Chemistry Practical: Experiment to Measure Heat Energy of Fuels
VerifiedAdded on 2023/06/15
|17
|2560
|334
Practical Assignment
AI Summary
This assignment details an experiment conducted to measure the heat energy of various fuels, specifically methanol, ethanol, and propanol. The experiment involves measuring the temperature change of water heated by the combustion of these alcohols and calculating the molar enthalpy change. The methodology includes precise measurements of initial and final temperatures and masses, with repeated trials to ensure accuracy. The results obtained are then compared with literature values, and error percentages are calculated. The report also includes a discussion of potential sources of error and suggests improvements for future experiments. Furthermore, the enthalpy of reaction is calculated using average bond enthalpies, and these values are compared to experimental results, demonstrating a comprehensive analysis of the heat energy of fuels. Desklib provides access to similar solved assignments and past papers for students.

MEASURING HEAT ENERGY OF FUELS 1
MEASURING HEAT ENERGY OF FUELS
By Name
Course
Instructor
Institution
Location
Date
MEASURING HEAT ENERGY OF FUELS
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

MEASURING HEAT ENERGY OF FUELS 2
ABSTRACT
There are numerous chemical reactions that are visible in our surrounding for example as
combustion. Combustion is like an oxidation reaction since oxygen is allowed to burn and in the
process, it combines with the substance being burnt. The substance will hence get oxidized in the
process while the oxygen is reduced to a different substance. For instance, combustion of
methanol leads to the formation of water vapor and carbon dioxide gas as in the below results.
All combustion reactions are exothermic reaction since it releases a large amount of energy
during the reaction. This energy change which occurs during the reaction is known as the
enthalpy of combustion.
INTRODUCTION
Enthalpy is best understood on the two terms;
• TEMPERATURE
Measures the kinetic energies of molecules present in a substance Independent of the
amount of substance present
• HEAT
A measure of the total energy of a substance, it depends on the amount of substance
present. For instance.A bucket full of water at 500C would have the same temperature as
a 250ml beaker of water at the same temperature, but the heat content of the bucket
would be bigger. And a bucket of oil and a bucket of water at 50ºC would have the same
temperature, but the heat content of the bucket of oil would be bigger.
ABSTRACT
There are numerous chemical reactions that are visible in our surrounding for example as
combustion. Combustion is like an oxidation reaction since oxygen is allowed to burn and in the
process, it combines with the substance being burnt. The substance will hence get oxidized in the
process while the oxygen is reduced to a different substance. For instance, combustion of
methanol leads to the formation of water vapor and carbon dioxide gas as in the below results.
All combustion reactions are exothermic reaction since it releases a large amount of energy
during the reaction. This energy change which occurs during the reaction is known as the
enthalpy of combustion.
INTRODUCTION
Enthalpy is best understood on the two terms;
• TEMPERATURE
Measures the kinetic energies of molecules present in a substance Independent of the
amount of substance present
• HEAT
A measure of the total energy of a substance, it depends on the amount of substance
present. For instance.A bucket full of water at 500C would have the same temperature as
a 250ml beaker of water at the same temperature, but the heat content of the bucket
would be bigger. And a bucket of oil and a bucket of water at 50ºC would have the same
temperature, but the heat content of the bucket of oil would be bigger.

MEASURING HEAT ENERGY OF FUELS 3
Therefore we can define enthalpy of combustion as the amount of heat released by a complete
combustion (burning between the organic compound and oxygen) of one mole of a substance.
Combustion is always exothermic i.e. the enthalpy for the combustion reaction is negative
(ΔHcombustion is negative). The heat of combustion is defined as a positive value i.e. the heat of
combustion = - ΔHcombustion. The heat of combustion can be measured experimentally (Tremaine,
2012).
The molar enthalpy change can always be calculated (Sato, 2014). A simple method to calculate
the enthalpy change of a reaction is to measure the temperature change caused by the reaction.
ΔH = m cp
ΔT……………………………………………………………………………………………1
ΔH = the enthalpy of the reaction
m = the mass of the sample which changes the temperature
Cp = the heat capacity of the substance which changes temperature, the heat capacity measures
how much energy is required to change the temperature of 1 g of the substance by 1 oC, the heat
capacity differs from substance to substance, water=4.2 J/g oC or ethanol=2.4 J/g oC
ΔT = temperature
This will give the energy in kJ released during the reaction to calculate the molar
enthalpy change, kJ mol-1, divide by the number of moles used in the reaction (Wohlfarth, 2016).
The experimental setup can be shown in the diagram below;
Therefore we can define enthalpy of combustion as the amount of heat released by a complete
combustion (burning between the organic compound and oxygen) of one mole of a substance.
Combustion is always exothermic i.e. the enthalpy for the combustion reaction is negative
(ΔHcombustion is negative). The heat of combustion is defined as a positive value i.e. the heat of
combustion = - ΔHcombustion. The heat of combustion can be measured experimentally (Tremaine,
2012).
The molar enthalpy change can always be calculated (Sato, 2014). A simple method to calculate
the enthalpy change of a reaction is to measure the temperature change caused by the reaction.
ΔH = m cp
ΔT……………………………………………………………………………………………1
ΔH = the enthalpy of the reaction
m = the mass of the sample which changes the temperature
Cp = the heat capacity of the substance which changes temperature, the heat capacity measures
how much energy is required to change the temperature of 1 g of the substance by 1 oC, the heat
capacity differs from substance to substance, water=4.2 J/g oC or ethanol=2.4 J/g oC
ΔT = temperature
This will give the energy in kJ released during the reaction to calculate the molar
enthalpy change, kJ mol-1, divide by the number of moles used in the reaction (Wohlfarth, 2016).
The experimental setup can be shown in the diagram below;
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
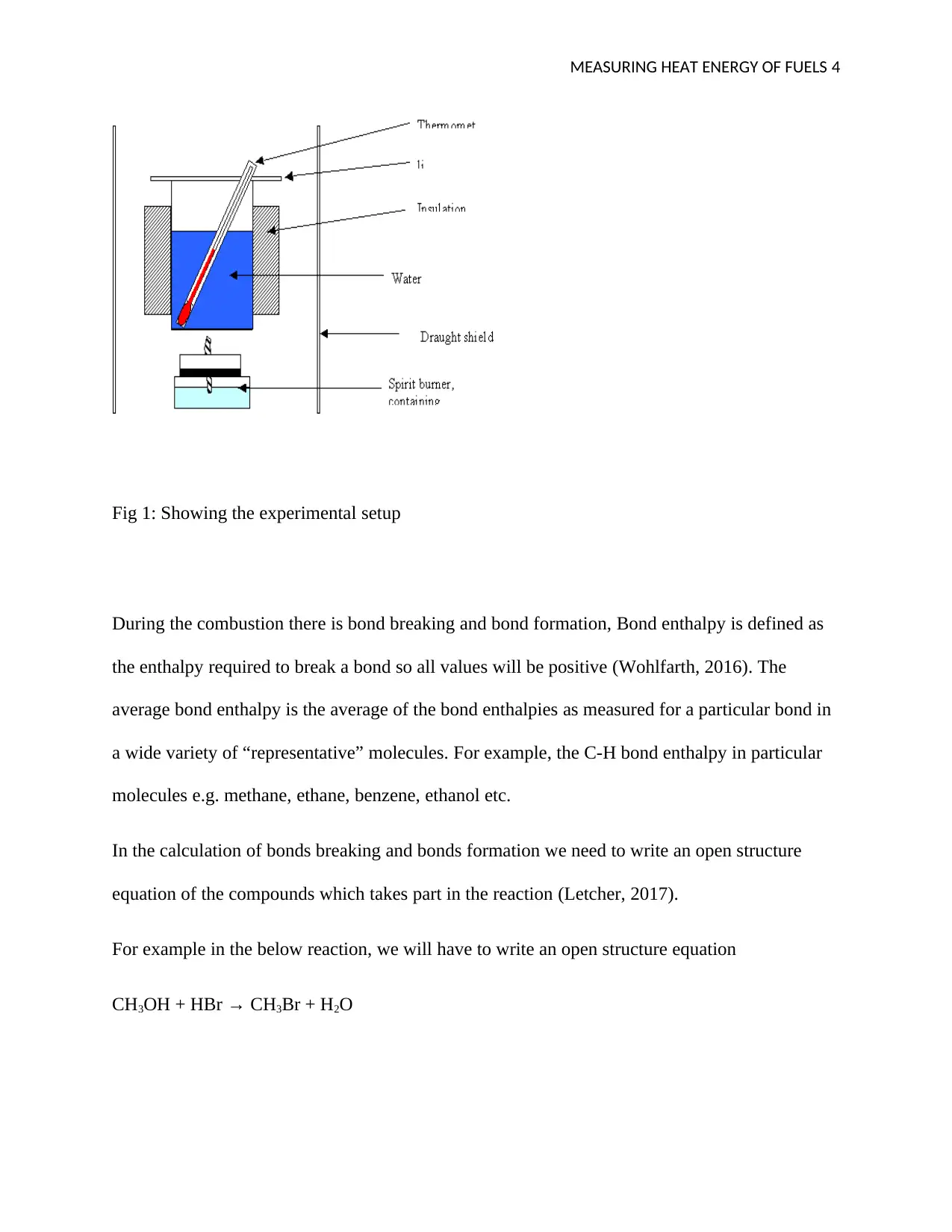
MEASURING HEAT ENERGY OF FUELS 4
Fig 1: Showing the experimental setup
During the combustion there is bond breaking and bond formation, Bond enthalpy is defined as
the enthalpy required to break a bond so all values will be positive (Wohlfarth, 2016). The
average bond enthalpy is the average of the bond enthalpies as measured for a particular bond in
a wide variety of “representative” molecules. For example, the C-H bond enthalpy in particular
molecules e.g. methane, ethane, benzene, ethanol etc.
In the calculation of bonds breaking and bonds formation we need to write an open structure
equation of the compounds which takes part in the reaction (Letcher, 2017).
For example in the below reaction, we will have to write an open structure equation
CH3OH + HBr → CH3Br + H2O
Fig 1: Showing the experimental setup
During the combustion there is bond breaking and bond formation, Bond enthalpy is defined as
the enthalpy required to break a bond so all values will be positive (Wohlfarth, 2016). The
average bond enthalpy is the average of the bond enthalpies as measured for a particular bond in
a wide variety of “representative” molecules. For example, the C-H bond enthalpy in particular
molecules e.g. methane, ethane, benzene, ethanol etc.
In the calculation of bonds breaking and bonds formation we need to write an open structure
equation of the compounds which takes part in the reaction (Letcher, 2017).
For example in the below reaction, we will have to write an open structure equation
CH3OH + HBr → CH3Br + H2O
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

MEASURING HEAT ENERGY OF FUELS 5
H H
l l
H- C - O-H + H - Br -> H- C - Br + H - O - H
l l
H H
METHODS
A suitable table was prepared to record materials used during the experiment and the
results obtained after conducting the experiment (Tsao, 2012). The conical flask was clamped at
a suitable height to allow room for the spirit burner to be placed below it. A gap of 2.5cm was
allowed between the base of the conical flask and the top of the spirit burner. The gap allowed
may need to be adjusted depending on the height of the flame. A cold water of 100cm3 was
measured into the conical flask using the measuring cylinder. The initial temperature of water in
the conical flask was measured using a thermometer (Mbadi, 2010). The sprite burner (with cap)
having alcohol was weighted and its result was recorded as initial mass and it was named
alcohol. The spirit burner was placed on the heat- resistant mat under the conical flask, the cap
was removed and the wick was the light.
The alcohol was allowed to heat the water, therefore, the temperature rose by about
400C. A glass rod or a thermometer is used to stir the water gently while the alcohol burns. The
cap on the spirit burner was replaced to extinguish the flame (Ahmed, 2011). The final
temperature of the water using thermometer was recorded. The temperature change was worked
out. The spirit burner and the cap were weighed and the final mass was recorded. The mass of
alcohol used was worked out. The experiment for different alcohols using 100cm3 of fresh cold
H H
l l
H- C - O-H + H - Br -> H- C - Br + H - O - H
l l
H H
METHODS
A suitable table was prepared to record materials used during the experiment and the
results obtained after conducting the experiment (Tsao, 2012). The conical flask was clamped at
a suitable height to allow room for the spirit burner to be placed below it. A gap of 2.5cm was
allowed between the base of the conical flask and the top of the spirit burner. The gap allowed
may need to be adjusted depending on the height of the flame. A cold water of 100cm3 was
measured into the conical flask using the measuring cylinder. The initial temperature of water in
the conical flask was measured using a thermometer (Mbadi, 2010). The sprite burner (with cap)
having alcohol was weighted and its result was recorded as initial mass and it was named
alcohol. The spirit burner was placed on the heat- resistant mat under the conical flask, the cap
was removed and the wick was the light.
The alcohol was allowed to heat the water, therefore, the temperature rose by about
400C. A glass rod or a thermometer is used to stir the water gently while the alcohol burns. The
cap on the spirit burner was replaced to extinguish the flame (Ahmed, 2011). The final
temperature of the water using thermometer was recorded. The temperature change was worked
out. The spirit burner and the cap were weighed and the final mass was recorded. The mass of
alcohol used was worked out. The experiment for different alcohols using 100cm3 of fresh cold

MEASURING HEAT ENERGY OF FUELS 6
water each time was repeated. The experiment for each alcohol was repeated at least twice. The
amount of energy transferred to the water from the burning alcohol was calculated.
Equipment and materials needed
Balance
Stand, clamp, and boss
Conical flask (250cm3)
Measuring cylinder (100cm3)
Spirit burner with cap
Stirring thermometer (0-1100c)
Glass rod
Heat resistant mat
Matches
Methanol – highly flammable, toxic
Ethanol- Highly flammable
Propanol – highly flammable, an irritant.
RESULT
water each time was repeated. The experiment for each alcohol was repeated at least twice. The
amount of energy transferred to the water from the burning alcohol was calculated.
Equipment and materials needed
Balance
Stand, clamp, and boss
Conical flask (250cm3)
Measuring cylinder (100cm3)
Spirit burner with cap
Stirring thermometer (0-1100c)
Glass rod
Heat resistant mat
Matches
Methanol – highly flammable, toxic
Ethanol- Highly flammable
Propanol – highly flammable, an irritant.
RESULT
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
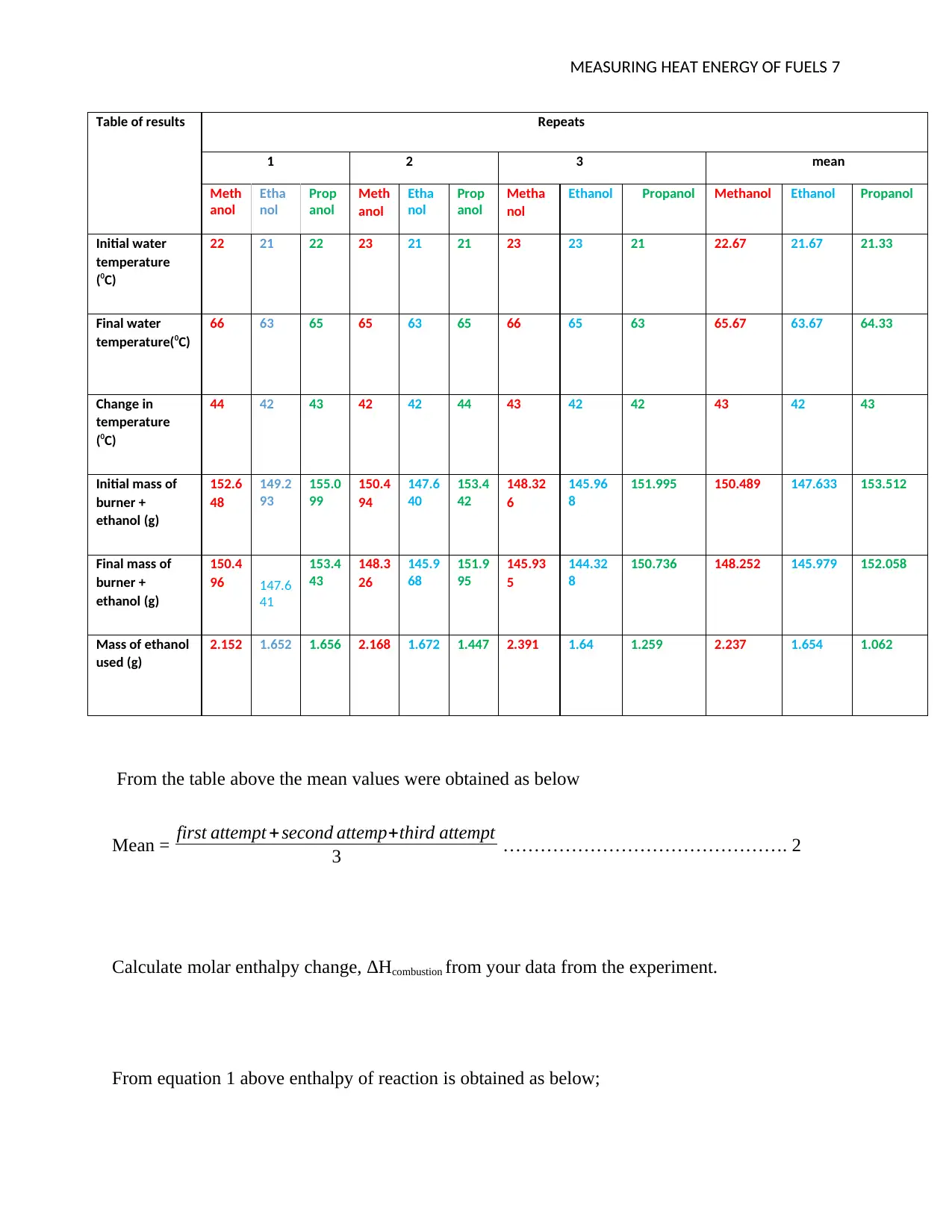
MEASURING HEAT ENERGY OF FUELS 7
Table of results Repeats
1 2 3 mean
Meth
anol
Etha
nol
Prop
anol
Meth
anol
Etha
nol
Prop
anol
Metha
nol
Ethanol Propanol Methanol Ethanol Propanol
Initial water
temperature
(0C)
22 21 22 23 21 21 23 23 21 22.67 21.67 21.33
Final water
temperature(0C)
66 63 65 65 63 65 66 65 63 65.67 63.67 64.33
Change in
temperature
(0C)
44 42 43 42 42 44 43 42 42 43 42 43
Initial mass of
burner +
ethanol (g)
152.6
48
149.2
93
155.0
99
150.4
94
147.6
40
153.4
42
148.32
6
145.96
8
151.995 150.489 147.633 153.512
Final mass of
burner +
ethanol (g)
150.4
96 147.6
41
153.4
43
148.3
26
145.9
68
151.9
95
145.93
5
144.32
8
150.736 148.252 145.979 152.058
Mass of ethanol
used (g)
2.152 1.652 1.656 2.168 1.672 1.447 2.391 1.64 1.259 2.237 1.654 1.062
From the table above the mean values were obtained as below
Mean = first attempt +second attemp+third attempt
3 ………………………………………. 2
Calculate molar enthalpy change, ΔHcombustion from your data from the experiment.
From equation 1 above enthalpy of reaction is obtained as below;
Table of results Repeats
1 2 3 mean
Meth
anol
Etha
nol
Prop
anol
Meth
anol
Etha
nol
Prop
anol
Metha
nol
Ethanol Propanol Methanol Ethanol Propanol
Initial water
temperature
(0C)
22 21 22 23 21 21 23 23 21 22.67 21.67 21.33
Final water
temperature(0C)
66 63 65 65 63 65 66 65 63 65.67 63.67 64.33
Change in
temperature
(0C)
44 42 43 42 42 44 43 42 42 43 42 43
Initial mass of
burner +
ethanol (g)
152.6
48
149.2
93
155.0
99
150.4
94
147.6
40
153.4
42
148.32
6
145.96
8
151.995 150.489 147.633 153.512
Final mass of
burner +
ethanol (g)
150.4
96 147.6
41
153.4
43
148.3
26
145.9
68
151.9
95
145.93
5
144.32
8
150.736 148.252 145.979 152.058
Mass of ethanol
used (g)
2.152 1.652 1.656 2.168 1.672 1.447 2.391 1.64 1.259 2.237 1.654 1.062
From the table above the mean values were obtained as below
Mean = first attempt +second attemp+third attempt
3 ………………………………………. 2
Calculate molar enthalpy change, ΔHcombustion from your data from the experiment.
From equation 1 above enthalpy of reaction is obtained as below;
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

MEASURING HEAT ENERGY OF FUELS 8
ΔH = m cp ΔT
Enthalpy for methanol
m= 2.237g
ΔT= 43
Cp= 2.51 J/g oC
ΔH = 2.237×2.237×43
ΔH= - 215.179 J ( since it is heat released).
Molar enthalpy hence is given as
Heat produced by burning 1 mol of methanol
Moles of methanol= Mass
molar mass …………………………………………………………..2
Moles of methanol= 2.237
32 = 0.0699 moles
Molar enthalpy = −215.179
0.0699
Molar enthalpy= -976.38kjmol-1
The literature value of molar enthalpy of methanol is 998kJ/mol
ΔH = m cp ΔT
Enthalpy for methanol
m= 2.237g
ΔT= 43
Cp= 2.51 J/g oC
ΔH = 2.237×2.237×43
ΔH= - 215.179 J ( since it is heat released).
Molar enthalpy hence is given as
Heat produced by burning 1 mol of methanol
Moles of methanol= Mass
molar mass …………………………………………………………..2
Moles of methanol= 2.237
32 = 0.0699 moles
Molar enthalpy = −215.179
0.0699
Molar enthalpy= -976.38kjmol-1
The literature value of molar enthalpy of methanol is 998kJ/mol

MEASURING HEAT ENERGY OF FUELS 9
Error= 998−976
998 × 100
Error= 2.2%
Enthalpy for ethanol
m= 1.654g
ΔT= 420C
Cp= 2.4 J/g oC
ΔH = 1.654×2.4×42
ΔH= - 166.7232J ( since it is heat released).
Molar enthalpy hence is given as
Heat produced by burning 1 mol of ethanol
Moles of ethanol= Mass
molar mass
Moles of ethanol= 1.654
46 = 0.035956 moles
Molar enthalpy = −166.723
0.035956
Error= 998−976
998 × 100
Error= 2.2%
Enthalpy for ethanol
m= 1.654g
ΔT= 420C
Cp= 2.4 J/g oC
ΔH = 1.654×2.4×42
ΔH= - 166.7232J ( since it is heat released).
Molar enthalpy hence is given as
Heat produced by burning 1 mol of ethanol
Moles of ethanol= Mass
molar mass
Moles of ethanol= 1.654
46 = 0.035956 moles
Molar enthalpy = −166.723
0.035956
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

MEASURING HEAT ENERGY OF FUELS 10
Molar enthalpy= -1223.2 jmol-1
The literature value of molar enthalpy of ethanol is 1289kJ/mol (Rao, 2010).
Error= 1289−1223.2
1289 × 100
Error= 5.1%
Enthalpy for propanol
m= 1.062g
ΔT= 43
Cp= 2.46 J/g oC
ΔH = 1.0627×2.46×43
ΔH= - 112.338 J (since it is heat released).
Molar enthalpy hence is given as
Heat produced by burning 1 mol of propanol
Moles of propanol = Mass
molar mass
Moles of propanol = 1.062
60 = 0.0177 moles
Molar enthalpy = −112.338
0.0177
Molar enthalpy= -1223.2 jmol-1
The literature value of molar enthalpy of ethanol is 1289kJ/mol (Rao, 2010).
Error= 1289−1223.2
1289 × 100
Error= 5.1%
Enthalpy for propanol
m= 1.062g
ΔT= 43
Cp= 2.46 J/g oC
ΔH = 1.0627×2.46×43
ΔH= - 112.338 J (since it is heat released).
Molar enthalpy hence is given as
Heat produced by burning 1 mol of propanol
Moles of propanol = Mass
molar mass
Moles of propanol = 1.062
60 = 0.0177 moles
Molar enthalpy = −112.338
0.0177
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

MEASURING HEAT ENERGY OF FUELS 11
Molar enthalpy= -1316.77kJmol-1
The literature value of molar enthalpy of propanol is 1350kJ/mol
Error= 1350−1316.77
1350 × 100
Error= 2.46%
Calculation of reaction enthalpy from average bond enthalpies and compare these to your values
Methanol
Obtaining the reaction enthalpy from bond breaking and formation
CH3OH+O2 CO2 + 2 H2O
Therefore the bonds broken are
3 (H-C)+ C-O + O-H + O=O
3(413) + 258 +463 + 495
1239 + 258+463+495
2455
Bonds formed are
Molar enthalpy= -1316.77kJmol-1
The literature value of molar enthalpy of propanol is 1350kJ/mol
Error= 1350−1316.77
1350 × 100
Error= 2.46%
Calculation of reaction enthalpy from average bond enthalpies and compare these to your values
Methanol
Obtaining the reaction enthalpy from bond breaking and formation
CH3OH+O2 CO2 + 2 H2O
Therefore the bonds broken are
3 (H-C)+ C-O + O-H + O=O
3(413) + 258 +463 + 495
1239 + 258+463+495
2455
Bonds formed are

MEASURING HEAT ENERGY OF FUELS 12
C¿O + C¿O + 2(O-H +O-H)
799+799+ 2(463+463)
1598+ 1852
3450
Reaction enthalpy
2455-3450
= - 995kJ/Mol
The literature value of molar enthalpy of methanol is 998kJ/mol
Error= 998−995
998 × 100
Error= 0.3%
Ethanol
C2H6O+3 O2 2CO2 +3H2O
Bond broken
5(C-H) + C-C + C-O + O-H + 3(O=O)
5(413)+ 348+ 358 + 463+ 3(495)
2065+1169+1485
C¿O + C¿O + 2(O-H +O-H)
799+799+ 2(463+463)
1598+ 1852
3450
Reaction enthalpy
2455-3450
= - 995kJ/Mol
The literature value of molar enthalpy of methanol is 998kJ/mol
Error= 998−995
998 × 100
Error= 0.3%
Ethanol
C2H6O+3 O2 2CO2 +3H2O
Bond broken
5(C-H) + C-C + C-O + O-H + 3(O=O)
5(413)+ 348+ 358 + 463+ 3(495)
2065+1169+1485
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 17
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





