezing in the shock
context(Figure 5F)or after the tone in a neutralenvironment
(Figure 5G)by mock-treated transgenic mice was slightly but not
significantlyhighercompared to controlsand treatmentwith
galantamineresulted in increased freezingirrespectiveof the
genotype.
In summary, the behavioral differences between transgenic and
non-transgenic mice at this early age of 4–5 months confirmed the
findings for the 7-month-old mice.The treatmentwith galanta-
mine improved the behavior in the open field significantly and
partially in the light-dark avoidance paradigm but was not able to
normalize the startle response. In the fear conditioning paradigm,
galantamine generally increased freezing potentially indicating a
side effect on anxiety.
Followingthe behavioraltests,we quantified in thesame
animals the plaque load in the hippocampus and entorhinal cortex
by thioflavin staining.Transgenic animals treated with the high
dose of galantamine showed a highly significant lower number of
plaquesin both areascompared to untreated controlmice (p#
0.0001 according to ANOVA) (Figure 6, 7). In the hippocampus of
high dose treated transgenic malesapproximately 19% and in
females approximately 25% less plaques were counted compared
to untreated controls.In the entorhinalcortex,32% less plaques
for malesand 33% lessfor femaleswere observed.These data
indicated that galantamine treatment might reduce the formation
of plaques in this modelsystem.
GFAP-positive reactive astrocytes are usually found in associ-
ation with Ab plaques (Figure 1).Therefore,we determined the
numberof GFAP-positive reactive astrocytesin 5XFAD trans-
genic mice and found a strong correlation between plaque density
and the number of reactive astrocytes (Figure 6C).
Discussion
In this study,the effectof chronicoral administration of
galantamine,an acetylcholinesteraseinhibitorand allosteric
nicotinic receptormodulator,in 5XFAD transgenic mice [20]
was investigated.
Time Course of Plaque Deposition in 5XFAD Mice
The 5XFAD model, due to its earlyplaquedevelopment
phenotype,has been investigated extensively to study the various
aspectsof early onsetAD [20–24,37].However,the pattern of
progressive plaque deposition and development has not yet been
described in a systematic manner. Here, we monitored for the first
time quantitatively the increase in plaque load over time between
the age of 3 to 14 months in the hippocampus and the entorhinal
cortex,because these plaques are known to closely resemble the
amyloid plaques in human AD patients [38]. Moreover, in AD loss
of pyramidalcells in lamina 2 of the entorhinalcortex and in the
CA1 region ofthe hippocampuswasdescribed [39],indicating
that these brain structures are severely affected by the disease.In
agreementwith previousstudies[20,21,40],we observed very
early onset of plaque deposition in 5XFAD mice beginning around
2 monthsof age. Furthermore,we show herethat plaque
deposition occurs differently in the two sexes with lower plaque
density reaching plateau levels at 10 months of age in males, whi
stillincreasing in females atleastuntilan age of14 months.A
higherplaque density in female transgenic mice hasalso been
noted in otherAD mousemodels[33–35]and is possibly a
consequence ofdecreased estrogen levels[41],modified BACE
activity,or altered metalion levels [42].
Behavior of Untreated 7-months-old 5XFAD Mice
As therapeutic intervention may be mostpromising atearly
stages,we investigated relatively young 5XFAD transgenic mice
for behavioralabnormalities.Although,5XFAD transgenic mice
displayed already at the age of seven months a significantly lower
body weightparalleling the weightloss always closely associated
with AD [43], neuromuscular functioning and motor-coordination
appeared normal,confirmingpreviousstudiesreportingthat
5XFAD mice do not exhibit sensory-motoric impairments in string
hanging and beam walking before 9 months[24]or abnormal
rota-rod coordination before 12 months[22] of age.Likewise,
severalother AD mouse models display normalmotor coordina-
tion and grip strength [44].In contrast,we detected behavioral
abnormalities in the anxiety addressing paradigms,namely,open
field, light-dark avoidance, and O-maze, similar to the tendency to
spend longer times in the center of the open field or the open arm
of the elevated plus maze reported previously for 5XFAD [22,23]
and severalother AD mouse models [45,46].In addition,in the
course ofthe light-dark avoidance test,5XFAD mice showed a
longer latency to enter the dark compartmentfor the firsttime.
Such behavior could be a consequence of reduced anxiety levels,
but in addition,it could also reflect the characteristic aversion to
darkness as noticed in AD patients.
Reduced hippocampus dependenttrace fear conditioning,but
normalhippocampus independent delay fear conditioning,which
is comparable to the tone memory assayed here, has been report
for 5XFAD mice [47]. During hippocampus dependent contextual
fear conditioningwe observed significantlymore freezingof
5XFAD mice in the context but also in the neutralsurrounding.
Therefore,the calculated context memory and the hippocampus
independent tone memory were not significantly different.It has
also been shown previously, that contextual fear conditioning afte
1 footshock is normal in 5XFAD mice younger than 4 months, but
impaired in the 6 month old animals,which could be overcome
using 3–5 footshocks [48]. Our paradigm used 2 footshocks, which
may have been sufficient to overcome a slight deficit.
Suppressionof sensorimotorgatinghas been repeatedly
reported in AD patients [49,50]. In a similar APP/PS1 transgenic
mouse model, sensorimotor gating deficits were reported for aged
mice using prepulse inhibition of the acoustic startle response [51
In the 5XFAD mice,we measured a very weak acoustic startle
response, indicating possible defects in processing or responding
the stimulus. Furthermore, prepulses of various intensities did not
inhibit this residual startle response indicating sensorimotor gatin
deficits.
In conclusion,5XFAD mice show at the age ofseven months
severalbehavioraldeficits,a phenotypewith mild cognitive
impairment, and progressive amyloid plaque deposition permittin
the detection of therapeutic effects.
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 10 February 2014 |Volume 9 |Issue 2 | e89454
Treatment with galantamine reduced the time spent in the light to
normallevels (Figure 5C),but reduced the number of transitions
even more (Figure 5D). The latency of transgenic mice compared
to non-transgenic mice was higher on the first exposure and not
significantly altered by galantamine treatment(data notshown).
The latency at the second encounter with the test box was reduced
irrespectiveof genotypeor treatmentindicatinglong-term
memory formation.The lower magnitude ofthe startle response
was reproduced with these younger transgenic mice (Figure 5E),
but the treatmentwith galantaminehad no effecton the
magnitude ofthe startle response,or itsinhibition by prepulses
(data not shown).During fear conditioning,freezing in the shock
context(Figure 5F)or after the tone in a neutralenvironment
(Figure 5G)by mock-treated transgenic mice was slightly but not
significantlyhighercompared to controlsand treatmentwith
galantamineresulted in increased freezingirrespectiveof the
genotype.
In summary, the behavioral differences between transgenic and
non-transgenic mice at this early age of 4–5 months confirmed the
findings for the 7-month-old mice.The treatmentwith galanta-
mine improved the behavior in the open field significantly and
partially in the light-dark avoidance paradigm but was not able to
normalize the startle response. In the fear conditioning paradigm,
galantamine generally increased freezing potentially indicating a
side effect on anxiety.
Followingthe behavioraltests,we quantified in thesame
animals the plaque load in the hippocampus and entorhinal cortex
by thioflavin staining.Transgenic animals treated with the high
dose of galantamine showed a highly significant lower number of
plaquesin both areascompared to untreated controlmice (p#
0.0001 according to ANOVA) (Figure 6, 7). In the hippocampus of
high dose treated transgenic malesapproximately 19% and in
females approximately 25% less plaques were counted compared
to untreated controls.In the entorhinalcortex,32% less plaques
for malesand 33% lessfor femaleswere observed.These data
indicated that galantamine treatment might reduce the formation
of plaques in this modelsystem.
GFAP-positive reactive astrocytes are usually found in associ-
ation with Ab plaques (Figure 1).Therefore,we determined the
numberof GFAP-positive reactive astrocytesin 5XFAD trans-
genic mice and found a strong correlation between plaque density
and the number of reactive astrocytes (Figure 6C).
Discussion
In this study,the effectof chronicoral administration of
galantamine,an acetylcholinesteraseinhibitorand allosteric
nicotinic receptormodulator,in 5XFAD transgenic mice [20]
was investigated.
Time Course of Plaque Deposition in 5XFAD Mice
The 5XFAD model, due to its earlyplaquedevelopment
phenotype,has been investigated extensively to study the various
aspectsof early onsetAD [20–24,37].However,the pattern of
progressive plaque deposition and development has not yet been
described in a systematic manner. Here, we monitored for the first
time quantitatively the increase in plaque load over time between
the age of 3 to 14 months in the hippocampus and the entorhinal
cortex,because these plaques are known to closely resemble the
amyloid plaques in human AD patients [38]. Moreover, in AD loss
of pyramidalcells in lamina 2 of the entorhinalcortex and in the
CA1 region ofthe hippocampuswasdescribed [39],indicating
that these brain structures are severely affected by the disease.In
agreementwith previousstudies[20,21,40],we observed very
early onset of plaque deposition in 5XFAD mice beginning around
2 monthsof age. Furthermore,we show herethat plaque
deposition occurs differently in the two sexes with lower plaque
density reaching plateau levels at 10 months of age in males, whi
stillincreasing in females atleastuntilan age of14 months.A
higherplaque density in female transgenic mice hasalso been
noted in otherAD mousemodels[33–35]and is possibly a
consequence ofdecreased estrogen levels[41],modified BACE
activity,or altered metalion levels [42].
Behavior of Untreated 7-months-old 5XFAD Mice
As therapeutic intervention may be mostpromising atearly
stages,we investigated relatively young 5XFAD transgenic mice
for behavioralabnormalities.Although,5XFAD transgenic mice
displayed already at the age of seven months a significantly lower
body weightparalleling the weightloss always closely associated
with AD [43], neuromuscular functioning and motor-coordination
appeared normal,confirmingpreviousstudiesreportingthat
5XFAD mice do not exhibit sensory-motoric impairments in string
hanging and beam walking before 9 months[24]or abnormal
rota-rod coordination before 12 months[22] of age.Likewise,
severalother AD mouse models display normalmotor coordina-
tion and grip strength [44].In contrast,we detected behavioral
abnormalities in the anxiety addressing paradigms,namely,open
field, light-dark avoidance, and O-maze, similar to the tendency to
spend longer times in the center of the open field or the open arm
of the elevated plus maze reported previously for 5XFAD [22,23]
and severalother AD mouse models [45,46].In addition,in the
course ofthe light-dark avoidance test,5XFAD mice showed a
longer latency to enter the dark compartmentfor the firsttime.
Such behavior could be a consequence of reduced anxiety levels,
but in addition,it could also reflect the characteristic aversion to
darkness as noticed in AD patients.
Reduced hippocampus dependenttrace fear conditioning,but
normalhippocampus independent delay fear conditioning,which
is comparable to the tone memory assayed here, has been report
for 5XFAD mice [47]. During hippocampus dependent contextual
fear conditioningwe observed significantlymore freezingof
5XFAD mice in the context but also in the neutralsurrounding.
Therefore,the calculated context memory and the hippocampus
independent tone memory were not significantly different.It has
also been shown previously, that contextual fear conditioning afte
1 footshock is normal in 5XFAD mice younger than 4 months, but
impaired in the 6 month old animals,which could be overcome
using 3–5 footshocks [48]. Our paradigm used 2 footshocks, which
may have been sufficient to overcome a slight deficit.
Suppressionof sensorimotorgatinghas been repeatedly
reported in AD patients [49,50]. In a similar APP/PS1 transgenic
mouse model, sensorimotor gating deficits were reported for aged
mice using prepulse inhibition of the acoustic startle response [51
In the 5XFAD mice,we measured a very weak acoustic startle
response, indicating possible defects in processing or responding
the stimulus. Furthermore, prepulses of various intensities did not
inhibit this residual startle response indicating sensorimotor gatin
deficits.
In conclusion,5XFAD mice show at the age ofseven months
severalbehavioraldeficits,a phenotypewith mild cognitive
impairment, and progressive amyloid plaque deposition permittin
the detection of therapeutic effects.
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 10 February 2014 |Volume 9 |Issue 2 | e89454
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Galantamine Treatment Affects Behavior and Plaque
Deposition
To investigate the potential effects on the retardation of disease
progression,we started with chronic galantamine treatmentat 3
months of age, when plaque deposition is considerable, assayed the
behavior 2 monthslater,and then determined the plaque load
before reaching saturation levels.Galantamine is known to have
side effects with respect to cholinergically mediated gastrointestinal
symptoms like other cholinesterase inhibitors [52].To mimic the
treatment regimen in humans, where the dosage is increased with
time to minimize negative side effects[53], we increased the
dosagegraduallyduring thechronictreatment,and chose2
different concentrations that were tolerated well by the mice. Also,
galantaminedid not alter the amountof waterconsumed,
indicating the absence of an aversive response.
The chronic treatment with galantamine had positive effects on
certain behavioral tasks but could not rescue all the abnormalities.
It did not modify the poorstartle response orthe lack ofits
prepulse inhibition in 5XFAD mice. At the moment, we can only
speculate thatthe low startle response results from a very early
event that cannot be cured by later treatment or that the dosage or
duration were notsufficientto protectthese neuronalcircuits.
Furthermore,the treatment increased the overallfreezing behav-
ior in the fear conditioning paradigm irrespective of the genotype,
which could be a consequence of the potential ‘‘nicotinic effect’’ of
the drug.However,in the open field paradigm galantamine
treatmentrestored thebehaviorof treated 5XFAD miceto
normal.Likewise,the abnormallight-dark avoidance behavior of
5XFAD mice was partiallynormalized.Accordingto the
cholinergic hypothesis,a decreased production ofacetylcholine
or an amplified acetylcholinesterase activity results in impairment
of the cholinergic transmission,in turn leading to the lossof
intellectual abilities [54]. Hence, the positive effect of galantamine
in restoring certain normalbehavioraltraits could result from its
acetylcholinesterase inhibitor activity.
On analyzing the plaque load in the hippocampalformation
and the entorhinalcortex,we found thattreated mice show a
significantlylower plaque densityin both the structures,
irrespective of the sex,when compared to the untreated controls.
Potentialmechanismsof action ofthe drug may be a reduced
deposition of Ab into plaques as opposed to clearance of already
existingdeposits.Importantly,acetylcholinesterasehas been
shown to promote the aggregation of b-amyloid peptide fragments
by forminga complexwith the growingfibrils [54]. Also,
galantamine has been shown to enhance microglialAb clearance
[16].Anotherpossible explanation could be thatgalantamine
binds to amyloid oligomers leading to a significant conformational
change atthe turn region (Asp23-Gly29)disrupting interactions
between individualb strands and promoting a nontoxic confor-
mation ofAb (1–40)to preventthe formation ofneurotoxic
oligomers [55]. A previous study by Unger et al. [56] reported that
galantamine at a concentration of 2mg/kg injected subcutaneously
for 10 days had no effect on the levels of soluble or insoluble forms
of Ab in 10 months old Tg2576 mice.In our study,we used a
much higherdose,chronicoral application formorethan 2
months,and assayed thenumberof plaquedepositsbefore
saturation.Hence,as an extension of this study,quantification of
the totalAb and its different isoforms in chronically treated mice
and their relation to plaque density may possibly be helpfulto
address the mechanisms of galantamine action.However,despite
the debate about the most toxic form of Ab, it is important to note
that a numberof currentstrategiese.g. glutaminylcyclase
inhibition and immunotherapy,involved in the treatmentand/
or prevention ofAD progression are indeed directed against the
deposition of plaques [6,57]. Fewer plaques could have a multifold
benefit through the reduced levels of all possible harmful forms of
Ab (monomer,oligomer,and fibril).A reduced plaque load has
been positively correlated with better performances in behavioral
tests [6,57].Additionally,regardless ofthe levels ofAb, reduced
plaque formation could have a role in preventing further cortical
atrophy in AD brain, as plaque deposition in multiple studies has
been correlatedwith acceleratedcortical atrophy[58,59].
Furthermore,a reduced plaque burden hasbeen shown to be
accompanied by a reduced glialactivation [6,60].In agreement,
we found a positive correlation between the number ofplaques
and the extentof gliosis,quantified by the numberof GFAP
positive astrocytes.This implies that chronic galantamine admin-
istration reduced the levelof astrogliosis in the AD mice brain.
Therapeutic targeting ofneuroinflammatory pathwaysin which
astrocytes have a prominent position may be a promising strategy
to cure AD [61]. The potential of galantamine to interfere with the
extentof gliosisis, therefore,anotherpositive outcome ofthe
treatment.
In summary,we reporta significantly delayed progression of
amyloid plaque deposition and improvement of certain behavioral
symptoms associated with AD in the 5XFAD Alzheimer’s disease
modelafter chronic treatment with galantamine.
In contrast to other cholinesterase inhibitors e.g. donepeziland
rivastigmine,the relatively hydrophilic galantamine poorly pene-
tratesthe blood brain barrier.However,the much more
hydrophobic Gln-1062 (Memogain),a pro-drug ofgalantamine,
possesses a more than 15-fold higher bioavailability in the brain
[62].Therefore,it willbe interesting to investigate Memogain in
comparison to galantamine in this AD modelin the future.
Acknowledgments
The authors gratefully acknowledge expert technical assistance by Angelik
Reichel,Karla Sowa,and Daniela Hill.This study was supported by the
German Ministry for Education and Research (BMBF)specialnetwork
program KMU-Innovativ-2.We thank allmembersof the Neurocure
network for collaboration and fruitfuldiscussions and Galantos Pharma
GmbH for providing galantamine hydrobromide.
Author Contributions
Conceived and designed theexperiments:DM AM. Performed the
experiments:DM SB CH. Analyzed the data:DM SB CH. Contributed
reagents/materials/analysis tools: DM AM. Wrote the paper: DM SB AM.
References
1. Karran E,Mercken M,De Strooper B (2011)The amyloid cascade hypothesis
for Alzheimer’s disease:an appraisalfor the development oftherapeutics.Nat
Rev Drug Discov 10:698–712.
2. Citron M (2004)Strategies for disease modification in Alzheimer’s disease.Nat
Rev Neurosci5: 677–685.
3. Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, et al.
(2009) Oligomeric amyloid b associates with postsynaptic densities and correlates
with excitatory synapse loss near senile plaques.Proc NatlAcad SciUSA 106:
4012–4017.
4. Spires TL,Meyer-Luehmann M,Stern EA,McLean PJ,Skoch J,et al.(2005)
Dendritic spine abnormalitiesin APP transgenic mice demonstrated by gene
transfer and intravitalmultiphoton microscopy.J Neurosci25:7278–7287.
5. Knowles RB,Wyart C,Buldyrev SV,Cruz L, Urbanc B,et al.(1999)Plaque-
induced neurite abnormalities: Implications for disruption of neural networks in
Alzheimer’s disease.Proc NatlAcad SciUSA 96:5274–5279.
6. Schilling S,ZeitschelU, Hoffmann T,HeiserU, Francke M,et al. (2008)
Glutaminylcyclase inhibition attenuatespyroglutamate Ab and Alzheimer’s
disease-like pathology in vivo.Nat Med 14:1106–1111.
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 11 February 2014 |Volume 9 |Issue 2 | e89454
Deposition
To investigate the potential effects on the retardation of disease
progression,we started with chronic galantamine treatmentat 3
months of age, when plaque deposition is considerable, assayed the
behavior 2 monthslater,and then determined the plaque load
before reaching saturation levels.Galantamine is known to have
side effects with respect to cholinergically mediated gastrointestinal
symptoms like other cholinesterase inhibitors [52].To mimic the
treatment regimen in humans, where the dosage is increased with
time to minimize negative side effects[53], we increased the
dosagegraduallyduring thechronictreatment,and chose2
different concentrations that were tolerated well by the mice. Also,
galantaminedid not alter the amountof waterconsumed,
indicating the absence of an aversive response.
The chronic treatment with galantamine had positive effects on
certain behavioral tasks but could not rescue all the abnormalities.
It did not modify the poorstartle response orthe lack ofits
prepulse inhibition in 5XFAD mice. At the moment, we can only
speculate thatthe low startle response results from a very early
event that cannot be cured by later treatment or that the dosage or
duration were notsufficientto protectthese neuronalcircuits.
Furthermore,the treatment increased the overallfreezing behav-
ior in the fear conditioning paradigm irrespective of the genotype,
which could be a consequence of the potential ‘‘nicotinic effect’’ of
the drug.However,in the open field paradigm galantamine
treatmentrestored thebehaviorof treated 5XFAD miceto
normal.Likewise,the abnormallight-dark avoidance behavior of
5XFAD mice was partiallynormalized.Accordingto the
cholinergic hypothesis,a decreased production ofacetylcholine
or an amplified acetylcholinesterase activity results in impairment
of the cholinergic transmission,in turn leading to the lossof
intellectual abilities [54]. Hence, the positive effect of galantamine
in restoring certain normalbehavioraltraits could result from its
acetylcholinesterase inhibitor activity.
On analyzing the plaque load in the hippocampalformation
and the entorhinalcortex,we found thattreated mice show a
significantlylower plaque densityin both the structures,
irrespective of the sex,when compared to the untreated controls.
Potentialmechanismsof action ofthe drug may be a reduced
deposition of Ab into plaques as opposed to clearance of already
existingdeposits.Importantly,acetylcholinesterasehas been
shown to promote the aggregation of b-amyloid peptide fragments
by forminga complexwith the growingfibrils [54]. Also,
galantamine has been shown to enhance microglialAb clearance
[16].Anotherpossible explanation could be thatgalantamine
binds to amyloid oligomers leading to a significant conformational
change atthe turn region (Asp23-Gly29)disrupting interactions
between individualb strands and promoting a nontoxic confor-
mation ofAb (1–40)to preventthe formation ofneurotoxic
oligomers [55]. A previous study by Unger et al. [56] reported that
galantamine at a concentration of 2mg/kg injected subcutaneously
for 10 days had no effect on the levels of soluble or insoluble forms
of Ab in 10 months old Tg2576 mice.In our study,we used a
much higherdose,chronicoral application formorethan 2
months,and assayed thenumberof plaquedepositsbefore
saturation.Hence,as an extension of this study,quantification of
the totalAb and its different isoforms in chronically treated mice
and their relation to plaque density may possibly be helpfulto
address the mechanisms of galantamine action.However,despite
the debate about the most toxic form of Ab, it is important to note
that a numberof currentstrategiese.g. glutaminylcyclase
inhibition and immunotherapy,involved in the treatmentand/
or prevention ofAD progression are indeed directed against the
deposition of plaques [6,57]. Fewer plaques could have a multifold
benefit through the reduced levels of all possible harmful forms of
Ab (monomer,oligomer,and fibril).A reduced plaque load has
been positively correlated with better performances in behavioral
tests [6,57].Additionally,regardless ofthe levels ofAb, reduced
plaque formation could have a role in preventing further cortical
atrophy in AD brain, as plaque deposition in multiple studies has
been correlatedwith acceleratedcortical atrophy[58,59].
Furthermore,a reduced plaque burden hasbeen shown to be
accompanied by a reduced glialactivation [6,60].In agreement,
we found a positive correlation between the number ofplaques
and the extentof gliosis,quantified by the numberof GFAP
positive astrocytes.This implies that chronic galantamine admin-
istration reduced the levelof astrogliosis in the AD mice brain.
Therapeutic targeting ofneuroinflammatory pathwaysin which
astrocytes have a prominent position may be a promising strategy
to cure AD [61]. The potential of galantamine to interfere with the
extentof gliosisis, therefore,anotherpositive outcome ofthe
treatment.
In summary,we reporta significantly delayed progression of
amyloid plaque deposition and improvement of certain behavioral
symptoms associated with AD in the 5XFAD Alzheimer’s disease
modelafter chronic treatment with galantamine.
In contrast to other cholinesterase inhibitors e.g. donepeziland
rivastigmine,the relatively hydrophilic galantamine poorly pene-
tratesthe blood brain barrier.However,the much more
hydrophobic Gln-1062 (Memogain),a pro-drug ofgalantamine,
possesses a more than 15-fold higher bioavailability in the brain
[62].Therefore,it willbe interesting to investigate Memogain in
comparison to galantamine in this AD modelin the future.
Acknowledgments
The authors gratefully acknowledge expert technical assistance by Angelik
Reichel,Karla Sowa,and Daniela Hill.This study was supported by the
German Ministry for Education and Research (BMBF)specialnetwork
program KMU-Innovativ-2.We thank allmembersof the Neurocure
network for collaboration and fruitfuldiscussions and Galantos Pharma
GmbH for providing galantamine hydrobromide.
Author Contributions
Conceived and designed theexperiments:DM AM. Performed the
experiments:DM SB CH. Analyzed the data:DM SB CH. Contributed
reagents/materials/analysis tools: DM AM. Wrote the paper: DM SB AM.
References
1. Karran E,Mercken M,De Strooper B (2011)The amyloid cascade hypothesis
for Alzheimer’s disease:an appraisalfor the development oftherapeutics.Nat
Rev Drug Discov 10:698–712.
2. Citron M (2004)Strategies for disease modification in Alzheimer’s disease.Nat
Rev Neurosci5: 677–685.
3. Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, et al.
(2009) Oligomeric amyloid b associates with postsynaptic densities and correlates
with excitatory synapse loss near senile plaques.Proc NatlAcad SciUSA 106:
4012–4017.
4. Spires TL,Meyer-Luehmann M,Stern EA,McLean PJ,Skoch J,et al.(2005)
Dendritic spine abnormalitiesin APP transgenic mice demonstrated by gene
transfer and intravitalmultiphoton microscopy.J Neurosci25:7278–7287.
5. Knowles RB,Wyart C,Buldyrev SV,Cruz L, Urbanc B,et al.(1999)Plaque-
induced neurite abnormalities: Implications for disruption of neural networks in
Alzheimer’s disease.Proc NatlAcad SciUSA 96:5274–5279.
6. Schilling S,ZeitschelU, Hoffmann T,HeiserU, Francke M,et al. (2008)
Glutaminylcyclase inhibition attenuatespyroglutamate Ab and Alzheimer’s
disease-like pathology in vivo.Nat Med 14:1106–1111.
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 11 February 2014 |Volume 9 |Issue 2 | e89454

7. Citron M (2010) Alzheimer’s disease: strategies for disease modification. Nat Rev
Drug Discov 9:387–398.
8. PatelL, Grossberg GT (2011)Combination therapy for Alzheimer’sdisease.
Drugs Aging 28:539–546.
9. Maelicke A, Samochock M, Jostock R, Fehrenbacher A, Ludwig J, et al. (2001)
AllostericSensitization ofNicotinic Receptorsby Galantamine,a New
Treatment Strategy for Alzheimer’s Disease.Biol Psychiatry 49:279–288.
10. Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J (1997) Chronic nicotine
treatmentup-regulatesalpha3 and alpha7 acetylcholinereceptorsubtypes
expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol 51:
776–784.
11. Court JA, Perry EK (1994) CNS nicotine receptors. Possible therapeutic targets
in neurodegenerative disorders.CNS Drugs 2:216–233.
12. Kihara T, Shimohama S,UrushitaniM, Sawada H,Kimura J, et al. (1998)
Stimulation ofalpha4beta2nicotinicacetylcholinereceptorsinhibitsbeta-
amyloid toxicity.Brain Res 792:331–334.
13. Villarroya M,Garcı´a AG, Marco-Contelles J, Lo´pez MG (2007)An update on
the pharmacology of galantamine. Expert Opin Investig Drugs 16:1987–1998.
14. Lenzken SC, Lanni C, Govoni S, Lucchelli A, Schettini G, et al. (2007) Nicotinic
componentof galantaminein the regulation ofamyloid precursorprotein
processing.Chem BiolInteract 165:138–145.
15. Matharu B,Gibson G,ParsonsR, Huckerby TN,Moore SA, et al. (2009)
Galantamine inhibits beta-amyloid aggregation and cytotoxicity.J Neurol Sci
280:49–58.
16. TakataK, KitamuraY, SaekiM, Terada M, KagitaniS, et al. (2010)
Galantamine-inducedAmyloid-b ClearanceMediatedvia Stimulation of
MicroglialNicotinic Acetylcholine Receptors.J Biol Chem 285:40180–40191.
17. Wallin A˚K, Wattmo C, Minthon L (2011) Galantaminetreatmentin
Alzheimer’sdisease:responseand long-term outcome in a routineclinical
setting.Neuropsychiatr Dis Treat 7:565–576.
18. Kavanagh S,Gaudig M,Van Baelen B,AdamiM, Delgado A,et al. (2011)
Galantamine and behavior in Alzheimer disease:analysisof four trials.Acta
NeurolScand 124:302–308.
19. Keller C, Kadir A, Forsberg A, Porras O, Nordberg A (2011) Long-term effects
of galantamine treatment on brain functionalactivities as measured by PET in
Alzheimer’s disease patients.J Alz Dis 24:109–123.
20. Oakley H,Cole SL,Logan S,Maus E,Shao P,et al.(2006)Intraneuronalb-
Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice
with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid
Plaque Formation.J Neurosci26:10129–10140.
21. Ohno M, Cole SL,Yasvoina M,Zhao J,Citron M,et al.(2007)BACE1 gene
deletion preventsneuron lossand memorydeficitsin 5XFAD APP/PS1
transgenic mice.NeurobiolDis 26:134–145.
22. Shukla V, Zheng YL, Mishra SK, Amin ND, Steiner J, et al. (2013) A truncated
peptide from p35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in
modelmice.FASEB J 27:174–186.
23. Wirths O, Erck C, MartensH, HarmeierA, Geumann C,et al. (2010)
Identificationof low molecularweightpyroglutamateAb oligomers in
Alzheimer’s disease:a noveltoolfor therapy and diagnosis.J Biol Chem 285:
41517–41524.
24. Jawhar S, Trawicka A, Jenneckens C, Bayer TA, Wirths O (2012) Motor deficits,
neuron loss,and reduced anxiety coinciding with axonaldegeneration and
intraneuronalAb aggregation in the 5XFAD mouse modelof Alzheimer’s
disease.NeurobiolAging 33:196.e29–40.
25. Montag-SallazM, SchachnerM, Montag D (2002) Misguidedaxonal
projections,NCAM180 mRNA upregulation,and altered behaviorin mice
deficient for the Close Homolog of L1 (CHL1).Mol Cell Biol 22:7967–7981.
26. Montag-Sallaz M,Montag D (2003)Severe cognitive and motor coordination
deficits in Tenascin-R-deficient mice.Genes Brain Behav 2:20–31.
27. Wishaw IQ, Haun F, Kolb B (1999) Analysis of behavior in laboratory rodents.
In : Windhorst, U. & Johansson, H., eds. Modern Techniques in Neurosci Berlin
Springer 1243–1275.
28. Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, et al. (1997) Behavioral
and functionalanalysis of mouse phenotype:SHIRPA, a proposed protocolfor
comprehensive phenotype assessment.Mamm Genome 8:711–713.
29. Montag-Sallaz M, Montag D (2003) Learning-induced arg 3.1 expression in the
mouse brain.Learn Mem 10:99–107.
30. Comery TA,Martone RL,AschmiesS, Atchison KP,DiamantidisG, et al.
(2005)Acute gamma-secretase inhibition improves contextualfear conditioning
in the Tg2576 mouse model of Alzheimer’s disease. J. Neurosci 25: 8898–8902.
31. Jacobsen JS,Wu CC, Redwine JM,Comery TA,Arias R,et al.(2006)Early-
onset behavioraland synaptic deficits in a mouse modelof Alzheimer’s disease.
Proc NatlAcad SciUSA 103:5161–5166.
32. Sun A, Nguyen XV, Bing G (2002)Comparative analysisof an improved
thioflavin-s stain,Gallyassilver stain,and immunohistochemistry for neurofi-
brillary tangle demonstration on the same sections.J Histochem Cytochem 50:
463–472.
33. Sturchler-PierratC, StaufenbielM (2000)Pathogenic mechanismsof Alzhei-
mer’s disease analyzed in the APP23 transgenic mouse model.Ann N Y Acad
Sci920:134–139.
34. Lewis J,Dickson DW,Lin WL, Chisholm L,CorralA, et al.(2001)Enhanced
Neurofbrillary Degeneration in Transgenic Mice Expressing MutantTau and
APP. Science 293:1487–1491.
35. Callahan MJ, LipinskiWJ, Bian F, Durham RA, Pack A, et al. (2001)
Augmented Senile Plaque Load in Aged Female b-Amyloid Precursor Protein-
Transgenic Mice.Am J Pathol158:1173–1177.
36. Guntern R,Bouras C,Hof PR, ValletPG (1992)An improved thioflavine S
method for staining neurofibrillary tanglesand senile plaquesin Alzheimer’s
disease.Experientia 48:8–10.
37. Annunziata I,Patterson A,Helton D, Hu H, Moshiach S,et al. (2013)
LysosomalNEU1 deficiencyaffectsamyloid precursorprotein levelsand
amyloid-b secretion via deregulated lysosomal exocytosis. Nat Commun 4: 2734.
38. Wengenack TM,ReyesDA, Curran GL, BorowskiBJ, Lin J, et al. (2011)
Regionaldifferencesin MRI detection ofamyloid plaquesin AD transgenic
mouse brain.Neuroimage 54:113–122.
39. Gomez-Isla T, Price JL, McKeelDW Jr, Morris JC, Growdon JH, et al. (1996)
Profound Lossof Layer II EntorhinalCortex NeuronsOccursin Very Mild
Alzheimer’s Disease.J Neurosci16:4491–4500.
40. Vassar RJ, Oakley H, Krishnamurthy S, Maus E, Shao P, et al. (2005) 5XFAD
Tg mice that express five FAD mutations have high-cerebralab42 levels, rapid
amyloid deposition,and intraneuronalab.Soc NeurosciAbstr 587:2.
41. Grimm A, Lim YA,Mensah-Nyagan AG,Go¨tz J,Eckert A (2012)Alzheimer’s
disease,Oestrogenand mitochondria:an ambiguousrelationship.Mol
Neurobiol46:151–160.
42. Schaeffer S,Wirths O,Multhaup G,Bayer TA (2007)Gender dependent APP
processing in a transgenic mouse model of Alzheimer’s disease. J Neural Transm
114:387–394.
43. SergiG, Rui MD, Coin A, Inelmen EM,Manzato E (2013)Weightloss and
Alzheimer’s disease:temporaland aetiologic connections.Proc NutriSoc 72:
160–165.
44. Webster SJ,Bachstetter AD,Van Eldik LJ (2013)Comprehensive behavioral
characterization of an APP/PS-1 double knock-in mouse modelof Alzheimer’s
disease.Alzheimers Res Ther 5:28.
45. Tong Y, Xu Y, Scearce-Levie K,Pta´cek LJ,Fu YH (2010)COL25A1 triggers
and promotes Alzheimer’s disease-like pathology in vivo. Neurogenet 11: 41–52.
46. Lalonde R,FukuchiK, Strazielle C (2012)APP transgenic mice for modelling
behavioral and psychological symptoms of dementia (BPSD). Neurosci Biobehav
Rev 36:1357–1375.
47. Ohno M, Chang L,Tseng W,Oakley H,Citron M, et al. (2006)Temporal
memory deficitsin Alzheimer’smouse models:rescue by genetic deletion of
BACE1.Eur Jour Neurosci23:251–260.
48. Kimura R, Ohno M (2009) Impairmentsin remotememorystabilization
precede hippocampalsynaptic and cognitive failuresin 5XFAD Alzheimer
mouse model.NeurobiolDis 33:229–235.
49. Jessen F,Kucharsk C,Fries T, Papassotiropoulos A,Hoenig K,et al. (2001)
Sensory Gating Deficit Expressed by a Disturbed Suppression of the P50 Event-
Related Potentialin Patients With Alzheimer’s Disease.Am J Psychiatry 158:
1319–1321.
50. Cancelli I, Cadore IP, Merlino G, Valentinis L, Moratti U, et al. (2006) Sensory
gating deficitassessed by P50/Pb middle latency eventrelated potentialin
Alzheimer’s disease.J Clin Neurophys 23:421–425.
51. Wang H, He J, Zhang R, Zhu S, Wang J, et al. (2012) Sensorimotor gating and
memory deficits in an APP/PS1 double transgenic mouse model of Alzheimer’s
disease.Beh Brain Res 233:237–243.
52. Prvulovic D,HampelH, PantelJ (2010)Galantamine for Alzheimer’s disease.
Expert Opin.Drug Metab Toxicol6: 345–354.
53. SeltzerB (2010) Galantamine-ER forthe treatmentof mild-to-moderate
Alzheimer’s disease.Clin Interv Aging 5:1–6.
54. Singh M, Kaur M, Kukreja H, Chugh R, Silakari O, et al. (2013)
Acetylcholinesterase inhibitorsas Alzheimertherapy:From nerve toxinsto
neuroprotection.Eur J Med Chem 70:165–188.
55. Rao PPN, Mohamed T, Osman W (2013) Investigating the binding interactions
of galantamine with b-amyloid peptide.Bioorg Med Chem Lett 23:239–243.
56. Unger C,Svedberg MM,Yu WF, Hedberg MM,Nordberg A (2006)Effect of
subchronic treatment ofmemantine,galantamine,and nicotine in the brain of
Tg2576 (APPswe)transgenic mice.J PharmacolExp Ther 317:30–36.
57. Demattos RB,Lu J, Tang Y,Racke MM,Delong CA,et al.(2012)A plaque-
specific antibody clears existing b-amyloid plaques in Alzheimer’s disease mice.
Neuron 76:908–920.
58. Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, et al. (2011) Amyloid- b
associated corticalthinning in clinically normalelderly.Ann Neurol69:1032–
1042.
59. Che´telatG, VillemagneVL, Villain N, JonesG, Ellis KA, et al. (2012)
Accelerated corticalatrophy in cognitively normalelderly with high b-amyloid
deposition.Neurol78:477–484.
60. Kalinin S, Polak PE,Lin SX, Sakharkar AJ,Pandey SC,et al. (2012)The
noradrenaline precursorL-DOPS reducespathology in a mousemodelof
Alzheimer’s disease.NeurobiolAging 33:1651–1663.
61. Li C, Zhao R, Gao K, Wei Z, Yin MY, et al. (2011) Astrocytes: implications for
neuroinflammatory pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 8:
67–80.
62. Maelicke A,Hoeffle-Maas A,Ludwig J,Maus A,SamochockiM, et al.(2010)
Memogain isa galantamine pro-drug having dramatically reduced adverse
effects and enhanced efficacy.J Mol Neurosci40:135–137.
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 12 February 2014 |Volume 9 |Issue 2 | e89454
Drug Discov 9:387–398.
8. PatelL, Grossberg GT (2011)Combination therapy for Alzheimer’sdisease.
Drugs Aging 28:539–546.
9. Maelicke A, Samochock M, Jostock R, Fehrenbacher A, Ludwig J, et al. (2001)
AllostericSensitization ofNicotinic Receptorsby Galantamine,a New
Treatment Strategy for Alzheimer’s Disease.Biol Psychiatry 49:279–288.
10. Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J (1997) Chronic nicotine
treatmentup-regulatesalpha3 and alpha7 acetylcholinereceptorsubtypes
expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol 51:
776–784.
11. Court JA, Perry EK (1994) CNS nicotine receptors. Possible therapeutic targets
in neurodegenerative disorders.CNS Drugs 2:216–233.
12. Kihara T, Shimohama S,UrushitaniM, Sawada H,Kimura J, et al. (1998)
Stimulation ofalpha4beta2nicotinicacetylcholinereceptorsinhibitsbeta-
amyloid toxicity.Brain Res 792:331–334.
13. Villarroya M,Garcı´a AG, Marco-Contelles J, Lo´pez MG (2007)An update on
the pharmacology of galantamine. Expert Opin Investig Drugs 16:1987–1998.
14. Lenzken SC, Lanni C, Govoni S, Lucchelli A, Schettini G, et al. (2007) Nicotinic
componentof galantaminein the regulation ofamyloid precursorprotein
processing.Chem BiolInteract 165:138–145.
15. Matharu B,Gibson G,ParsonsR, Huckerby TN,Moore SA, et al. (2009)
Galantamine inhibits beta-amyloid aggregation and cytotoxicity.J Neurol Sci
280:49–58.
16. TakataK, KitamuraY, SaekiM, Terada M, KagitaniS, et al. (2010)
Galantamine-inducedAmyloid-b ClearanceMediatedvia Stimulation of
MicroglialNicotinic Acetylcholine Receptors.J Biol Chem 285:40180–40191.
17. Wallin A˚K, Wattmo C, Minthon L (2011) Galantaminetreatmentin
Alzheimer’sdisease:responseand long-term outcome in a routineclinical
setting.Neuropsychiatr Dis Treat 7:565–576.
18. Kavanagh S,Gaudig M,Van Baelen B,AdamiM, Delgado A,et al. (2011)
Galantamine and behavior in Alzheimer disease:analysisof four trials.Acta
NeurolScand 124:302–308.
19. Keller C, Kadir A, Forsberg A, Porras O, Nordberg A (2011) Long-term effects
of galantamine treatment on brain functionalactivities as measured by PET in
Alzheimer’s disease patients.J Alz Dis 24:109–123.
20. Oakley H,Cole SL,Logan S,Maus E,Shao P,et al.(2006)Intraneuronalb-
Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice
with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid
Plaque Formation.J Neurosci26:10129–10140.
21. Ohno M, Cole SL,Yasvoina M,Zhao J,Citron M,et al.(2007)BACE1 gene
deletion preventsneuron lossand memorydeficitsin 5XFAD APP/PS1
transgenic mice.NeurobiolDis 26:134–145.
22. Shukla V, Zheng YL, Mishra SK, Amin ND, Steiner J, et al. (2013) A truncated
peptide from p35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in
modelmice.FASEB J 27:174–186.
23. Wirths O, Erck C, MartensH, HarmeierA, Geumann C,et al. (2010)
Identificationof low molecularweightpyroglutamateAb oligomers in
Alzheimer’s disease:a noveltoolfor therapy and diagnosis.J Biol Chem 285:
41517–41524.
24. Jawhar S, Trawicka A, Jenneckens C, Bayer TA, Wirths O (2012) Motor deficits,
neuron loss,and reduced anxiety coinciding with axonaldegeneration and
intraneuronalAb aggregation in the 5XFAD mouse modelof Alzheimer’s
disease.NeurobiolAging 33:196.e29–40.
25. Montag-SallazM, SchachnerM, Montag D (2002) Misguidedaxonal
projections,NCAM180 mRNA upregulation,and altered behaviorin mice
deficient for the Close Homolog of L1 (CHL1).Mol Cell Biol 22:7967–7981.
26. Montag-Sallaz M,Montag D (2003)Severe cognitive and motor coordination
deficits in Tenascin-R-deficient mice.Genes Brain Behav 2:20–31.
27. Wishaw IQ, Haun F, Kolb B (1999) Analysis of behavior in laboratory rodents.
In : Windhorst, U. & Johansson, H., eds. Modern Techniques in Neurosci Berlin
Springer 1243–1275.
28. Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, et al. (1997) Behavioral
and functionalanalysis of mouse phenotype:SHIRPA, a proposed protocolfor
comprehensive phenotype assessment.Mamm Genome 8:711–713.
29. Montag-Sallaz M, Montag D (2003) Learning-induced arg 3.1 expression in the
mouse brain.Learn Mem 10:99–107.
30. Comery TA,Martone RL,AschmiesS, Atchison KP,DiamantidisG, et al.
(2005)Acute gamma-secretase inhibition improves contextualfear conditioning
in the Tg2576 mouse model of Alzheimer’s disease. J. Neurosci 25: 8898–8902.
31. Jacobsen JS,Wu CC, Redwine JM,Comery TA,Arias R,et al.(2006)Early-
onset behavioraland synaptic deficits in a mouse modelof Alzheimer’s disease.
Proc NatlAcad SciUSA 103:5161–5166.
32. Sun A, Nguyen XV, Bing G (2002)Comparative analysisof an improved
thioflavin-s stain,Gallyassilver stain,and immunohistochemistry for neurofi-
brillary tangle demonstration on the same sections.J Histochem Cytochem 50:
463–472.
33. Sturchler-PierratC, StaufenbielM (2000)Pathogenic mechanismsof Alzhei-
mer’s disease analyzed in the APP23 transgenic mouse model.Ann N Y Acad
Sci920:134–139.
34. Lewis J,Dickson DW,Lin WL, Chisholm L,CorralA, et al.(2001)Enhanced
Neurofbrillary Degeneration in Transgenic Mice Expressing MutantTau and
APP. Science 293:1487–1491.
35. Callahan MJ, LipinskiWJ, Bian F, Durham RA, Pack A, et al. (2001)
Augmented Senile Plaque Load in Aged Female b-Amyloid Precursor Protein-
Transgenic Mice.Am J Pathol158:1173–1177.
36. Guntern R,Bouras C,Hof PR, ValletPG (1992)An improved thioflavine S
method for staining neurofibrillary tanglesand senile plaquesin Alzheimer’s
disease.Experientia 48:8–10.
37. Annunziata I,Patterson A,Helton D, Hu H, Moshiach S,et al. (2013)
LysosomalNEU1 deficiencyaffectsamyloid precursorprotein levelsand
amyloid-b secretion via deregulated lysosomal exocytosis. Nat Commun 4: 2734.
38. Wengenack TM,ReyesDA, Curran GL, BorowskiBJ, Lin J, et al. (2011)
Regionaldifferencesin MRI detection ofamyloid plaquesin AD transgenic
mouse brain.Neuroimage 54:113–122.
39. Gomez-Isla T, Price JL, McKeelDW Jr, Morris JC, Growdon JH, et al. (1996)
Profound Lossof Layer II EntorhinalCortex NeuronsOccursin Very Mild
Alzheimer’s Disease.J Neurosci16:4491–4500.
40. Vassar RJ, Oakley H, Krishnamurthy S, Maus E, Shao P, et al. (2005) 5XFAD
Tg mice that express five FAD mutations have high-cerebralab42 levels, rapid
amyloid deposition,and intraneuronalab.Soc NeurosciAbstr 587:2.
41. Grimm A, Lim YA,Mensah-Nyagan AG,Go¨tz J,Eckert A (2012)Alzheimer’s
disease,Oestrogenand mitochondria:an ambiguousrelationship.Mol
Neurobiol46:151–160.
42. Schaeffer S,Wirths O,Multhaup G,Bayer TA (2007)Gender dependent APP
processing in a transgenic mouse model of Alzheimer’s disease. J Neural Transm
114:387–394.
43. SergiG, Rui MD, Coin A, Inelmen EM,Manzato E (2013)Weightloss and
Alzheimer’s disease:temporaland aetiologic connections.Proc NutriSoc 72:
160–165.
44. Webster SJ,Bachstetter AD,Van Eldik LJ (2013)Comprehensive behavioral
characterization of an APP/PS-1 double knock-in mouse modelof Alzheimer’s
disease.Alzheimers Res Ther 5:28.
45. Tong Y, Xu Y, Scearce-Levie K,Pta´cek LJ,Fu YH (2010)COL25A1 triggers
and promotes Alzheimer’s disease-like pathology in vivo. Neurogenet 11: 41–52.
46. Lalonde R,FukuchiK, Strazielle C (2012)APP transgenic mice for modelling
behavioral and psychological symptoms of dementia (BPSD). Neurosci Biobehav
Rev 36:1357–1375.
47. Ohno M, Chang L,Tseng W,Oakley H,Citron M, et al. (2006)Temporal
memory deficitsin Alzheimer’smouse models:rescue by genetic deletion of
BACE1.Eur Jour Neurosci23:251–260.
48. Kimura R, Ohno M (2009) Impairmentsin remotememorystabilization
precede hippocampalsynaptic and cognitive failuresin 5XFAD Alzheimer
mouse model.NeurobiolDis 33:229–235.
49. Jessen F,Kucharsk C,Fries T, Papassotiropoulos A,Hoenig K,et al. (2001)
Sensory Gating Deficit Expressed by a Disturbed Suppression of the P50 Event-
Related Potentialin Patients With Alzheimer’s Disease.Am J Psychiatry 158:
1319–1321.
50. Cancelli I, Cadore IP, Merlino G, Valentinis L, Moratti U, et al. (2006) Sensory
gating deficitassessed by P50/Pb middle latency eventrelated potentialin
Alzheimer’s disease.J Clin Neurophys 23:421–425.
51. Wang H, He J, Zhang R, Zhu S, Wang J, et al. (2012) Sensorimotor gating and
memory deficits in an APP/PS1 double transgenic mouse model of Alzheimer’s
disease.Beh Brain Res 233:237–243.
52. Prvulovic D,HampelH, PantelJ (2010)Galantamine for Alzheimer’s disease.
Expert Opin.Drug Metab Toxicol6: 345–354.
53. SeltzerB (2010) Galantamine-ER forthe treatmentof mild-to-moderate
Alzheimer’s disease.Clin Interv Aging 5:1–6.
54. Singh M, Kaur M, Kukreja H, Chugh R, Silakari O, et al. (2013)
Acetylcholinesterase inhibitorsas Alzheimertherapy:From nerve toxinsto
neuroprotection.Eur J Med Chem 70:165–188.
55. Rao PPN, Mohamed T, Osman W (2013) Investigating the binding interactions
of galantamine with b-amyloid peptide.Bioorg Med Chem Lett 23:239–243.
56. Unger C,Svedberg MM,Yu WF, Hedberg MM,Nordberg A (2006)Effect of
subchronic treatment ofmemantine,galantamine,and nicotine in the brain of
Tg2576 (APPswe)transgenic mice.J PharmacolExp Ther 317:30–36.
57. Demattos RB,Lu J, Tang Y,Racke MM,Delong CA,et al.(2012)A plaque-
specific antibody clears existing b-amyloid plaques in Alzheimer’s disease mice.
Neuron 76:908–920.
58. Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, et al. (2011) Amyloid- b
associated corticalthinning in clinically normalelderly.Ann Neurol69:1032–
1042.
59. Che´telatG, VillemagneVL, Villain N, JonesG, Ellis KA, et al. (2012)
Accelerated corticalatrophy in cognitively normalelderly with high b-amyloid
deposition.Neurol78:477–484.
60. Kalinin S, Polak PE,Lin SX, Sakharkar AJ,Pandey SC,et al. (2012)The
noradrenaline precursorL-DOPS reducespathology in a mousemodelof
Alzheimer’s disease.NeurobiolAging 33:1651–1663.
61. Li C, Zhao R, Gao K, Wei Z, Yin MY, et al. (2011) Astrocytes: implications for
neuroinflammatory pathogenesis of Alzheimer’s disease. Curr Alzheimer Res 8:
67–80.
62. Maelicke A,Hoeffle-Maas A,Ludwig J,Maus A,SamochockiM, et al.(2010)
Memogain isa galantamine pro-drug having dramatically reduced adverse
effects and enhanced efficacy.J Mol Neurosci40:135–137.
Galantamine 5xFAD Mice
PLOS ONE |www.plosone.org 12 February 2014 |Volume 9 |Issue 2 | e89454
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 12
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.