Global Business Assignment
VerifiedAdded on 2023/06/07
|19
|3928
|303
AI Summary
The AUSMED Company is willing to expand its business in a foreign market. There are two countries where the company is willing to expand its business such as China or South Africa. The report provides a comparative analysis of the market situation of the two countries. Based on this analysis, the report also suggests the best country that the AUSMED Company should opt for and the market entry strategy for conducting its new business establishments.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running Head: Global Business Assignment
Global Business Assignment
Name of the Student:
Name of University:
Author Note:
Global Business Assignment
Name of the Student:
Name of University:
Author Note:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1Global Business Assignment
Executive Summary
AUSMED is an Australian pharmaceutical company that wants to expand its business in a
foreign country due to facing stagnation in its domestic business operations. In response to that
China or South Africa are the two best possible markets where the company intends to set up its
new business establishments. Therefore, the aim of this report is to guide the company in
decision making through a comparative analysis of the market situation of the two countries.
Moreover, the report suggests the joint venturing method as a new market entry tool for the
company to enter into the South African pharmaceutical market.
Executive Summary
AUSMED is an Australian pharmaceutical company that wants to expand its business in a
foreign country due to facing stagnation in its domestic business operations. In response to that
China or South Africa are the two best possible markets where the company intends to set up its
new business establishments. Therefore, the aim of this report is to guide the company in
decision making through a comparative analysis of the market situation of the two countries.
Moreover, the report suggests the joint venturing method as a new market entry tool for the
company to enter into the South African pharmaceutical market.

2Global Business Assignment
Table of Contents
Introduction......................................................................................................................................4
Discussion........................................................................................................................................4
Risk and opportunities in the countries.......................................................................................4
Political....................................................................................................................................4
Economic.................................................................................................................................5
Social.......................................................................................................................................6
Technological..........................................................................................................................6
Legal........................................................................................................................................7
Environmental..........................................................................................................................8
SWOT analysis............................................................................................................................9
Selection of the destination country..............................................................................................10
Justification for the proposed entry...............................................................................................11
Conclusion.....................................................................................................................................12
Reference.......................................................................................................................................14
Appendices....................................................................................................................................17
Appendix 1.................................................................................................................................17
Appendix 2.................................................................................................................................17
Appendix 3.................................................................................................................................18
Appendix 4.................................................................................................................................18
Table of Contents
Introduction......................................................................................................................................4
Discussion........................................................................................................................................4
Risk and opportunities in the countries.......................................................................................4
Political....................................................................................................................................4
Economic.................................................................................................................................5
Social.......................................................................................................................................6
Technological..........................................................................................................................6
Legal........................................................................................................................................7
Environmental..........................................................................................................................8
SWOT analysis............................................................................................................................9
Selection of the destination country..............................................................................................10
Justification for the proposed entry...............................................................................................11
Conclusion.....................................................................................................................................12
Reference.......................................................................................................................................14
Appendices....................................................................................................................................17
Appendix 1.................................................................................................................................17
Appendix 2.................................................................................................................................17
Appendix 3.................................................................................................................................18
Appendix 4.................................................................................................................................18

3Global Business Assignment
Introduction
The AUSMED Company is highly popular for its pharmaceutical products in the
Australian market. However, recently the company faces stagnation in its business and willing to
expand the business in a foreign market. There are two countries where the company is willing to
expand its business such as China or South Africa. Therefore, the purpose of this report is to
enlighten the company’s decision in order to choose the right country where it can start its new
operations. In course of the discussion, the report is going to use the PESTLE analysis and the
SWOT matrix in order to understand the scope and risk factors exist in both the country. For the
betterment of the understanding the report focuses on the comparative assessment of Chinese and
South African pharmaceutical market. Based on this analysis, the report also suggests the best
country that the AUSMED Company should opt for and the market entry strategy for conducting
its new business establishments.
Discussion
Risk and opportunities in the countries
Political
The pharmaceutical industry in China is considered to be one of the leading sectors in the
country. There are number of domestic and multinational pharmaceutical companies in China
that are responsible to deliver a steady growth in the Chinese market (Mossialos et al. 2016). In
this regard, with the growth in the Chinese population the government is willing to create a better
framework in the healthcare facilities across the world. As per the report of DBS Group
Introduction
The AUSMED Company is highly popular for its pharmaceutical products in the
Australian market. However, recently the company faces stagnation in its business and willing to
expand the business in a foreign market. There are two countries where the company is willing to
expand its business such as China or South Africa. Therefore, the purpose of this report is to
enlighten the company’s decision in order to choose the right country where it can start its new
operations. In course of the discussion, the report is going to use the PESTLE analysis and the
SWOT matrix in order to understand the scope and risk factors exist in both the country. For the
betterment of the understanding the report focuses on the comparative assessment of Chinese and
South African pharmaceutical market. Based on this analysis, the report also suggests the best
country that the AUSMED Company should opt for and the market entry strategy for conducting
its new business establishments.
Discussion
Risk and opportunities in the countries
Political
The pharmaceutical industry in China is considered to be one of the leading sectors in the
country. There are number of domestic and multinational pharmaceutical companies in China
that are responsible to deliver a steady growth in the Chinese market (Mossialos et al. 2016). In
this regard, with the growth in the Chinese population the government is willing to create a better
framework in the healthcare facilities across the world. As per the report of DBS Group
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

4Global Business Assignment
Research, the Chinese government intends to ensure the public medical insurance and as a result
of that the sales growth in the pharmaceutical sector has declined from 3.9% in 2012-2015 to
0.6% in 2016 (Kong and Lam 2017).
On the other hand, the South African government firmly depends on the multinational
companies in order to expand their pharmaceutical market all over the country. As far as the
government report in 2015, it was accounted that 85% of the pharmaceutical products were
imported (IPASA, 2018). In fact, South Africa is the only country in the Southern African
Development Community (SADC) that only meets the Manufacturing Practice Standards
prescribed by the World Health Organisation (WHO) (polity.org.za 2018)
Economic
As far as the economic situation is concerned, the Chinese pharmaceutical sector is
referred as the second largest market in the world and the fastest emerging market. According to
the CNBC report in 2017, the Chinese pharmaceutical market was valued $122.6 billion and
expected to be reached $145 billion to $175 billion by 2022 (Deloitte 2018). In compare to this,
the annual growth in Chinese pharmaceutical market was accounted 9.4% between 2013 and
2017 (fmprc.gov.cn 2018).
On the other hand, the South African pharmaceutical market is also promising for the
new multinational entrants. The pharmaceutical market in South Africa was calculated R11.7
billion in 2015 (sanews.gov.za 2018). Furthermore, there are approximately 276 companies that
are listed in the South African market with the purpose to import, export and distributing
pharmaceutical products (polity.org.za 2018). As a matter of fact, the South African market is
Research, the Chinese government intends to ensure the public medical insurance and as a result
of that the sales growth in the pharmaceutical sector has declined from 3.9% in 2012-2015 to
0.6% in 2016 (Kong and Lam 2017).
On the other hand, the South African government firmly depends on the multinational
companies in order to expand their pharmaceutical market all over the country. As far as the
government report in 2015, it was accounted that 85% of the pharmaceutical products were
imported (IPASA, 2018). In fact, South Africa is the only country in the Southern African
Development Community (SADC) that only meets the Manufacturing Practice Standards
prescribed by the World Health Organisation (WHO) (polity.org.za 2018)
Economic
As far as the economic situation is concerned, the Chinese pharmaceutical sector is
referred as the second largest market in the world and the fastest emerging market. According to
the CNBC report in 2017, the Chinese pharmaceutical market was valued $122.6 billion and
expected to be reached $145 billion to $175 billion by 2022 (Deloitte 2018). In compare to this,
the annual growth in Chinese pharmaceutical market was accounted 9.4% between 2013 and
2017 (fmprc.gov.cn 2018).
On the other hand, the South African pharmaceutical market is also promising for the
new multinational entrants. The pharmaceutical market in South Africa was calculated R11.7
billion in 2015 (sanews.gov.za 2018). Furthermore, there are approximately 276 companies that
are listed in the South African market with the purpose to import, export and distributing
pharmaceutical products (polity.org.za 2018). As a matter of fact, the South African market is

5Global Business Assignment
also interested to attract multinational pharmaceutical companies to develop the infrastructure of
manufacturing pharmaceutical products and creating new employments for the citizens.
Social
In case of China, the population growth has become an escalating trend in the country
with 247 million in 2015 (Kong and Lam 2017). Therefore, it can be argued that there is ample
of opportunities for the pharmaceutical companies to expand their business in the China where
they can expect a dynamic market with enough customers. As a matter of fact, the Chinese
government has planned to ensure health care facilities for all the citizens. It requires adequate
infrastructure as well as enough pharmaceutical products. According to the 12th Five-Year Plan
of China, the authority was proposed to expand the distribution of pharmaceutical products from
the Eastern province to the rests of the country (Li and Hamblin 2016). Therefore, for AUSMAD
it creates high prospect to intervene into the dynamic Chinese pharmaceutical market.
As far as the South African pharmaceutical market is concerned, the country suffers with
enough diseases and epidemics. Besides this, the rapid urbanisation, sedentary lifestyles and the
dietary trends facilitates the demand for pharmaceutical product to strengthen the healthcare
facilities (sanews.gov.za 2018). The South African people face lots of life causing diseases and
epidemics that have to be stopped with the urgent need of pharmaceutical products.
Technological
Technological advancement fosters a more lucrative market opportunities for the
multinational pharmaceutical Companies in China. The Chinese authority expresses grave
concern regarding the role of the quality of the pharmaceutical products and intends to imply
more transparency in the drug approval process. As per the ‘Made in China 2025’ industrial plan
also interested to attract multinational pharmaceutical companies to develop the infrastructure of
manufacturing pharmaceutical products and creating new employments for the citizens.
Social
In case of China, the population growth has become an escalating trend in the country
with 247 million in 2015 (Kong and Lam 2017). Therefore, it can be argued that there is ample
of opportunities for the pharmaceutical companies to expand their business in the China where
they can expect a dynamic market with enough customers. As a matter of fact, the Chinese
government has planned to ensure health care facilities for all the citizens. It requires adequate
infrastructure as well as enough pharmaceutical products. According to the 12th Five-Year Plan
of China, the authority was proposed to expand the distribution of pharmaceutical products from
the Eastern province to the rests of the country (Li and Hamblin 2016). Therefore, for AUSMAD
it creates high prospect to intervene into the dynamic Chinese pharmaceutical market.
As far as the South African pharmaceutical market is concerned, the country suffers with
enough diseases and epidemics. Besides this, the rapid urbanisation, sedentary lifestyles and the
dietary trends facilitates the demand for pharmaceutical product to strengthen the healthcare
facilities (sanews.gov.za 2018). The South African people face lots of life causing diseases and
epidemics that have to be stopped with the urgent need of pharmaceutical products.
Technological
Technological advancement fosters a more lucrative market opportunities for the
multinational pharmaceutical Companies in China. The Chinese authority expresses grave
concern regarding the role of the quality of the pharmaceutical products and intends to imply
more transparency in the drug approval process. As per the ‘Made in China 2025’ industrial plan

6Global Business Assignment
the Xi Jinping government seeks to upgrade the existing technology in the pharmaceutical sector
(Deloitte 2018). In this context, it can be argued that most of the domestic pharmaceutical
companies are still relied on the generic drugs or the therapeutic medicines. Therefore, they only
invest 5% of their profit for the R&D projects in compare to US where the pharmaceutical
companies invest 20% (Mossialos et al. 2016). Keeping in mind of this drawback the Chinese
government takes the responsibility to upgrade the existing system by aiding $1.5 million in the
pharmaceutical sector (Kong and Lam 2017).
The implementation of new technologies in the South African market was low in the past
years. Nevertheless, in recent times some impressive and optimistic initiatives taken by the SA
government drive better opportunities for technological intervention in the pharmaceutical
sectors as well. The introduction of the Innovative Pharmaceutical Association South Africa
(IPASA) in 2013 further bolstered the process. The purpose of this organisation is to encourage
the pharmaceutical companies to research and develop novel medication, medical device and
diagnostic tools (IPASA, 2018). With the assistance from IPASA now both the public and
private pharmaceutical companies are able to provide better medication facilities for deadly
diseases like small pox, diabetes and heart attacks which are growing in a rapid pace in the
country.
Legal
In case of the legal framework for maintaining the quality of the pharmaceutical products
and licensing of drugs the Medicine Control Council of the Department of Health of South
Africa enacts some regulations. The guidelines follow the Good Manufacturing Practices (GMP)
and the Good Distribution Practices (GDP) in order to safeguard the life and mortality of the
South African citizens (Statutory Mandate 2018). Besides this, there are some important laws
the Xi Jinping government seeks to upgrade the existing technology in the pharmaceutical sector
(Deloitte 2018). In this context, it can be argued that most of the domestic pharmaceutical
companies are still relied on the generic drugs or the therapeutic medicines. Therefore, they only
invest 5% of their profit for the R&D projects in compare to US where the pharmaceutical
companies invest 20% (Mossialos et al. 2016). Keeping in mind of this drawback the Chinese
government takes the responsibility to upgrade the existing system by aiding $1.5 million in the
pharmaceutical sector (Kong and Lam 2017).
The implementation of new technologies in the South African market was low in the past
years. Nevertheless, in recent times some impressive and optimistic initiatives taken by the SA
government drive better opportunities for technological intervention in the pharmaceutical
sectors as well. The introduction of the Innovative Pharmaceutical Association South Africa
(IPASA) in 2013 further bolstered the process. The purpose of this organisation is to encourage
the pharmaceutical companies to research and develop novel medication, medical device and
diagnostic tools (IPASA, 2018). With the assistance from IPASA now both the public and
private pharmaceutical companies are able to provide better medication facilities for deadly
diseases like small pox, diabetes and heart attacks which are growing in a rapid pace in the
country.
Legal
In case of the legal framework for maintaining the quality of the pharmaceutical products
and licensing of drugs the Medicine Control Council of the Department of Health of South
Africa enacts some regulations. The guidelines follow the Good Manufacturing Practices (GMP)
and the Good Distribution Practices (GDP) in order to safeguard the life and mortality of the
South African citizens (Statutory Mandate 2018). Besides this, there are some important laws
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7Global Business Assignment
that the private pharmaceutical companies have to follow such as the Medicines and Related
Substances Act 101 of 1965, Occupational Health and Safety Act 85 of 1993 and the Pharmacy
Act of 1974 (Legislation 2018).
Subsequently, the Chinese authorities also put some monitoring process and reflect grave
concern for the medicines and other pharmaceutical products. As far as the Chinese regulation is
concerned, the China Food and Drug Administration (CFDA) is solely responsible for the
regulation of medical devices and pharmaceutical products in the Chinese mainland
(fmprc.gov.cn 2018). For a better healthcare service in March 2018 the CFDA was merged with
the National Market Supervision Administration (Li and Hamblin 2016). Moreover, the
multinational pharmaceutical companies have enjoyed a reduction of R&D costs since the new
guidelines advocated for the elimination of the unnecessary and costly clinical trials in China. In
this context, it will be a beneficial factor for the AUSMED to expand its pharmaceutical business
by entering in the Chinese market.
Environmental
Since 2013 after XI Jinping Came to power in China the government expressed their top
concern on the question of environmental pollution. In this regard, the government intended to
implement strict enforcement of pollution control. In fact, it was in the agenda of the new
government under the leadership of Xi to maintain the global standards of environmental
performance. A major push was started since last year where the authority tried to put more
emphasis on the detrimental impact of the producers of Active Pharmaceutical Ingredients (API)
(Kong, M. and Lam 2017). As a matter of fact, the government initiates a program of monitoring
the pharmaceutical plants and measures the intensity of pollution.
that the private pharmaceutical companies have to follow such as the Medicines and Related
Substances Act 101 of 1965, Occupational Health and Safety Act 85 of 1993 and the Pharmacy
Act of 1974 (Legislation 2018).
Subsequently, the Chinese authorities also put some monitoring process and reflect grave
concern for the medicines and other pharmaceutical products. As far as the Chinese regulation is
concerned, the China Food and Drug Administration (CFDA) is solely responsible for the
regulation of medical devices and pharmaceutical products in the Chinese mainland
(fmprc.gov.cn 2018). For a better healthcare service in March 2018 the CFDA was merged with
the National Market Supervision Administration (Li and Hamblin 2016). Moreover, the
multinational pharmaceutical companies have enjoyed a reduction of R&D costs since the new
guidelines advocated for the elimination of the unnecessary and costly clinical trials in China. In
this context, it will be a beneficial factor for the AUSMED to expand its pharmaceutical business
by entering in the Chinese market.
Environmental
Since 2013 after XI Jinping Came to power in China the government expressed their top
concern on the question of environmental pollution. In this regard, the government intended to
implement strict enforcement of pollution control. In fact, it was in the agenda of the new
government under the leadership of Xi to maintain the global standards of environmental
performance. A major push was started since last year where the authority tried to put more
emphasis on the detrimental impact of the producers of Active Pharmaceutical Ingredients (API)
(Kong, M. and Lam 2017). As a matter of fact, the government initiates a program of monitoring
the pharmaceutical plants and measures the intensity of pollution.
8Global Business Assignment
Simultaneously, in South Africa also the government tries to follow the global mandate
of maintaining environmental sustainability. The country is full of natural diversities in terms of
both flora and fauna. Therefore, it is pertinent to restore some eco-friendly attitude for sustaining
in the South African market.
SWOT analysis
Strength Opportunity
China South Africa China South Africa
The Chinese market
has a promising
prospect in future.
Huge consumer
strength.
High technological
advancement.
Government
endorsement.
Huge government
initiatives.
The continuous
growth in the
market.
International aids
for developing
the health
infrastructure.
Encouragement
of R&D projects.
The population
growth increases
the opportunity
for more
Pharmaceutical
products.
Government
provides further
financial aids in
Pharmaceutical
sector.
Following the
international
standards.
Government
always encourage
foreign
Pharmaceutical
companies to
invest in the
country.
The growing
market with less
competitiveness.
Need of
medication for
combating with
deadly diseases.
Ample scope for
R&D projects with
Simultaneously, in South Africa also the government tries to follow the global mandate
of maintaining environmental sustainability. The country is full of natural diversities in terms of
both flora and fauna. Therefore, it is pertinent to restore some eco-friendly attitude for sustaining
in the South African market.
SWOT analysis
Strength Opportunity
China South Africa China South Africa
The Chinese market
has a promising
prospect in future.
Huge consumer
strength.
High technological
advancement.
Government
endorsement.
Huge government
initiatives.
The continuous
growth in the
market.
International aids
for developing
the health
infrastructure.
Encouragement
of R&D projects.
The population
growth increases
the opportunity
for more
Pharmaceutical
products.
Government
provides further
financial aids in
Pharmaceutical
sector.
Following the
international
standards.
Government
always encourage
foreign
Pharmaceutical
companies to
invest in the
country.
The growing
market with less
competitiveness.
Need of
medication for
combating with
deadly diseases.
Ample scope for
R&D projects with
9Global Business Assignment
the help of IPASA.
Weakness Threats
China South Africa China South Africa
Government puts
more focus on the
local companies.
High taxation.
Less initiative in
R&D projects.
Popularisation of
the traditional
Chinese
medication.
Lack of
international
standards of
infrastructure.
The
Pharmaceutical
market is not so
large.
Intense
competition from
the local
companies.
Low pricing
strategy
implemented by
the government.
Corruption.
Volatile market
with dearth of
proper
infrastructure.
Presence of many
international
Pharmaceutical
companies
increases the
competition.
Selection of the destination country
On the basis of the above discussion it can be argued that the AUSMED Company should
go for expanding its business in South Africa. It is true that the South African market is growing
and the standard of infrastructure is not of international standard. Despite of this issue, it will be
better for the AUSMED Company to put focus on the South African market. One of the major
factors is the government willingness. The South African government is keen to have more
multinational pharmaceutical companies to invest in the country (IPASA, 2018). On the
contrary, in China the government policy is not advantageous for the international companies.
the help of IPASA.
Weakness Threats
China South Africa China South Africa
Government puts
more focus on the
local companies.
High taxation.
Less initiative in
R&D projects.
Popularisation of
the traditional
Chinese
medication.
Lack of
international
standards of
infrastructure.
The
Pharmaceutical
market is not so
large.
Intense
competition from
the local
companies.
Low pricing
strategy
implemented by
the government.
Corruption.
Volatile market
with dearth of
proper
infrastructure.
Presence of many
international
Pharmaceutical
companies
increases the
competition.
Selection of the destination country
On the basis of the above discussion it can be argued that the AUSMED Company should
go for expanding its business in South Africa. It is true that the South African market is growing
and the standard of infrastructure is not of international standard. Despite of this issue, it will be
better for the AUSMED Company to put focus on the South African market. One of the major
factors is the government willingness. The South African government is keen to have more
multinational pharmaceutical companies to invest in the country (IPASA, 2018). On the
contrary, in China the government policy is not advantageous for the international companies.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

10Global Business Assignment
The Chinese government always encourages the local pharmaceutical companies rather than the
foreign counterparts (Kong, M. and Lam 2017). As a matter of fact, the AUSMED Company is
not so big to compete in the high competitive market in China. In addition to this, apparently the
Chinese market looks very lucrative and full of opportunities but corruption and the way of
business in China are far different from the rest of the world. Moreover, in South Africa the
AUSMED Company can get a near similar market environment as in Australia where the
government and the international organisations can help to establish business profoundly. As a
matter of fact, the South African market is more relaxed and transparent in compare to the
Chinese market.
Justification for the proposed entry
There are number of strategies to enter into the new market for a foreign company.
Strategies like direct exporting, licensing, franchising, partnering, joint ventures, buying a
company and turnkey projects. However, for the AUSMED Company it will be a better strategy
to follow the joint venture strategy to enter into the South African market at first. The Company
is not so big to establish new set up in South Africa. Moreover, there are a number of advantages
for joint venture strategies.
According to Hitt, Li and Xu (2016) it is better for the not so big companies to expand
their business by utilising external knowledge on technological development and innovation. As
a matter of fact, procuring the joint venture strategy is a purposeful technique of the foreign
companies to achieve long term technological benefits (Chanu and Dhir 2016). Based on this
understanding, it can be argued that the AUSMED Company should focus on the joint venture
strategy. The Company has no previous experience in international market and business
The Chinese government always encourages the local pharmaceutical companies rather than the
foreign counterparts (Kong, M. and Lam 2017). As a matter of fact, the AUSMED Company is
not so big to compete in the high competitive market in China. In addition to this, apparently the
Chinese market looks very lucrative and full of opportunities but corruption and the way of
business in China are far different from the rest of the world. Moreover, in South Africa the
AUSMED Company can get a near similar market environment as in Australia where the
government and the international organisations can help to establish business profoundly. As a
matter of fact, the South African market is more relaxed and transparent in compare to the
Chinese market.
Justification for the proposed entry
There are number of strategies to enter into the new market for a foreign company.
Strategies like direct exporting, licensing, franchising, partnering, joint ventures, buying a
company and turnkey projects. However, for the AUSMED Company it will be a better strategy
to follow the joint venture strategy to enter into the South African market at first. The Company
is not so big to establish new set up in South Africa. Moreover, there are a number of advantages
for joint venture strategies.
According to Hitt, Li and Xu (2016) it is better for the not so big companies to expand
their business by utilising external knowledge on technological development and innovation. As
a matter of fact, procuring the joint venture strategy is a purposeful technique of the foreign
companies to achieve long term technological benefits (Chanu and Dhir 2016). Based on this
understanding, it can be argued that the AUSMED Company should focus on the joint venture
strategy. The Company has no previous experience in international market and business

11Global Business Assignment
operations. Taking direct entry in the South African market may cause a catastrophe for the
company. On the other hand, the joint venture strategy can provide them proper understanding of
the market trend in South Africa and the AUSMED Company can use its experience in
expanding business through direct investment in future.
Moreover, as per the study of Hearn (2015) from the perspective of profit and stock
market holding it will be an advantage for the companies to go for joint ventures to establish a
strong market capitalisation in the developing countries. As a matter of fact, van der Meer-
Kooistra and Kamminga (2015) opined that joint venture strategy is highly beneficial for the
healthcare firms in expanding their business in foreign country. It can be argued that the role of
the joint ventures in new market entry can facilitate product innovation through sharing products,
expertise and knowledge (Romeli et al. 2016). The strategic flexibility that the joint venture
strategy provides will be highly effective for the AUSMED Company for its future sustainability.
It will give AUSMED a strategic position to think about further expansion of its business.
On the other hand, Shah (2015) explained the joint venture strategy on the basis of new
trend in the global market. It can be asserted that with the growth in the communication and
transportation system the market competition has been escalated intensely (Klijn et al. 2014).
Moreover, there is a transformation in the global business orientation. Khamaksorn, Kurul and
Tah (2017) showed that fifty years ago business was largely localised and only the large
multinational companies had the ability to take part in the global business. However, presently
the multinational enterprises also play significant role in the global market (Link, Ruhm and
Siegel 2014). As a result of that, the business orientation has changed its shape and incorporates
more effective measures by introducing joint ventures as a strategic measure (Nakandala and Lau
2015). Therefore, the AUSMED Company should take the opportunity to penetrate in the
operations. Taking direct entry in the South African market may cause a catastrophe for the
company. On the other hand, the joint venture strategy can provide them proper understanding of
the market trend in South Africa and the AUSMED Company can use its experience in
expanding business through direct investment in future.
Moreover, as per the study of Hearn (2015) from the perspective of profit and stock
market holding it will be an advantage for the companies to go for joint ventures to establish a
strong market capitalisation in the developing countries. As a matter of fact, van der Meer-
Kooistra and Kamminga (2015) opined that joint venture strategy is highly beneficial for the
healthcare firms in expanding their business in foreign country. It can be argued that the role of
the joint ventures in new market entry can facilitate product innovation through sharing products,
expertise and knowledge (Romeli et al. 2016). The strategic flexibility that the joint venture
strategy provides will be highly effective for the AUSMED Company for its future sustainability.
It will give AUSMED a strategic position to think about further expansion of its business.
On the other hand, Shah (2015) explained the joint venture strategy on the basis of new
trend in the global market. It can be asserted that with the growth in the communication and
transportation system the market competition has been escalated intensely (Klijn et al. 2014).
Moreover, there is a transformation in the global business orientation. Khamaksorn, Kurul and
Tah (2017) showed that fifty years ago business was largely localised and only the large
multinational companies had the ability to take part in the global business. However, presently
the multinational enterprises also play significant role in the global market (Link, Ruhm and
Siegel 2014). As a result of that, the business orientation has changed its shape and incorporates
more effective measures by introducing joint ventures as a strategic measure (Nakandala and Lau
2015). Therefore, the AUSMED Company should take the opportunity to penetrate in the

12Global Business Assignment
international market through the medium of joint venture. By taking part in the joint venture
strategy the company can dodge the threats and competencies in the dynamic global market and
ensure an healthy business environment for its future sustainability.
Conclusion
The report clearly tried to figure out the best possibilities and risk factors for the
AUSMED Company for its new expansion in the international market. As the company is
intended to expand the business in China or South Africa therefore the report identified the risk
factors and opportunities by conducting a comparative analysis for the pharmaceutical market in
both the countries. In fact, the report incorporated the PESTLE analysis and SWOT tools to get a
clear picture of the condition of the pharmaceutical market in both China and South Africa. After
an in-depth analysis the report advised the AUSMED Company to opt for the South African
market because it is more flexible with high exposure for future sustainability of the company.
Moreover, the discussion also suggested using joint venture strategy keeping on mind about the
strength and weakness of the company. Henceforth, it can be concluded that the report portrayed
a thorough understanding related to the business expansion of AUSMED and is relevant and
rational with contextual analysis.
international market through the medium of joint venture. By taking part in the joint venture
strategy the company can dodge the threats and competencies in the dynamic global market and
ensure an healthy business environment for its future sustainability.
Conclusion
The report clearly tried to figure out the best possibilities and risk factors for the
AUSMED Company for its new expansion in the international market. As the company is
intended to expand the business in China or South Africa therefore the report identified the risk
factors and opportunities by conducting a comparative analysis for the pharmaceutical market in
both the countries. In fact, the report incorporated the PESTLE analysis and SWOT tools to get a
clear picture of the condition of the pharmaceutical market in both China and South Africa. After
an in-depth analysis the report advised the AUSMED Company to opt for the South African
market because it is more flexible with high exposure for future sustainability of the company.
Moreover, the discussion also suggested using joint venture strategy keeping on mind about the
strength and weakness of the company. Henceforth, it can be concluded that the report portrayed
a thorough understanding related to the business expansion of AUSMED and is relevant and
rational with contextual analysis.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

13Global Business Assignment
Reference
Chanu, O.R. and Dhir, S., 2016. A Perspective of Strategic Flexibility in a Joint Venture
Healthcare Firms for Innovative Products. Proceedings of GLOGIFT 16, pp. 473-487
Deloitte, 2016. The next phase: Opportunities in China's pharmaceuticals market. National
Industry Program, [online] pp.3-22. Available at:
https://www2.deloitte.com/content/dam/Deloitte/ch/Documents/life-sciences-health-care/
ch_Studie_Pharmaceutical_China_05052014.pdf [Accessed 29 Aug. 2018]
fmprc.gov.cn, 2018. Pharmaceutical Administration Law of the People's Republic of China.
[online] fmprc.gov.cn. Available at:
http://www.fmprc.gov.cn/ce/cgvienna/eng/dbtyw/jdwt/crimelaw/t209042.htm [Accessed 29 Aug.
2018].
Hearn, B., 2015. Institutional influences on board composition of international joint venture
firms listing on emerging stock exchanges: Evidence from Africa. Journal of World
Business, 50(1), pp.205-219.
Hitt, M.A., Li, D. and Xu, K., 2016. International strategy: From local to global and
beyond. Journal of World Business, 51(1), pp.58-73.
IPASA, 2018. IPASA | The Innovative Pharmaceutical Association South Africa. [online]
Ipasa.co.za. Available at: http://ipasa.co.za/ [Accessed 29 Aug. 2018].
Reference
Chanu, O.R. and Dhir, S., 2016. A Perspective of Strategic Flexibility in a Joint Venture
Healthcare Firms for Innovative Products. Proceedings of GLOGIFT 16, pp. 473-487
Deloitte, 2016. The next phase: Opportunities in China's pharmaceuticals market. National
Industry Program, [online] pp.3-22. Available at:
https://www2.deloitte.com/content/dam/Deloitte/ch/Documents/life-sciences-health-care/
ch_Studie_Pharmaceutical_China_05052014.pdf [Accessed 29 Aug. 2018]
fmprc.gov.cn, 2018. Pharmaceutical Administration Law of the People's Republic of China.
[online] fmprc.gov.cn. Available at:
http://www.fmprc.gov.cn/ce/cgvienna/eng/dbtyw/jdwt/crimelaw/t209042.htm [Accessed 29 Aug.
2018].
Hearn, B., 2015. Institutional influences on board composition of international joint venture
firms listing on emerging stock exchanges: Evidence from Africa. Journal of World
Business, 50(1), pp.205-219.
Hitt, M.A., Li, D. and Xu, K., 2016. International strategy: From local to global and
beyond. Journal of World Business, 51(1), pp.58-73.
IPASA, 2018. IPASA | The Innovative Pharmaceutical Association South Africa. [online]
Ipasa.co.za. Available at: http://ipasa.co.za/ [Accessed 29 Aug. 2018].

14Global Business Assignment
Khamaksorn, A., Kurul, E. and Tah, J.H.M., 2017, December. Factors Affecting Knowledge
Transfer in International Construction Joint Venture Projects. In International Conference on
Civil, Architecture and Sustainable Development (pp. 1-2).
Klijn, E., Reuer, J.J., Buckley, P.J. and Glaister, K.W., 2014. Combinations of partners’ joint
venture formation motives. In The Multinational Enterprise and the Emergence of the Global
Factory (pp. 203-219). Palgrave Macmillan, London.
Kong, M. and Lam, D., 2017. China Pharmaceutical Sector Inflection Point Emerging. DBS
Asian Insight, [online] 42, pp.4-12. Available at:
https://www.dbs.com.sg/sme/.../pdfController.page?.../052017/...china_pharma.. [Accessed 29
Aug. 2018].
Legislation, 2018. SAPC - South African Pharmacy Council. [online] Pharmcouncil.co.za.
Available at: https://www.pharmcouncil.co.za/G_PublicationsE.asp [Accessed 29 Aug. 2018].
Li, X. and Hamblin, D., 2016. Factors impacting on cleaner production: case studies of Chinese
pharmaceutical manufacturers in Tianjin, China. Journal of Cleaner Production, 131, pp.121-
132.
Link, A.N., Ruhm, C.J. and Siegel, D.S., 2014. Private equity and the innovation strategies of
entrepreneurial firms: Empirical evidence from the Small Business Innovation Research
Program. Managerial and Decision Economics, 35(2), pp.103-113.
Mossialos, E., Ge, Y., Hu, J. and Wang, L. (2016). Pharmaceutical policy in China: challenges
and opportunities for reform. Development Research Center of the State Council of China,
pp.15-125.
Khamaksorn, A., Kurul, E. and Tah, J.H.M., 2017, December. Factors Affecting Knowledge
Transfer in International Construction Joint Venture Projects. In International Conference on
Civil, Architecture and Sustainable Development (pp. 1-2).
Klijn, E., Reuer, J.J., Buckley, P.J. and Glaister, K.W., 2014. Combinations of partners’ joint
venture formation motives. In The Multinational Enterprise and the Emergence of the Global
Factory (pp. 203-219). Palgrave Macmillan, London.
Kong, M. and Lam, D., 2017. China Pharmaceutical Sector Inflection Point Emerging. DBS
Asian Insight, [online] 42, pp.4-12. Available at:
https://www.dbs.com.sg/sme/.../pdfController.page?.../052017/...china_pharma.. [Accessed 29
Aug. 2018].
Legislation, 2018. SAPC - South African Pharmacy Council. [online] Pharmcouncil.co.za.
Available at: https://www.pharmcouncil.co.za/G_PublicationsE.asp [Accessed 29 Aug. 2018].
Li, X. and Hamblin, D., 2016. Factors impacting on cleaner production: case studies of Chinese
pharmaceutical manufacturers in Tianjin, China. Journal of Cleaner Production, 131, pp.121-
132.
Link, A.N., Ruhm, C.J. and Siegel, D.S., 2014. Private equity and the innovation strategies of
entrepreneurial firms: Empirical evidence from the Small Business Innovation Research
Program. Managerial and Decision Economics, 35(2), pp.103-113.
Mossialos, E., Ge, Y., Hu, J. and Wang, L. (2016). Pharmaceutical policy in China: challenges
and opportunities for reform. Development Research Center of the State Council of China,
pp.15-125.

15Global Business Assignment
Nakandala, D. and Lau, H., 2015. A technology management strategy selection method for firms
in joint venture partnerships. International Journal of Management and Decision Making, 14(2),
pp.112-129.
polity.org.za, 2018. The South African pharmaceutical regulatory environment. [online]
Polity.org.za. Available at: http://www.polity.org.za/article/the-south-african-pharmaceutical-
regulatory-environment-2018-06-20 [Accessed 29 Aug. 2018].
Romeli, N., Halil, F.M., Ismail, F. and Shukor, A.S.A., 2016. Economic Challenges in Joint
Venture Infrastructure Projects: Towards Contractor's Quality of Life. Procedia-Social and
Behavioral Sciences, 234, pp.19-27.
sanews.gov.za, 2018. SA pharmaceutical sector provides unlimited opportunities. [online]
SAnews. Available at: https://www.sanews.gov.za/south-africa/sa-pharmaceutical-sector-
provides-unlimited-opportunities [Accessed 29 Aug. 2018].
Shah, K.U., 2015. Choice and control of international joint venture partners to improve corporate
environmental performance. Journal of Cleaner Production, 89, pp.32-40.
Statutory Mandate, 2018. SAPC - South African Pharmacy Council. [online] Pharmcouncil.co.za.
Available at: https://www.pharmcouncil.co.za/A_Statutory.asp [Accessed 29 Aug. 2018].
van der Meer-Kooistra, J. and Kamminga, P.E., 2015. Joint venture dynamics: The effects of
decisions made within a parent company and the role of joint venture management
control. Management Accounting Research, 26, pp.23-39.
Nakandala, D. and Lau, H., 2015. A technology management strategy selection method for firms
in joint venture partnerships. International Journal of Management and Decision Making, 14(2),
pp.112-129.
polity.org.za, 2018. The South African pharmaceutical regulatory environment. [online]
Polity.org.za. Available at: http://www.polity.org.za/article/the-south-african-pharmaceutical-
regulatory-environment-2018-06-20 [Accessed 29 Aug. 2018].
Romeli, N., Halil, F.M., Ismail, F. and Shukor, A.S.A., 2016. Economic Challenges in Joint
Venture Infrastructure Projects: Towards Contractor's Quality of Life. Procedia-Social and
Behavioral Sciences, 234, pp.19-27.
sanews.gov.za, 2018. SA pharmaceutical sector provides unlimited opportunities. [online]
SAnews. Available at: https://www.sanews.gov.za/south-africa/sa-pharmaceutical-sector-
provides-unlimited-opportunities [Accessed 29 Aug. 2018].
Shah, K.U., 2015. Choice and control of international joint venture partners to improve corporate
environmental performance. Journal of Cleaner Production, 89, pp.32-40.
Statutory Mandate, 2018. SAPC - South African Pharmacy Council. [online] Pharmcouncil.co.za.
Available at: https://www.pharmcouncil.co.za/A_Statutory.asp [Accessed 29 Aug. 2018].
van der Meer-Kooistra, J. and Kamminga, P.E., 2015. Joint venture dynamics: The effects of
decisions made within a parent company and the role of joint venture management
control. Management Accounting Research, 26, pp.23-39.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
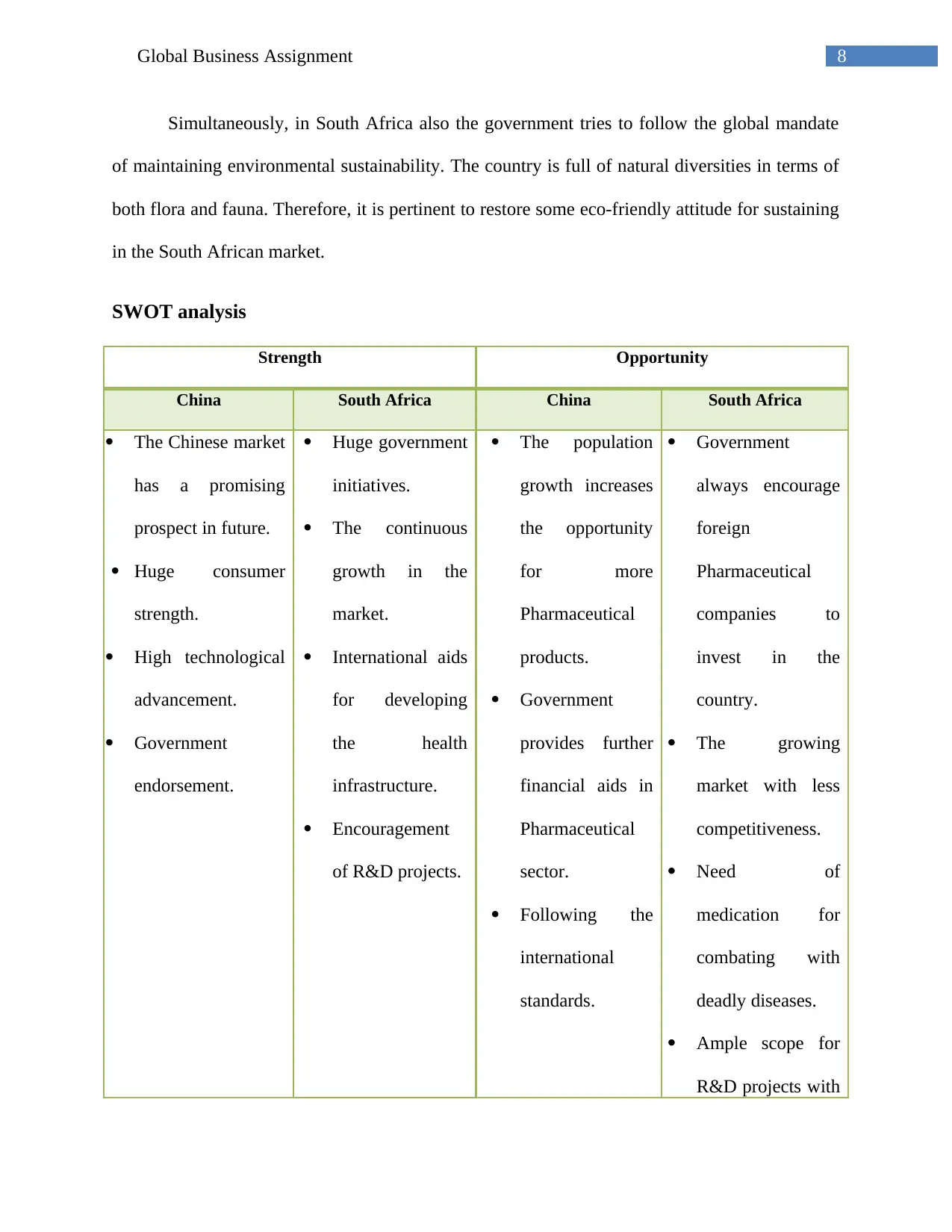
16Global Business Assignment
Appendices
Appendix 1
Strategic buyer deal and volume in China (2014-2017)
(Mossialos et al. 2016)
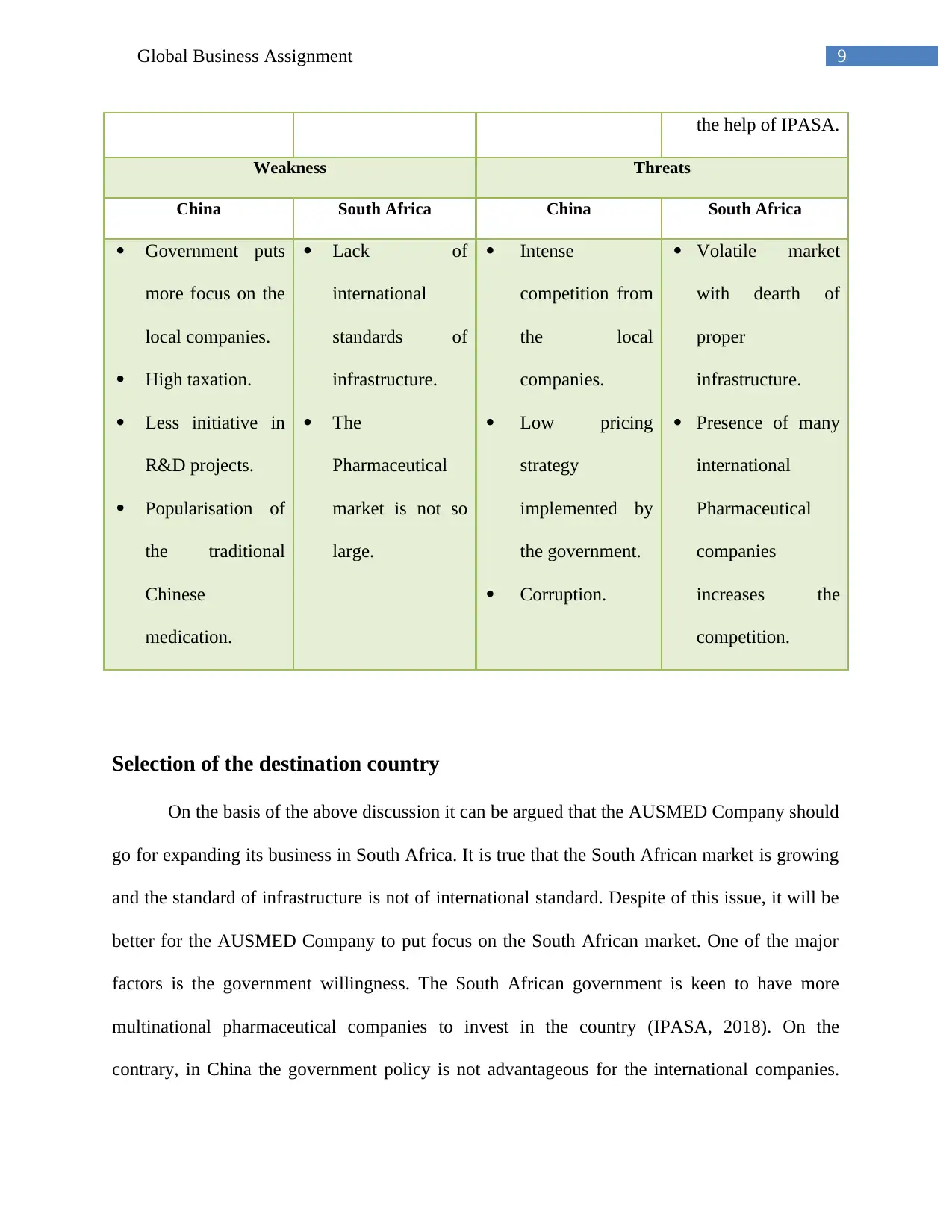
Appendix 2
SA pharmaceutical industry statistics
Appendices
Appendix 1
Strategic buyer deal and volume in China (2014-2017)
(Mossialos et al. 2016)
Appendix 2
SA pharmaceutical industry statistics
17Global Business Assignment
(sanews.gov.za 2018)
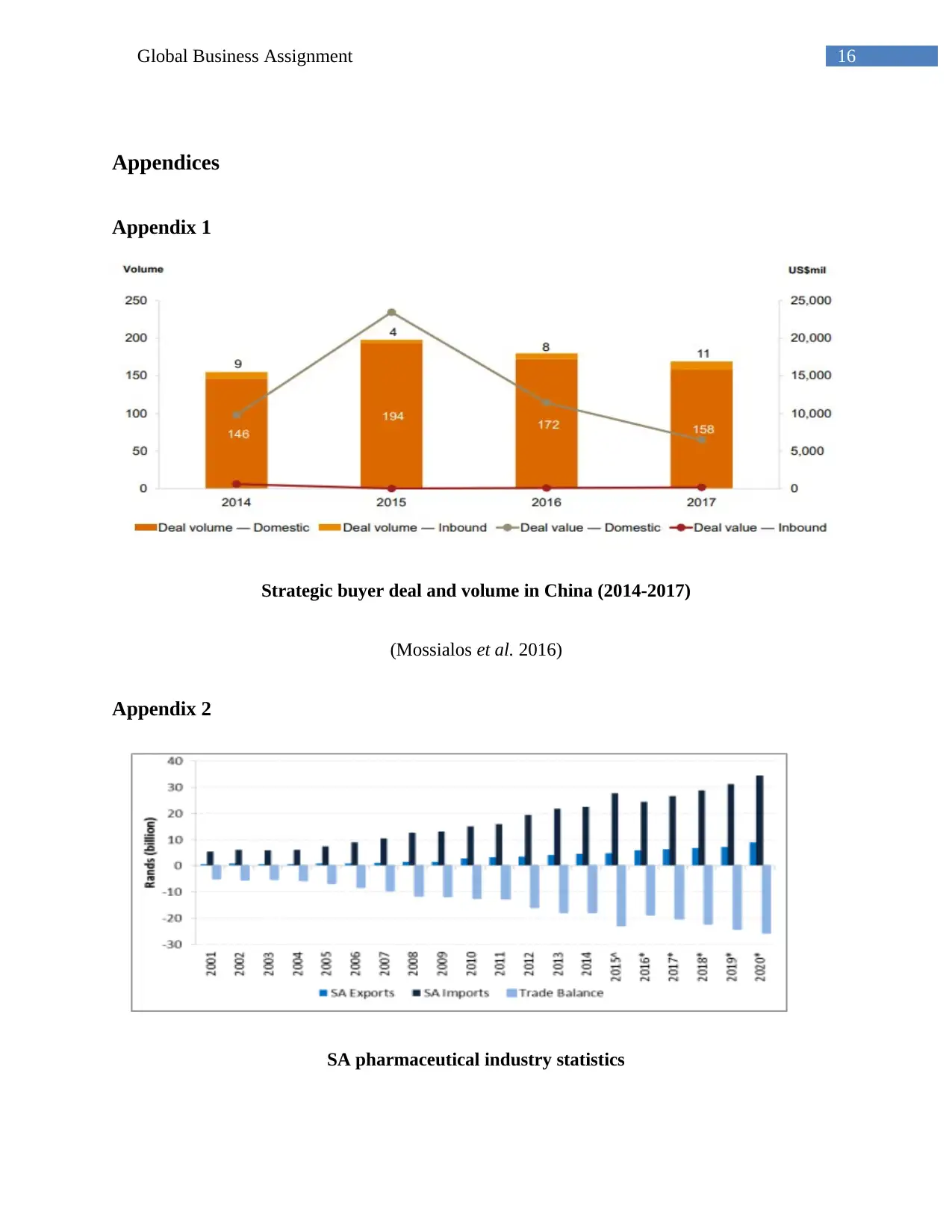
Appendix 3
Pharmaceutical sales statistics in China (2007-2015)
(Deloitte 2016)
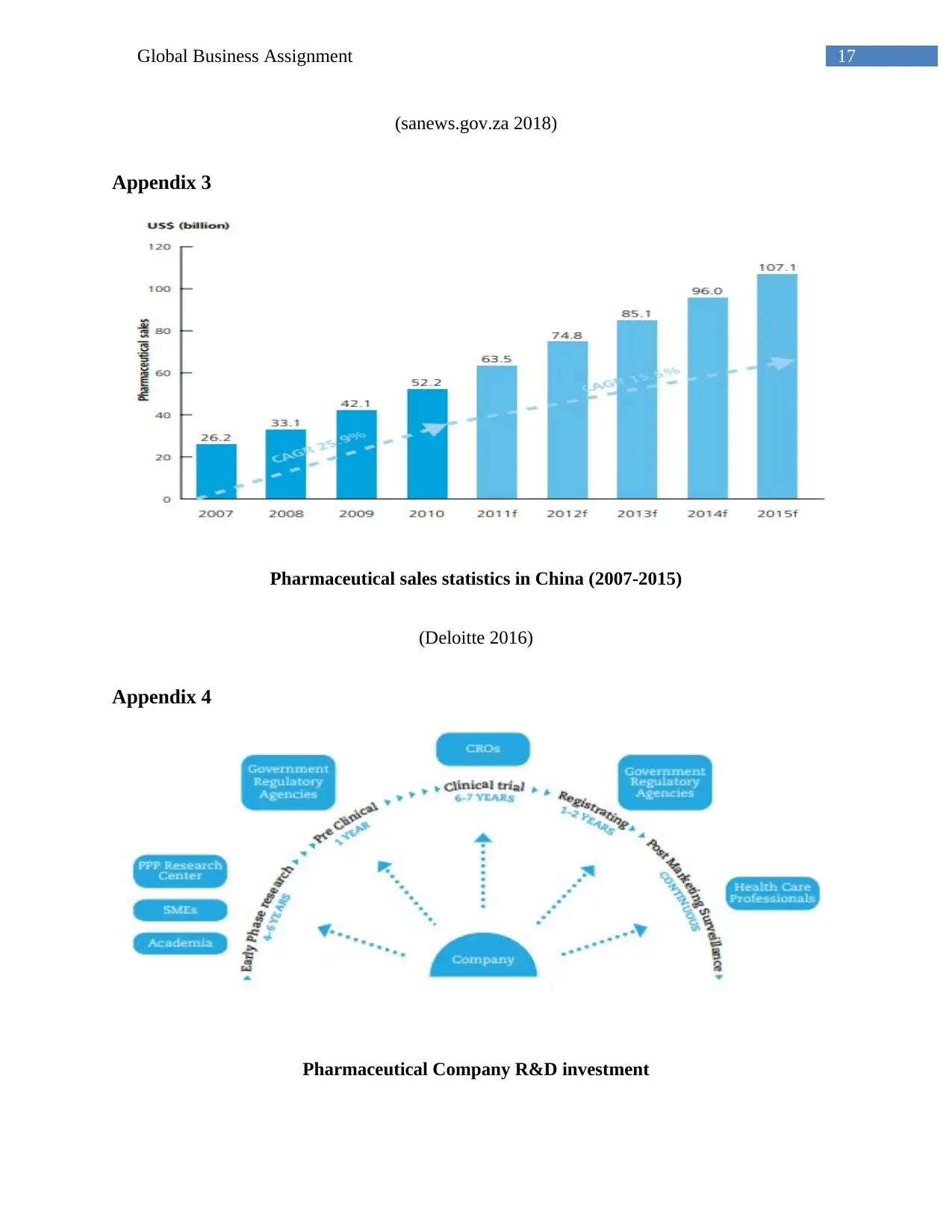
Appendix 4
Pharmaceutical Company R&D investment
(sanews.gov.za 2018)
Appendix 3
Pharmaceutical sales statistics in China (2007-2015)
(Deloitte 2016)
Appendix 4
Pharmaceutical Company R&D investment

18Global Business Assignment
(Created by the author)
(Created by the author)
1 out of 19
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.



