Experiment 1: Preparation and Analysis of a Gel Drug Product PF2
VerifiedAdded on 2023/06/15
|8
|2568
|295
Report
AI Summary
This report details an experiment focused on the preparation and analysis of a gel drug product, specifically a Lidocaine-based formulation. The experiment covers several key aspects, including the preparation of an isotonic aqueous formulation using methylcellulose and sodium chloride, API (Active ...

Experiment 1: Preparation and Analysis of a Gel Drug Product
PF2: Laboratory Report
_____________________________________________________________
Name:
Date:
Introduction
With the new era of medicine gels have been used as a form of making drugs and
applying to local action where they are absorbed into the body. Gels often dissolve into the
body while others are dispelled into the body. Gels have been proffered due to their ability to
penetrate easily into the skin and take over effects.
Gels have been defined and established as semi solids systems with liquid phase and
are often constrained in a polymeric matrix, with a high degree of physical and chemical
cross linking. Gels have been classified based won their colloidal phases , nature of solvent,
physical nature and rheological properties, (Ayatollahi, 2006).
Gels have often been described based on the colloidal phases. These are the inorganic
and organic phases. The inorganic phase system entails a partial size being dispersed in a
relatively large and uniform 3-diemnsional shape in the gel outline in systems such as those
consisting of small particles other than large molecules and the formation of the overall gel
structure. The disadvantage with this system is they are not always stable. They are
thyrotrophic in nature forming the semisolids which change to liquid form upon agitations.
The organic system consists of larger organic particles of molecules which the strands
when twisted dissolves in continues phase. These bigger and huge molecules are often
referred to as gel formers and are often natural or synthetic, they entangle on each other in the
random motion or in a bound manner by Vander walls forces, (Ono, 1998).
Gels can at time, they are based on their nature of solvent it dissolves. The hydro gels
or water based often contain continues liquid phase. Some of the hydrous liquids are gelatine,
cellulose derivatives, car-pooler and poloxamer gels. Organic gels are those that contain non
aqueous solutions. Common examples include the plastibase, olag gel and sterile oils.
Xerogels are solid gels with low solvent concentration. they are produced by
evaporation of solvent and drying in a freezer compartments. Often this leaves a framework
of gel behind which is in contact with fresh fluids which often swell and can be reproduces.
An example of such gels is acacaia tear, polystyrene and dry cellulose.
1
PF2: Laboratory Report
_____________________________________________________________
Name:
Date:
Introduction
With the new era of medicine gels have been used as a form of making drugs and
applying to local action where they are absorbed into the body. Gels often dissolve into the
body while others are dispelled into the body. Gels have been proffered due to their ability to
penetrate easily into the skin and take over effects.
Gels have been defined and established as semi solids systems with liquid phase and
are often constrained in a polymeric matrix, with a high degree of physical and chemical
cross linking. Gels have been classified based won their colloidal phases , nature of solvent,
physical nature and rheological properties, (Ayatollahi, 2006).
Gels have often been described based on the colloidal phases. These are the inorganic
and organic phases. The inorganic phase system entails a partial size being dispersed in a
relatively large and uniform 3-diemnsional shape in the gel outline in systems such as those
consisting of small particles other than large molecules and the formation of the overall gel
structure. The disadvantage with this system is they are not always stable. They are
thyrotrophic in nature forming the semisolids which change to liquid form upon agitations.
The organic system consists of larger organic particles of molecules which the strands
when twisted dissolves in continues phase. These bigger and huge molecules are often
referred to as gel formers and are often natural or synthetic, they entangle on each other in the
random motion or in a bound manner by Vander walls forces, (Ono, 1998).
Gels can at time, they are based on their nature of solvent it dissolves. The hydro gels
or water based often contain continues liquid phase. Some of the hydrous liquids are gelatine,
cellulose derivatives, car-pooler and poloxamer gels. Organic gels are those that contain non
aqueous solutions. Common examples include the plastibase, olag gel and sterile oils.
Xerogels are solid gels with low solvent concentration. they are produced by
evaporation of solvent and drying in a freezer compartments. Often this leaves a framework
of gel behind which is in contact with fresh fluids which often swell and can be reproduces.
An example of such gels is acacaia tear, polystyrene and dry cellulose.
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Aims
1. To prepare an isotonic aqueous formulation
2. To conduct gel product testing.
Method
In preparing the isotonic gel formulation, 5 mls of ultrapure water was added into a 100
ml beaker, with 0.25g of methylcellulose and stirred at room temperature. In another beaker,
0.09 g of NaCl is placed in a beaker and 4 ml of ultra pure water is stirred to dissolve the
solution. In this beaker, 0.025g of API compound is stirred for 10 minutes.
The solution of Nacl in which the API solution was added, methyl cellulose solution are
mixed in a step wise manner, the temperature is adjusted to pH between 6.0-6.5 using either
HCL or base –Naoh- 3M to standardize. Then finally enough water is added approximately
2mls to make the gel viscous.
With the test being done, API identification was done, and the physical appearances of
the product noted. Further the pH of the product was measured with the gel density. Further
the viscosity of the gel solution was calculated.
To finalize the testing on drug release test, a large vial has been used. 30mls of gel was
weighed and transferred into a larger sample vial. 2 ml of phosphate buffer at 0.03 M at a pH
of 7.4 is added as receptor phase and incubated at 37 degrees Celsius in an oven. The UV/VS
spectrum was recording in a predetermined velocity while the sample drawn is replaced
immediately.
The determined maximum velocity was determined through preparation of100ppm
solution of phosphate buffer as used in the receptor phase above was used. This was
recording in a table in order to calculate the absorbance rate.
Results
API identification
Purity status of the API used in gel formulation was identified through taking the
measurement of temperature levels of the product using the glass veil and melting point
equipment. The sample temperature is taken when the sample has heated which it gives
temperature recording of 72degreed Celsius. The theoretical temperature of both API’s that is
Lidocaine, procaine, shows that the temperature is Lidocaine as its theoretical temperature is
69degreed celcius.
Infra Red identification methodologies was carried out on the API used using the
Potassium bromide agent methods and found out that Lidocaine is 97 parts of Potassium
bromide utilised.
2
1. To prepare an isotonic aqueous formulation
2. To conduct gel product testing.
Method
In preparing the isotonic gel formulation, 5 mls of ultrapure water was added into a 100
ml beaker, with 0.25g of methylcellulose and stirred at room temperature. In another beaker,
0.09 g of NaCl is placed in a beaker and 4 ml of ultra pure water is stirred to dissolve the
solution. In this beaker, 0.025g of API compound is stirred for 10 minutes.
The solution of Nacl in which the API solution was added, methyl cellulose solution are
mixed in a step wise manner, the temperature is adjusted to pH between 6.0-6.5 using either
HCL or base –Naoh- 3M to standardize. Then finally enough water is added approximately
2mls to make the gel viscous.
With the test being done, API identification was done, and the physical appearances of
the product noted. Further the pH of the product was measured with the gel density. Further
the viscosity of the gel solution was calculated.
To finalize the testing on drug release test, a large vial has been used. 30mls of gel was
weighed and transferred into a larger sample vial. 2 ml of phosphate buffer at 0.03 M at a pH
of 7.4 is added as receptor phase and incubated at 37 degrees Celsius in an oven. The UV/VS
spectrum was recording in a predetermined velocity while the sample drawn is replaced
immediately.
The determined maximum velocity was determined through preparation of100ppm
solution of phosphate buffer as used in the receptor phase above was used. This was
recording in a table in order to calculate the absorbance rate.
Results
API identification
Purity status of the API used in gel formulation was identified through taking the
measurement of temperature levels of the product using the glass veil and melting point
equipment. The sample temperature is taken when the sample has heated which it gives
temperature recording of 72degreed Celsius. The theoretical temperature of both API’s that is
Lidocaine, procaine, shows that the temperature is Lidocaine as its theoretical temperature is
69degreed celcius.
Infra Red identification methodologies was carried out on the API used using the
Potassium bromide agent methods and found out that Lidocaine is 97 parts of Potassium
bromide utilised.
2
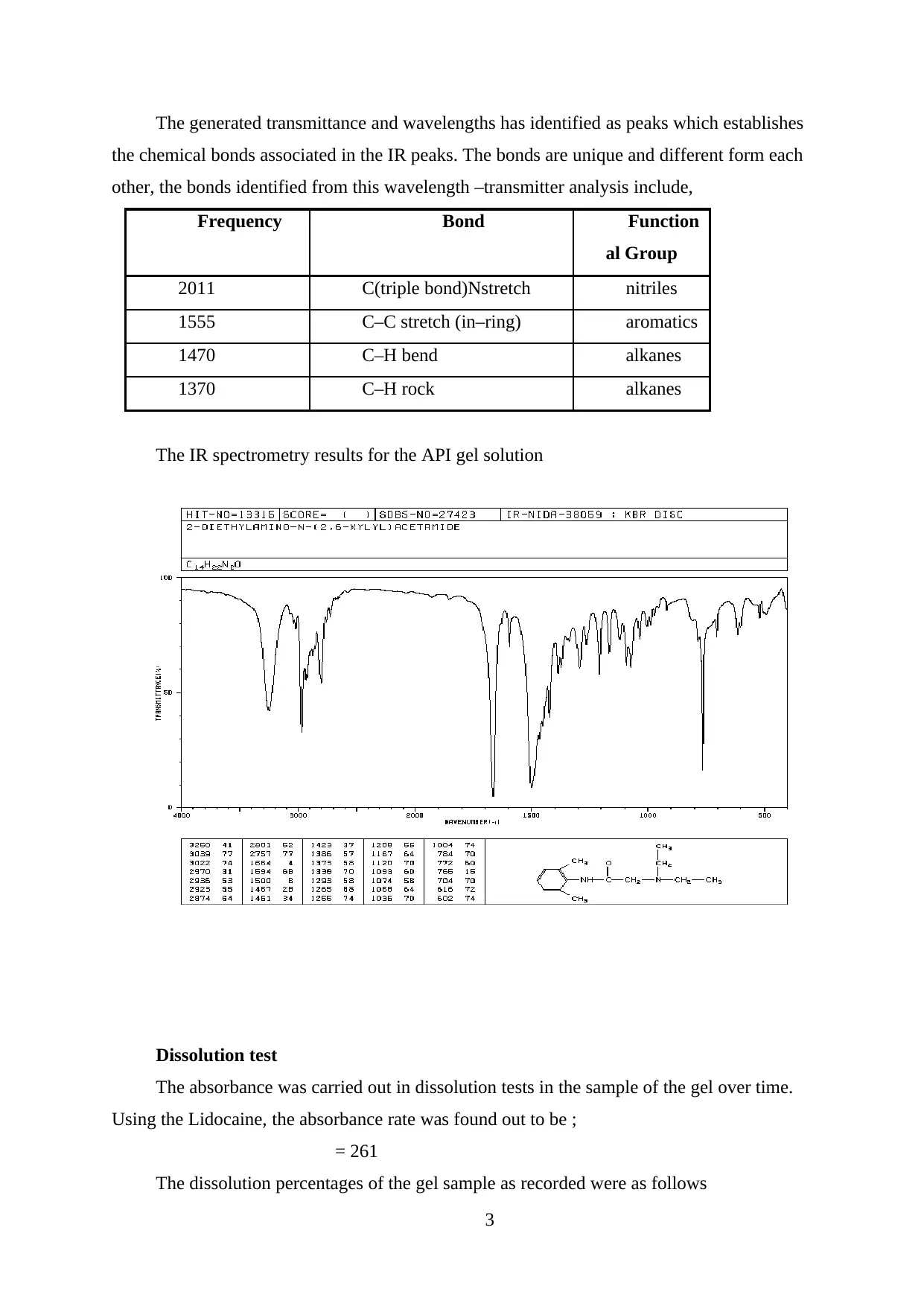
The generated transmittance and wavelengths has identified as peaks which establishes
the chemical bonds associated in the IR peaks. The bonds are unique and different form each
other, the bonds identified from this wavelength –transmitter analysis include,
Frequency Bond Function
al Group
2011 C(triple bond)Nstretch nitriles
1555 C–C stretch (in–ring) aromatics
1470 C–H bend alkanes
1370 C–H rock alkanes
The IR spectrometry results for the API gel solution
Dissolution test
The absorbance was carried out in dissolution tests in the sample of the gel over time.
Using the Lidocaine, the absorbance rate was found out to be ;
= 261
The dissolution percentages of the gel sample as recorded were as follows
3
the chemical bonds associated in the IR peaks. The bonds are unique and different form each
other, the bonds identified from this wavelength –transmitter analysis include,
Frequency Bond Function
al Group
2011 C(triple bond)Nstretch nitriles
1555 C–C stretch (in–ring) aromatics
1470 C–H bend alkanes
1370 C–H rock alkanes
The IR spectrometry results for the API gel solution
Dissolution test
The absorbance was carried out in dissolution tests in the sample of the gel over time.
Using the Lidocaine, the absorbance rate was found out to be ;
= 261
The dissolution percentages of the gel sample as recorded were as follows
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
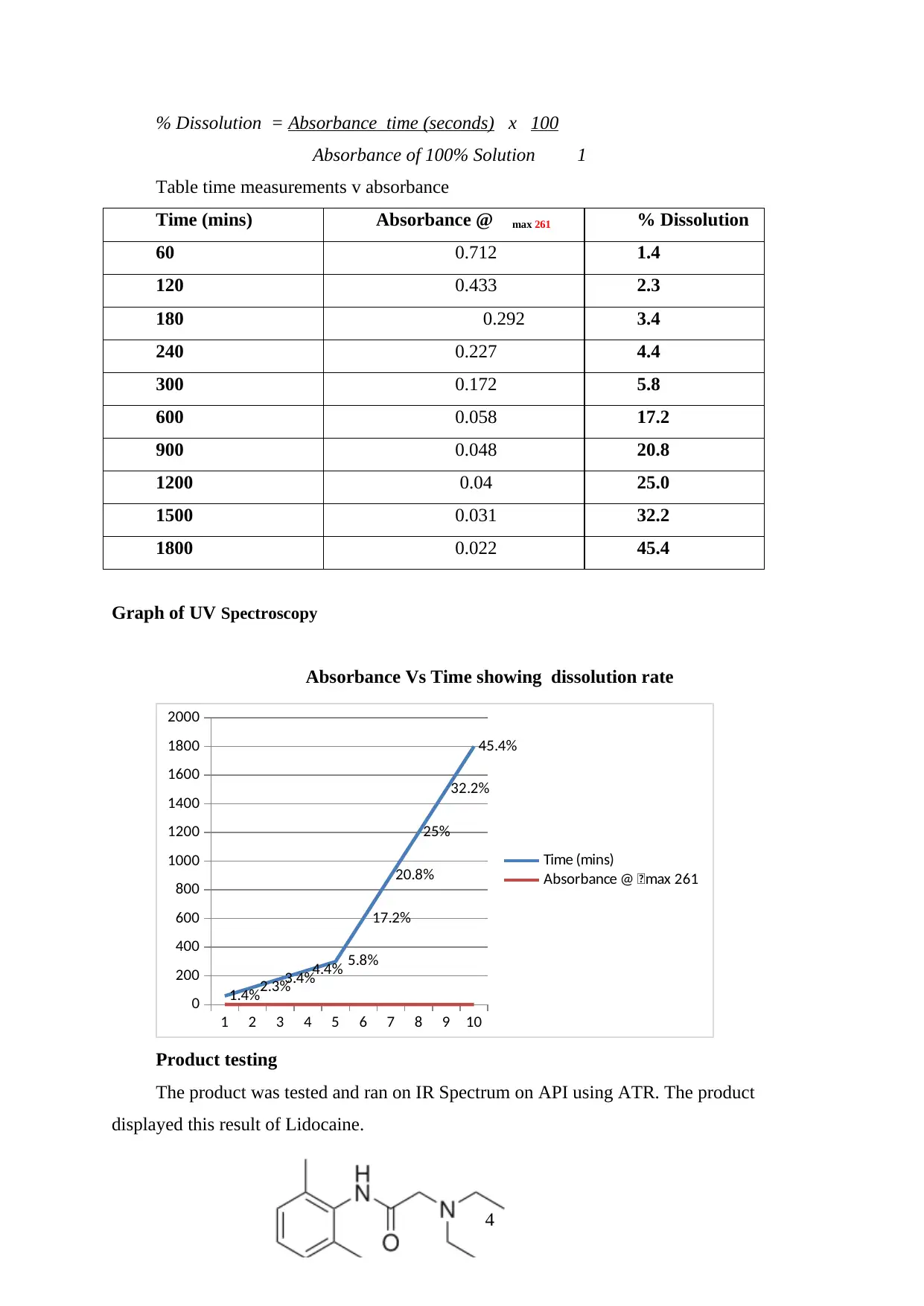
% Dissolution = Absorbance time (seconds) x 100
Absorbance of 100% Solution 1
Table time measurements v absorbance
Time (mins) Absorbance @ max 261 % Dissolution
60 0.712 1.4
120 0.433 2.3
180 0.292 3.4
240 0.227 4.4
300 0.172 5.8
600 0.058 17.2
900 0.048 20.8
1200 0.04 25.0
1500 0.031 32.2
1800 0.022 45.4
Graph of UV Spectroscopy
Absorbance Vs Time showing dissolution rate
1 2 3 4 5 6 7 8 9 10
0
200
400
600
800
1000
1200
1400
1600
1800
2000
1.4%2.3%
3.4%
4.4% 5.8%
17.2%
20.8%
25%
32.2%
45.4%
Time (mins)
Absorbance @ max 261
Product testing
The product was tested and ran on IR Spectrum on API using ATR. The product
displayed this result of Lidocaine.
4
Absorbance of 100% Solution 1
Table time measurements v absorbance
Time (mins) Absorbance @ max 261 % Dissolution
60 0.712 1.4
120 0.433 2.3
180 0.292 3.4
240 0.227 4.4
300 0.172 5.8
600 0.058 17.2
900 0.048 20.8
1200 0.04 25.0
1500 0.031 32.2
1800 0.022 45.4
Graph of UV Spectroscopy
Absorbance Vs Time showing dissolution rate
1 2 3 4 5 6 7 8 9 10
0
200
400
600
800
1000
1200
1400
1600
1800
2000
1.4%2.3%
3.4%
4.4% 5.8%
17.2%
20.8%
25%
32.2%
45.4%
Time (mins)
Absorbance @ max 261
Product testing
The product was tested and ran on IR Spectrum on API using ATR. The product
displayed this result of Lidocaine.
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

The product physical appearance had no any suspension colour which resulted in light
grey, while the pH recorded was at 7. The density calculation of the gel product was 0.6069
density.
The viscosity of the suspension using the viscometer shows that that
Viscosity of gel: 724 mPa.s
Discussion
From the results there is evidence of various groups as shown from the API sample
showing the different functional groups. The infra red spectrum picked up the peak
frequencies and its characteristics as identified in lab manual. The functional groups
represented here is nitriles, alkanes and aromatic compounds. This indicates the spectrum of
Ludcoane and shows that the API gel solution was Lidocane. The carbonyl peaks have
shifted in the lower wave numbers when the carbonyl function has been passed through
conjugation to another system. The nitro groups show intense peaks in the Infra red spectrum.
Peaks due to amine groups often have different appearances, the presence of (NH2) and the
secondary groups show one peak at this range. Alcohols on the other end often stretch with
OH with rounded peak in the area.
A notable observation is that the left side of the spectrum indicates the presence was
obtained from KBr pellet, which indicates a solid compound.
The dissolution tests of the gel Lidocane is often performed in industries for exploration
of physiological characteristics and physiochemical properties to use identified forms as
central to its development process. In drug testing micro dissolution techniques often require
small amounts of gel or the active API ingredient in this case Lidocane.
The lower levels of bioavailability of the gels was lower, this may be attributed to
errors caused in the experiment, when calculating the rate of dissolution.
In this study, real time reporting of the dissolution process was undertaken. Infrared
imaging has provided spatial and temporal information on techniques of drug dissolution,
(Aaltonen & Rdaes, 2009). The UV imaging and the selected wavelength of light in the UV
range which passes through the cells which is measure as function of time. UV imaging
enables the recording of temporal drug substances detection in the adjacent API solutions,
which in this experiment, it was founds out to be Lidocaaine.
5
grey, while the pH recorded was at 7. The density calculation of the gel product was 0.6069
density.
The viscosity of the suspension using the viscometer shows that that
Viscosity of gel: 724 mPa.s
Discussion
From the results there is evidence of various groups as shown from the API sample
showing the different functional groups. The infra red spectrum picked up the peak
frequencies and its characteristics as identified in lab manual. The functional groups
represented here is nitriles, alkanes and aromatic compounds. This indicates the spectrum of
Ludcoane and shows that the API gel solution was Lidocane. The carbonyl peaks have
shifted in the lower wave numbers when the carbonyl function has been passed through
conjugation to another system. The nitro groups show intense peaks in the Infra red spectrum.
Peaks due to amine groups often have different appearances, the presence of (NH2) and the
secondary groups show one peak at this range. Alcohols on the other end often stretch with
OH with rounded peak in the area.
A notable observation is that the left side of the spectrum indicates the presence was
obtained from KBr pellet, which indicates a solid compound.
The dissolution tests of the gel Lidocane is often performed in industries for exploration
of physiological characteristics and physiochemical properties to use identified forms as
central to its development process. In drug testing micro dissolution techniques often require
small amounts of gel or the active API ingredient in this case Lidocane.
The lower levels of bioavailability of the gels was lower, this may be attributed to
errors caused in the experiment, when calculating the rate of dissolution.
In this study, real time reporting of the dissolution process was undertaken. Infrared
imaging has provided spatial and temporal information on techniques of drug dissolution,
(Aaltonen & Rdaes, 2009). The UV imaging and the selected wavelength of light in the UV
range which passes through the cells which is measure as function of time. UV imaging
enables the recording of temporal drug substances detection in the adjacent API solutions,
which in this experiment, it was founds out to be Lidocaaine.
5

The melting point upon testing of the gel API solution found out that its melting point
of standard API solution is 70degrees Celsius, however in the experiment a temperature of
69degrees Celsius was found out, (Persson et al., 2008).
The theoretical melting point of Lidocaine gel is estimated to be at 70 degreed Celsius.
The dissolution analysis of the test found that it is increased as the time in seconds was
increased. The rate of the dissolution process, is reduced and then it increase gradually. The
results shown may be inaccurate due to control of errors (Shen & Burges, 2012).
The formulation of the gel in the medium viscous API gel has already a pH of 6.0
meaning it is in its aqueous state. The expecient used in the analysis and the sodium chloride
ensures that the pH is stabilized. The Gel sample density at 0.8mg/11ml, thus the final gel
concentration after mixing was 100 ppm.
At times gels can be prepared based on their physical properties. Elastic gels which
include pectin, guar gum, often exhibit behaviour of elasticity by forming weak bonds like
hydrogen bonds and dipole attractions in their fibrous attraction. The molecule often posses
free –cooh group, which when additional bonds takes effect when placed on salt bridge in the
adjacent strand networks.
Rigid gels on the other side are formed in a process of macromolecule where the
framework is linked to the primary valence bond. A case example is the silica gel and acid
molecules which are often held by strong Si-O-Si-O bonds which provide it with polymer
structures having pores.
Gels are prepared by industrial methods under room temperatures. They can be
prepared through two methods which include thermal changes and flocculation methods.
Thermal changes are often solvated polymers which when subjected to thermal heat causes
gelatine formation. Often the hydrogen formers are soluble in hot liquids like water. When
there is a reduction the water temperature, then the degree of hydration is lowered and
gelatinization occurs, (Yasuda, et al., 1996).
In flocculation methods, gelatine is formed through the addition of sufficient amounts
of salt for precipitation to occur, which produce an state of age, which is often no enough to
produce complete precipitation, thus it is important to consider through mixing to minimize
high precipitation (Kakihana, 1996).
In the chemical reaction formation of gel, often chemical inter action of solvent and
solute occurs. An example is when aluminium hydroxide is prepared in a form of interaction
in an aqueous state in a aluminium salt and sodium carbonated chloride which produces the
gel structure.
6
of standard API solution is 70degrees Celsius, however in the experiment a temperature of
69degrees Celsius was found out, (Persson et al., 2008).
The theoretical melting point of Lidocaine gel is estimated to be at 70 degreed Celsius.
The dissolution analysis of the test found that it is increased as the time in seconds was
increased. The rate of the dissolution process, is reduced and then it increase gradually. The
results shown may be inaccurate due to control of errors (Shen & Burges, 2012).
The formulation of the gel in the medium viscous API gel has already a pH of 6.0
meaning it is in its aqueous state. The expecient used in the analysis and the sodium chloride
ensures that the pH is stabilized. The Gel sample density at 0.8mg/11ml, thus the final gel
concentration after mixing was 100 ppm.
At times gels can be prepared based on their physical properties. Elastic gels which
include pectin, guar gum, often exhibit behaviour of elasticity by forming weak bonds like
hydrogen bonds and dipole attractions in their fibrous attraction. The molecule often posses
free –cooh group, which when additional bonds takes effect when placed on salt bridge in the
adjacent strand networks.
Rigid gels on the other side are formed in a process of macromolecule where the
framework is linked to the primary valence bond. A case example is the silica gel and acid
molecules which are often held by strong Si-O-Si-O bonds which provide it with polymer
structures having pores.
Gels are prepared by industrial methods under room temperatures. They can be
prepared through two methods which include thermal changes and flocculation methods.
Thermal changes are often solvated polymers which when subjected to thermal heat causes
gelatine formation. Often the hydrogen formers are soluble in hot liquids like water. When
there is a reduction the water temperature, then the degree of hydration is lowered and
gelatinization occurs, (Yasuda, et al., 1996).
In flocculation methods, gelatine is formed through the addition of sufficient amounts
of salt for precipitation to occur, which produce an state of age, which is often no enough to
produce complete precipitation, thus it is important to consider through mixing to minimize
high precipitation (Kakihana, 1996).
In the chemical reaction formation of gel, often chemical inter action of solvent and
solute occurs. An example is when aluminium hydroxide is prepared in a form of interaction
in an aqueous state in a aluminium salt and sodium carbonated chloride which produces the
gel structure.
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

In this experiment the bioavilability fo the gel formulation, the bioavailbility was lower
compared to other gels performed. The attribution factor on this could be due to errors
performed in the lab prerpation methods [performded in the Lidocaine API sample used.
Conclusion
The Licoaine API solution has hydrochloride combine with with methylcellulose in the
product. In medication, it provides and gives ,maximum contact on the mucosa and provides
lubrication purposes. The addition of HCL and NaoH, gives it the state of pH between 6.2 to
6.8. The tests done in the experiment showed the identity of the API gel product, the positive
identification through infrared analysis shows the presence of Lidocane formulation which is
dissolved in an effective gel which is more appropriate.
The plasma biding effect of the drug shows slows with an increase concentration. Thus
its lab formulation has to take keen interest on the molarity concentration of the substrates.
Thus test done in this lab report shows that the purity of the drug and the API shows infrared
analysis, with the melting point being ascertained to be 70 degrees Celsius near to the
standard values of commercial API ingredient.
For medical purposes Lidocaine has been utilized in the absorption if topical
administration mucous. The absorption depend on the rate of dose administration and its
topical exposures. Lidocaine is often absorbed in the liver and 90% administered is often
excreted in the form of various metabolites and often less than 10% is excreted unchanged.
7
compared to other gels performed. The attribution factor on this could be due to errors
performed in the lab prerpation methods [performded in the Lidocaine API sample used.
Conclusion
The Licoaine API solution has hydrochloride combine with with methylcellulose in the
product. In medication, it provides and gives ,maximum contact on the mucosa and provides
lubrication purposes. The addition of HCL and NaoH, gives it the state of pH between 6.2 to
6.8. The tests done in the experiment showed the identity of the API gel product, the positive
identification through infrared analysis shows the presence of Lidocane formulation which is
dissolved in an effective gel which is more appropriate.
The plasma biding effect of the drug shows slows with an increase concentration. Thus
its lab formulation has to take keen interest on the molarity concentration of the substrates.
Thus test done in this lab report shows that the purity of the drug and the API shows infrared
analysis, with the melting point being ascertained to be 70 degrees Celsius near to the
standard values of commercial API ingredient.
For medical purposes Lidocaine has been utilized in the absorption if topical
administration mucous. The absorption depend on the rate of dose administration and its
topical exposures. Lidocaine is often absorbed in the liver and 90% administered is often
excreted in the form of various metabolites and often less than 10% is excreted unchanged.
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
Aaltonen, J. and Rades, T., 2009. Commentary: Towards physicorelevant dissolution testing:
The importance of solid-state analysis in dissolution. Dissolut. Technol, 16, pp.47-54.
Ayatollahi, J., 2015. Sporotrichoid Cutaneous Leishmaniasis in Central Iran. Iranian Journal
of Medical Sciences, 31(3).
Kakihana, M., 1996. Invited review “sol-gel” preparation of high temperature
superconducting oxides. Journal of Sol-Gel Science and Technology, 6(1), pp.7-55.
Ono, Y., Nakashima, K., Sano, M., Kanekiyo, Y., Inoue, K., Shinkai, S. and Hojo, J., 1998.
Organic gels are useful as a template for the preparation of hollow fiber silica.
Chemical Communications, (14), pp.1477-1478.
Persson, A.M., Baumann, K., Sundelöf, L.O., Lindberg, W., Sokolowski, A. and Pettersson,
C., 2008. Design and characterization of a new miniaturized rotating disk equipment for
in vitro dissolution rate studies. Journal of pharmaceutical sciences, 97(8), pp.3344-
3355.
Shen, J. and Burgess, D.J., 2012. Accelerated in‐vitro release testing methods for extended‐
release parenteral dosage forms. Journal of Pharmacy and Pharmacology, 64(7),
pp.986-996.
van Gorp, J.J., Vekemans, J.A. and Meijer, E.W., 2002. C 3-symmetrical supramolecular
architectures: Fibers and organic gels from discotic trisamides and trisureas. Journal of
the American Chemical Society, 124(49), pp.14759-14769.
Yasuda, Y., Iishi, E., Inada, H. and Shirota, Y., 1996. Novel low-molecular-weight organic
gels: N, N′, N ″-tristearyltrimesamide/organic solvent system. Chemistry letters, 25(7),
pp.575-576.
8
Aaltonen, J. and Rades, T., 2009. Commentary: Towards physicorelevant dissolution testing:
The importance of solid-state analysis in dissolution. Dissolut. Technol, 16, pp.47-54.
Ayatollahi, J., 2015. Sporotrichoid Cutaneous Leishmaniasis in Central Iran. Iranian Journal
of Medical Sciences, 31(3).
Kakihana, M., 1996. Invited review “sol-gel” preparation of high temperature
superconducting oxides. Journal of Sol-Gel Science and Technology, 6(1), pp.7-55.
Ono, Y., Nakashima, K., Sano, M., Kanekiyo, Y., Inoue, K., Shinkai, S. and Hojo, J., 1998.
Organic gels are useful as a template for the preparation of hollow fiber silica.
Chemical Communications, (14), pp.1477-1478.
Persson, A.M., Baumann, K., Sundelöf, L.O., Lindberg, W., Sokolowski, A. and Pettersson,
C., 2008. Design and characterization of a new miniaturized rotating disk equipment for
in vitro dissolution rate studies. Journal of pharmaceutical sciences, 97(8), pp.3344-
3355.
Shen, J. and Burgess, D.J., 2012. Accelerated in‐vitro release testing methods for extended‐
release parenteral dosage forms. Journal of Pharmacy and Pharmacology, 64(7),
pp.986-996.
van Gorp, J.J., Vekemans, J.A. and Meijer, E.W., 2002. C 3-symmetrical supramolecular
architectures: Fibers and organic gels from discotic trisamides and trisureas. Journal of
the American Chemical Society, 124(49), pp.14759-14769.
Yasuda, Y., Iishi, E., Inada, H. and Shirota, Y., 1996. Novel low-molecular-weight organic
gels: N, N′, N ″-tristearyltrimesamide/organic solvent system. Chemistry letters, 25(7),
pp.575-576.
8
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
