Heat Transfer and Combustion
VerifiedAdded on 2023/05/29
|10
|2014
|133
AI Summary
This article discusses the balanced chemical equations for fuel components, need for excess air, actual fuel: air ratio, net calorific value, determination of flue gases composition, maximum flame temperature, furnace efficiency, amount of steam produced per hour, and dew point temperature for flue gas pressure.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.
Heat Transfer and Combustion Student Code: Tutor Marked Assignment
1.
(a) Balanced chemical equations for the fuel components.
Butane, C4 H10 C4 H10+6.5 02 → 4 CO2+ 5 H2 O
Propane, C3 H8
C3 H8 +5 O2 → 3 CO2 +4 H2 O
Butene,C4 H8
C4 H8+ 6 O2 → 4 CO2+4 H2 O
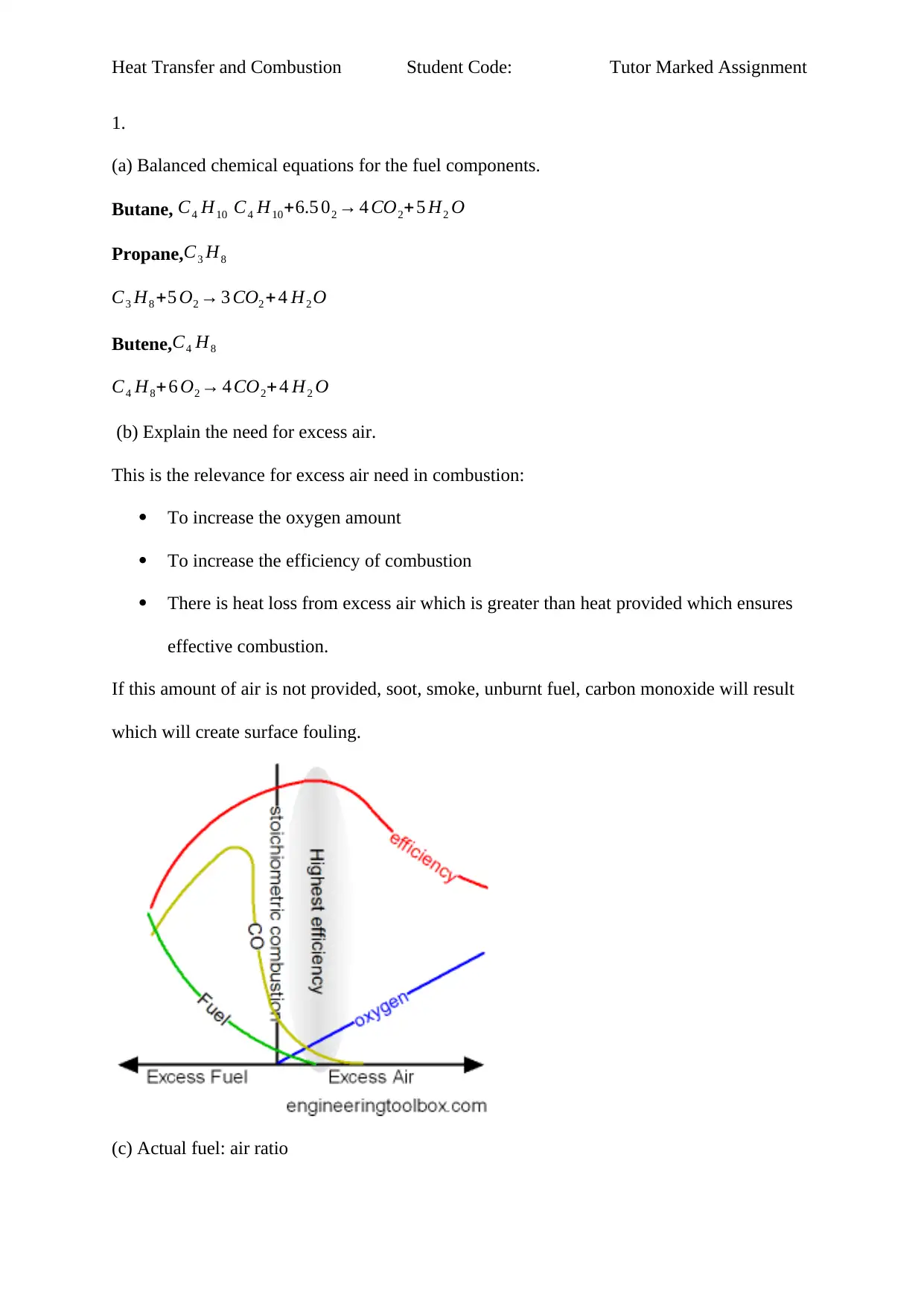
(b) Explain the need for excess air.
This is the relevance for excess air need in combustion:
To increase the oxygen amount
To increase the efficiency of combustion
There is heat loss from excess air which is greater than heat provided which ensures
effective combustion.
If this amount of air is not provided, soot, smoke, unburnt fuel, carbon monoxide will result
which will create surface fouling.
(c) Actual fuel: air ratio
1.
(a) Balanced chemical equations for the fuel components.
Butane, C4 H10 C4 H10+6.5 02 → 4 CO2+ 5 H2 O
Propane, C3 H8
C3 H8 +5 O2 → 3 CO2 +4 H2 O
Butene,C4 H8
C4 H8+ 6 O2 → 4 CO2+4 H2 O
(b) Explain the need for excess air.
This is the relevance for excess air need in combustion:
To increase the oxygen amount
To increase the efficiency of combustion
There is heat loss from excess air which is greater than heat provided which ensures
effective combustion.
If this amount of air is not provided, soot, smoke, unburnt fuel, carbon monoxide will result
which will create surface fouling.
(c) Actual fuel: air ratio
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Heat Transfer and Combustion Student Code: Tutor Marked Assignment
(i) By volume.
6.9025(1+3.76)
1
FAr =32.86
(ii) By mass.
0.75 ( 4∗12+10 )+¿ ¿ 0.75∗58+0.1∗44+0.15∗56
6.9025 ( 137.28 ) FAr=0.0594
(d) Calculate: (i) The net calorific value/m3
From the calorific value provided for individual components, the net calorific value/ m3 can
be obtained as follows:
Net calorific value= calorific value of butane + calorific value for propane + calorific value of
butene
Calorific value of butane =0.75∗111.7
Calorific value for propane = 0.1∗85.5
Calorific value of butane = 0.15∗105.2
=83.775+8.55+15.78Net calorific value¿ 108.105 MJ/m3
(ii) Net calorific value / kmol of fuel/air mix.
NCV per kilomole = ∑ N P ( hf ° ) p−Nr ( hf ° )r
Where,
N P ( hf ° ) p= calorific value of products
Nr ( hf ° )r= calorific value of reactants
1 mole of the fuel gas = 0.75 moles butane + 0.15 moles butane + 0.1 moles propane
= 0.75 (4 CO2 + 5H2O) +0.1 (3CO2 + 4H2O) + 0.15 (4CO2 + 4H2O)
= 3.9 CO2 + 4.75 H2O
(i) By volume.
6.9025(1+3.76)
1
FAr =32.86
(ii) By mass.
0.75 ( 4∗12+10 )+¿ ¿ 0.75∗58+0.1∗44+0.15∗56
6.9025 ( 137.28 ) FAr=0.0594
(d) Calculate: (i) The net calorific value/m3
From the calorific value provided for individual components, the net calorific value/ m3 can
be obtained as follows:
Net calorific value= calorific value of butane + calorific value for propane + calorific value of
butene
Calorific value of butane =0.75∗111.7
Calorific value for propane = 0.1∗85.5
Calorific value of butane = 0.15∗105.2
=83.775+8.55+15.78Net calorific value¿ 108.105 MJ/m3
(ii) Net calorific value / kmol of fuel/air mix.
NCV per kilomole = ∑ N P ( hf ° ) p−Nr ( hf ° )r
Where,
N P ( hf ° ) p= calorific value of products
Nr ( hf ° )r= calorific value of reactants
1 mole of the fuel gas = 0.75 moles butane + 0.15 moles butane + 0.1 moles propane
= 0.75 (4 CO2 + 5H2O) +0.1 (3CO2 + 4H2O) + 0.15 (4CO2 + 4H2O)
= 3.9 CO2 + 4.75 H2O

Heat Transfer and Combustion Student Code: Tutor Marked Assignment
NCV per kilomole=
[ 3.9 ( hf ° ) CO2 + 4.75 ( hf ° ) H 2 O ] − [ 0.75 ( hf ° ) C 4 H 10 +0.1 ( hf ° ) C 3 H 8 +0.15 ( hf ° ) C 4 H 8 ]
= [ 3.9(−393520)+4.75 (−285820) ] − [ 0.75(−126150)+0.1(−122876)+0.15 (−122000) ]
= 2,667.19 MJ/kMol
(e) Determination of the flue gases composition by volume if the inlet air is dry.
(i) Wet basis
On a wet basis, the composition of the flue gases will be butane, propane and butene’s
combustion products together with excess O2 and N2.
1 mole of the fuel gas produces the following moles of the constituents:
= 0.75 (4 CO2 + 5H2O) +0.1 (3CO2 + 4H2O) + 0.15 (4CO2 + 4H2O) + N2 + O2
Butane C4 H10 C4 H10+1.1∗6.5 02 ( 02 +3.76 N2 ) → 4 CO2 +5 H2 O+0.6 5 02 +26.88 N2
Propane C3 H8
C3 H8 +1.1∗5 02 ( 02+ 3.76 N 2 ) → 3 CO2 +4 H2 0+ 0.5 02 +20 . 6 8 N2
Butene C4 H8
C4 H8+ 1.1∗6 02 ( 02 +3.76 N2 ) → 4 CO2+ 4 H2 +0. 6 02 +24.82 N 2
Overall combustion reaction becomes
0.75 C4 H 10+0.1 C3 H8 +0.15 C4 H8+ 6.903 ¿
The volume yield = 3.9 CO2 +4.75 H2 O+0.64 5 02 +26.03 N2
(ii) Dry basis
On a dry basis, carbon dioxide, nitrogen and oxygen will be present in the following amount:
= 3.9 CO2 +0.64 5 02+26.03 N2
(f) Determination of the maximum flame temperature.
Butane C4 H10 C4 H10+6.5 02 → 4 CO2+ 5 H2 O+24.5 N 2
Propane C3 H8
NCV per kilomole=
[ 3.9 ( hf ° ) CO2 + 4.75 ( hf ° ) H 2 O ] − [ 0.75 ( hf ° ) C 4 H 10 +0.1 ( hf ° ) C 3 H 8 +0.15 ( hf ° ) C 4 H 8 ]
= [ 3.9(−393520)+4.75 (−285820) ] − [ 0.75(−126150)+0.1(−122876)+0.15 (−122000) ]
= 2,667.19 MJ/kMol
(e) Determination of the flue gases composition by volume if the inlet air is dry.
(i) Wet basis
On a wet basis, the composition of the flue gases will be butane, propane and butene’s
combustion products together with excess O2 and N2.
1 mole of the fuel gas produces the following moles of the constituents:
= 0.75 (4 CO2 + 5H2O) +0.1 (3CO2 + 4H2O) + 0.15 (4CO2 + 4H2O) + N2 + O2
Butane C4 H10 C4 H10+1.1∗6.5 02 ( 02 +3.76 N2 ) → 4 CO2 +5 H2 O+0.6 5 02 +26.88 N2
Propane C3 H8
C3 H8 +1.1∗5 02 ( 02+ 3.76 N 2 ) → 3 CO2 +4 H2 0+ 0.5 02 +20 . 6 8 N2
Butene C4 H8
C4 H8+ 1.1∗6 02 ( 02 +3.76 N2 ) → 4 CO2+ 4 H2 +0. 6 02 +24.82 N 2
Overall combustion reaction becomes
0.75 C4 H 10+0.1 C3 H8 +0.15 C4 H8+ 6.903 ¿
The volume yield = 3.9 CO2 +4.75 H2 O+0.64 5 02 +26.03 N2
(ii) Dry basis
On a dry basis, carbon dioxide, nitrogen and oxygen will be present in the following amount:
= 3.9 CO2 +0.64 5 02+26.03 N2
(f) Determination of the maximum flame temperature.
Butane C4 H10 C4 H10+6.5 02 → 4 CO2+ 5 H2 O+24.5 N 2
Propane C3 H8
Heat Transfer and Combustion Student Code: Tutor Marked Assignment
C3 H8 +5 O2 → 3 CO2 +4 H2 0+18.8 N 2
Butene C4 H8
C4 H8+ 6 O2 → 4 CO2+4 H2 +22.69 N2
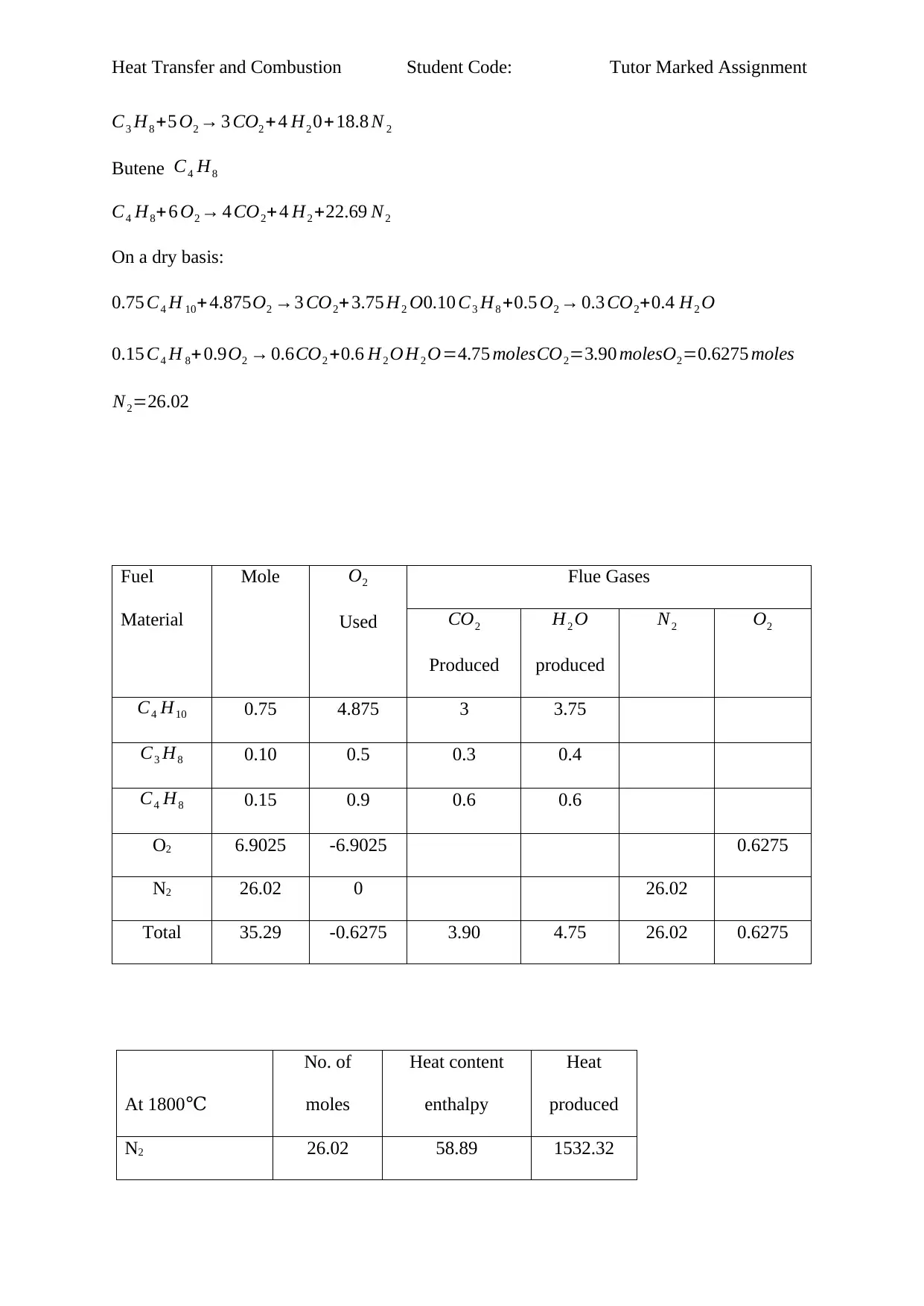
On a dry basis:
0.75 C4 H 10+ 4.875O2 →3 CO2+ 3.75 H2 O0.10 C3 H8 +0.5 O2 → 0.3 CO2+0.4 H2 O
0.15 C4 H 8+ 0.9O2 → 0.6CO2 +0.6 H2 O H2 O=4.75 molesCO2=3.90 molesO2=0.6275 moles
N2=26.02
Fuel
Material
Mole O2
Used
Flue Gases
CO2
Produced
H2 O
produced
N2 O2
C4 H10 0.75 4.875 3 3.75
C3 H8 0.10 0.5 0.3 0.4
C4 H8 0.15 0.9 0.6 0.6
O2 6.9025 -6.9025 0.6275
N2 26.02 0 26.02
Total 35.29 -0.6275 3.90 4.75 26.02 0.6275
At 1800℃
No. of
moles
Heat content
enthalpy
Heat
produced
N2 26.02 58.89 1532.32
C3 H8 +5 O2 → 3 CO2 +4 H2 0+18.8 N 2
Butene C4 H8
C4 H8+ 6 O2 → 4 CO2+4 H2 +22.69 N2
On a dry basis:
0.75 C4 H 10+ 4.875O2 →3 CO2+ 3.75 H2 O0.10 C3 H8 +0.5 O2 → 0.3 CO2+0.4 H2 O
0.15 C4 H 8+ 0.9O2 → 0.6CO2 +0.6 H2 O H2 O=4.75 molesCO2=3.90 molesO2=0.6275 moles
N2=26.02
Fuel
Material
Mole O2
Used
Flue Gases
CO2
Produced
H2 O
produced
N2 O2
C4 H10 0.75 4.875 3 3.75
C3 H8 0.10 0.5 0.3 0.4
C4 H8 0.15 0.9 0.6 0.6
O2 6.9025 -6.9025 0.6275
N2 26.02 0 26.02
Total 35.29 -0.6275 3.90 4.75 26.02 0.6275
At 1800℃
No. of
moles
Heat content
enthalpy
Heat
produced
N2 26.02 58.89 1532.32
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
Heat Transfer and Combustion Student Code: Tutor Marked Assignment
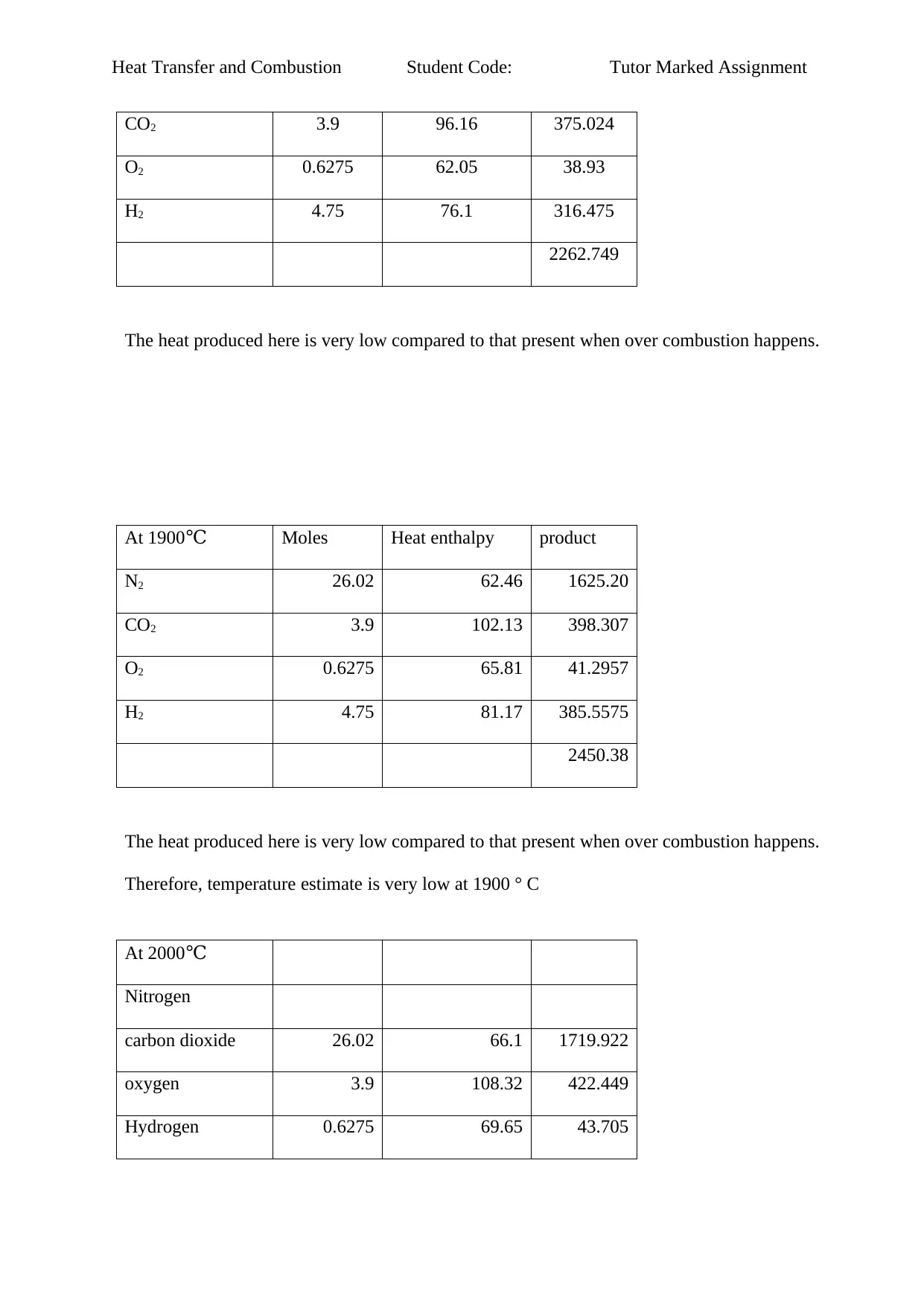
CO2 3.9 96.16 375.024
O2 0.6275 62.05 38.93
H2 4.75 76.1 316.475
2262.749
The heat produced here is very low compared to that present when over combustion happens.
At 1900℃ Moles Heat enthalpy product
N2 26.02 62.46 1625.20
CO2 3.9 102.13 398.307
O2 0.6275 65.81 41.2957
H2 4.75 81.17 385.5575
2450.38
The heat produced here is very low compared to that present when over combustion happens.
Therefore, temperature estimate is very low at 1900 ° C
At 2000℃
Nitrogen
carbon dioxide 26.02 66.1 1719.922
oxygen 3.9 108.32 422.449
Hydrogen 0.6275 69.65 43.705
CO2 3.9 96.16 375.024
O2 0.6275 62.05 38.93
H2 4.75 76.1 316.475
2262.749
The heat produced here is very low compared to that present when over combustion happens.
At 1900℃ Moles Heat enthalpy product
N2 26.02 62.46 1625.20
CO2 3.9 102.13 398.307
O2 0.6275 65.81 41.2957
H2 4.75 81.17 385.5575
2450.38
The heat produced here is very low compared to that present when over combustion happens.
Therefore, temperature estimate is very low at 1900 ° C
At 2000℃
Nitrogen
carbon dioxide 26.02 66.1 1719.922
oxygen 3.9 108.32 422.449
Hydrogen 0.6275 69.65 43.705
Heat Transfer and Combustion Student Code: Tutor Marked Assignment
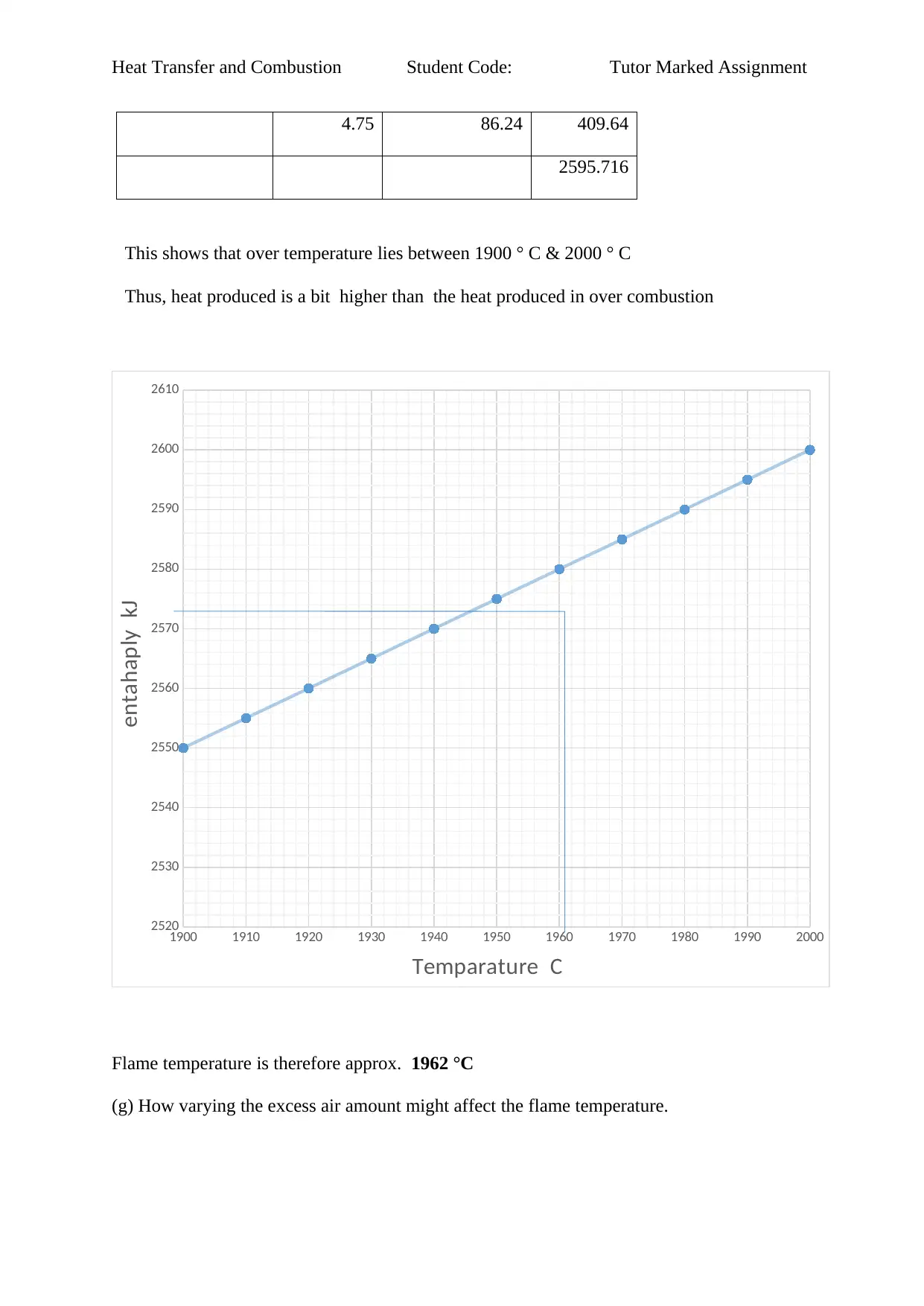
4.75 86.24 409.64
2595.716
This shows that over temperature lies between 1900 ° C & 2000 ° C
Thus, heat produced is a bit higher than the heat produced in over combustion
1900 1910 1920 1930 1940 1950 1960 1970 1980 1990 2000
2520
2530
2540
2550
2560
2570
2580
2590
2600
2610
Temparature C
entahaply kJ
Flame temperature is therefore approx. 1962 °C
(g) How varying the excess air amount might affect the flame temperature.
4.75 86.24 409.64
2595.716
This shows that over temperature lies between 1900 ° C & 2000 ° C
Thus, heat produced is a bit higher than the heat produced in over combustion
1900 1910 1920 1930 1940 1950 1960 1970 1980 1990 2000
2520
2530
2540
2550
2560
2570
2580
2590
2600
2610
Temparature C
entahaply kJ
Flame temperature is therefore approx. 1962 °C
(g) How varying the excess air amount might affect the flame temperature.
Heat Transfer and Combustion Student Code: Tutor Marked Assignment
Increasing the amount of excess air, there will be a large surface area to volume ratio
meaning that combustion efficiency is increased thus high flame temperature. When the air is
too little, the fuel will be lacking oxygen hence incomplete combustion to water and carbon
dioxide hence less energy will be released (lower heat of combustion). Flame temperature
will be reduced through this process. Heat losses to the surroundings may also reduce the
flame temperature.
(h) ‘Furnace efficiency’ if the exit temperature of flue gases is 300ºC from the boiler.
For the gases exiting the furnace at 300 °C, their heating values are:
Flue gases No. of Moles Enthalpy at 300 °C Heating value
CO2 3.9 26.61 103.779
O2 0.645 18.33 11.823
H2O 4.75 20.89 99.23
N2 26.03 17.4 452.92
TOTAL 667.75
The heating value = 667.75 MJ/Kg-Mol
Therefore the furnace efficiency= heating value
net calorific value∗100
= 667.75
2667.19∗100
Furnace efficiency = 25.04 %
(i) Amount of steam produced per hour
The heat available from the flue gases is the net calorific value =2667.19 MJ/k-Mol
Heat used to make steam is 95 % since 5% is lost
=0.95*2667.19
=2533.8 MJ/k-Mol
Increasing the amount of excess air, there will be a large surface area to volume ratio
meaning that combustion efficiency is increased thus high flame temperature. When the air is
too little, the fuel will be lacking oxygen hence incomplete combustion to water and carbon
dioxide hence less energy will be released (lower heat of combustion). Flame temperature
will be reduced through this process. Heat losses to the surroundings may also reduce the
flame temperature.
(h) ‘Furnace efficiency’ if the exit temperature of flue gases is 300ºC from the boiler.
For the gases exiting the furnace at 300 °C, their heating values are:
Flue gases No. of Moles Enthalpy at 300 °C Heating value
CO2 3.9 26.61 103.779
O2 0.645 18.33 11.823
H2O 4.75 20.89 99.23
N2 26.03 17.4 452.92
TOTAL 667.75
The heating value = 667.75 MJ/Kg-Mol
Therefore the furnace efficiency= heating value
net calorific value∗100
= 667.75
2667.19∗100
Furnace efficiency = 25.04 %
(i) Amount of steam produced per hour
The heat available from the flue gases is the net calorific value =2667.19 MJ/k-Mol
Heat used to make steam is 95 % since 5% is lost
=0.95*2667.19
=2533.8 MJ/k-Mol
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Heat Transfer and Combustion Student Code: Tutor Marked Assignment
At 5 bars, the steam enthalpy= 49.464 MJ/k-Mol
At 90°C, the saturated enthalpy= 6.93 MJ/k-Mol
Heat utilized in generating steam= 49.464 - 6.93
=42.53 MJ/k-Mol
The flow rate of the flue gases= 1400 k-Mol/hr
Amount of steam produced hourly= (heat provided/heat consumed)
= (1400¿¿ 42.53)∗2533.8 ¿
=~83,407 kg/hr.
(j) The dew point temperature when the flue gas pressure is one bar & the inlet air:
(i) is dry
The moles of the constituents in the reaction are as follows:
3.90 mole CO24.75 mole H 2 O
26.02 mole N20.6275 mole O2
Total mole of fuel =26.02 + 3.9 + 4.75 + 0.6275
=35.30
molar fraction= mole H2 O
total moles % mole= mols .Water
total mols ∗100
These will give the flue gases composition as % mole and molar fraction respectively.
% mole of H2 O= mols H2 O
total mols .¿ 4.75
35.30∗100
¿ 13.45 %molar fraction= 13.45
100 =0.1345
Partial pressure = molar fraction * total pressure provided
The pressure provided is 1 bar, so;
∂ pressure= ( mole fraction ) H 2 O∗total pressure¿ 0.1345∗1¿ 0.1345
At 5 bars, the steam enthalpy= 49.464 MJ/k-Mol
At 90°C, the saturated enthalpy= 6.93 MJ/k-Mol
Heat utilized in generating steam= 49.464 - 6.93
=42.53 MJ/k-Mol
The flow rate of the flue gases= 1400 k-Mol/hr
Amount of steam produced hourly= (heat provided/heat consumed)
= (1400¿¿ 42.53)∗2533.8 ¿
=~83,407 kg/hr.
(j) The dew point temperature when the flue gas pressure is one bar & the inlet air:
(i) is dry
The moles of the constituents in the reaction are as follows:
3.90 mole CO24.75 mole H 2 O
26.02 mole N20.6275 mole O2
Total mole of fuel =26.02 + 3.9 + 4.75 + 0.6275
=35.30
molar fraction= mole H2 O
total moles % mole= mols .Water
total mols ∗100
These will give the flue gases composition as % mole and molar fraction respectively.
% mole of H2 O= mols H2 O
total mols .¿ 4.75
35.30∗100
¿ 13.45 %molar fraction= 13.45
100 =0.1345
Partial pressure = molar fraction * total pressure provided
The pressure provided is 1 bar, so;
∂ pressure= ( mole fraction ) H 2 O∗total pressure¿ 0.1345∗1¿ 0.1345

Heat Transfer and Combustion Student Code: Tutor Marked Assignment
The dew points read from the charts of water vapour = 53 °C
(ii) 0.8 kg water per kmol of air.
Actual amount of air in the intake = 32.945 kg mole
Hence water present = 0.8*32.945
=26.356 kg
Actual kg-mole of water is increased proportionately with this amount
26.356 kg/molar mass of water
26.356 kg/18.0
= 1.46 kg-mole
Total water vapour = 1.46 + 4.75 = 6.21 kg-mole
= (6.21/ 3.9 + 4.75 + 0.645 + 26.03) + 1.46
= 16.9% by volume
The dew point read from the charts = 58°C
(k) At 300 °C, water has completely turned into steam, the water federate would be:
=enthalpy of vaporization (5bar) + 5 bar + liquid heat (90 °C)
= 2107 + 2.32 + 4.208
=2113 kg/hr
But heat content * flow rate of the flue gases = preheat sum/0.9 * flow rate (flue gases)..eqn 1
=2113*4.2*62
=555 kilojoules/hr.
= 31 kJ
kMol
0.9 ∗1400 kMol /hr
=48.2 kilojoules/hr.
Equating the two as per equation above
= 555
48.2 =11.4
The dew points read from the charts of water vapour = 53 °C
(ii) 0.8 kg water per kmol of air.
Actual amount of air in the intake = 32.945 kg mole
Hence water present = 0.8*32.945
=26.356 kg
Actual kg-mole of water is increased proportionately with this amount
26.356 kg/molar mass of water
26.356 kg/18.0
= 1.46 kg-mole
Total water vapour = 1.46 + 4.75 = 6.21 kg-mole
= (6.21/ 3.9 + 4.75 + 0.645 + 26.03) + 1.46
= 16.9% by volume
The dew point read from the charts = 58°C
(k) At 300 °C, water has completely turned into steam, the water federate would be:
=enthalpy of vaporization (5bar) + 5 bar + liquid heat (90 °C)
= 2107 + 2.32 + 4.208
=2113 kg/hr
But heat content * flow rate of the flue gases = preheat sum/0.9 * flow rate (flue gases)..eqn 1
=2113*4.2*62
=555 kilojoules/hr.
= 31 kJ
kMol
0.9 ∗1400 kMol /hr
=48.2 kilojoules/hr.
Equating the two as per equation above
= 555
48.2 =11.4

Heat Transfer and Combustion Student Code: Tutor Marked Assignment
11.4Mw = 300- Tf
Outlet temperature, (Tf) = 300-11.4(Mw) where,
Mass of water, Mw in kg per kg-mol will be proportional to outlet temperature Tf
Dew point will be achieved for the two cases since the preheat temperature gets to 90 °C at 5
bars hence it cannot evaporate.
(l) 2 advantages and one disadvantage of preheating the water in this manner.
Advantages
High overall efficiency of heat is maintained since the latent heat of vaporisation is
recovered from the water vapour.
This practice has environmental benefits since the toxic nature of flue gases is
reduced through the combustion and the preheating process.
Disadvantages
At such a temperature, the repair and maintenances of the stand pipes will have to be
done regularly.
(m) Reasons why sulphur presence in the fuel mix will be undesirable.
Sulphur in the fuel mix will lead to formation of sulphur dioxide which interferes with
the catalytic converters installed in the boiler.
The sulphur dioxide produced is further oxidised to sulphur trioxide which react with
water vapour forming sulphuric acid which will reduce the amount of water vapour
and also corrode stand pipes. Presence of sulphuric acid in the system will also affect
the dew point.
11.4Mw = 300- Tf
Outlet temperature, (Tf) = 300-11.4(Mw) where,
Mass of water, Mw in kg per kg-mol will be proportional to outlet temperature Tf
Dew point will be achieved for the two cases since the preheat temperature gets to 90 °C at 5
bars hence it cannot evaporate.
(l) 2 advantages and one disadvantage of preheating the water in this manner.
Advantages
High overall efficiency of heat is maintained since the latent heat of vaporisation is
recovered from the water vapour.
This practice has environmental benefits since the toxic nature of flue gases is
reduced through the combustion and the preheating process.
Disadvantages
At such a temperature, the repair and maintenances of the stand pipes will have to be
done regularly.
(m) Reasons why sulphur presence in the fuel mix will be undesirable.
Sulphur in the fuel mix will lead to formation of sulphur dioxide which interferes with
the catalytic converters installed in the boiler.
The sulphur dioxide produced is further oxidised to sulphur trioxide which react with
water vapour forming sulphuric acid which will reduce the amount of water vapour
and also corrode stand pipes. Presence of sulphuric acid in the system will also affect
the dew point.
1 out of 10
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.
