HI5015: Gilead Sciences Inc. - Legal Aspects of International Business
VerifiedAdded on 2024/07/22
|10
|2533
|494
Report
AI Summary
This report provides a comprehensive analysis of the legal aspects impacting Gilead Sciences Inc., an American biopharmaceutical company, in its international business operations. It identifies key legislations and regulatory frameworks, such as the Corporations Act 2001, Healthcare Quality Improvement Act of 1986, HIPAA, Affordable Care Act of 2010, Patient Safety and Quality Improvement Act of 2005, Patent Act 1990, Trademarks Act 1995, Gene Technology Act 2000, and Therapeutic Goods Act 1989, that govern the company's activities. The report also discusses international conventions, including the Convention on Biological Diversity, and agreements related to research, acquisitions, and collaborations, using the Kite Pharma acquisition as a case study. Furthermore, it examines a landmark case involving Gilead Sciences Inc. and the Ukraine’s Ministry of Health, highlighting the significance of bilateral investment treaties in protecting intellectual property rights. The analysis emphasizes the importance of these legal considerations for Gilead Sciences to ensure compliance, foster international collaborations, and achieve sustainable growth in the global market.

HI5015: LEGAL ASPECTS OF
INTERNATIONAL BUSINESS &
ENTERPRISE
1
INTERNATIONAL BUSINESS &
ENTERPRISE
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Contents
Question 1................................................................................................................................................... 3
Question 2................................................................................................................................................... 4
Question 3................................................................................................................................................... 7
References.................................................................................................................................................10
2
Question 1................................................................................................................................................... 3
Question 2................................................................................................................................................... 4
Question 3................................................................................................................................................... 7
References.................................................................................................................................................10
2
Question 1
Gilead Sciences Inc is also called as Gilead that is American Biopharmaceutical Company
which develops researches and commercializes drugs. The company mainly focuses on antiviral
drugs which are used in the treatment of Hepatitis B and C, HIV and Influenza involves with
Sovaldi and Harvoni. The Headquarters of the company established in 1987 in Foster City of
California and Gilead is considered as the member of NASDAQ Biotechnology Index and the
S&P 500. The company has 10000 employees in January 2018 across six continents of the world
and company has become largest biopharmaceutical company of the world.
Gilead Sciences Inc has challenge to create or acquire more blockbuster medicines before
existing revenue producers and the expiry of patent protection. Company also gets benefits from
the vast expansion of Medicaid in ACA, the HIV drugs of company faces pressure of funds
under restructuring proposals where Gilead has $32 billion cash.
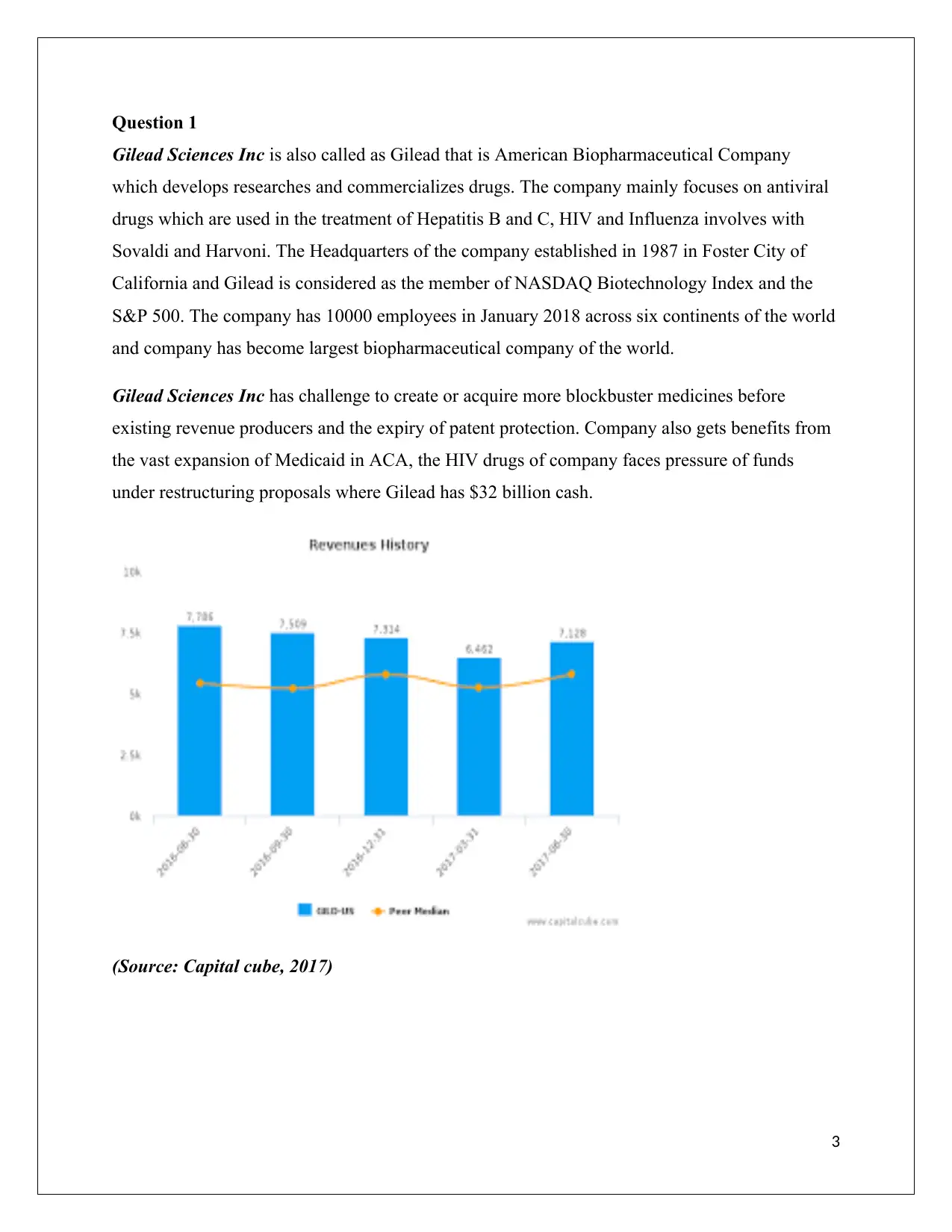
(Source: Capital cube, 2017)
3
Gilead Sciences Inc is also called as Gilead that is American Biopharmaceutical Company
which develops researches and commercializes drugs. The company mainly focuses on antiviral
drugs which are used in the treatment of Hepatitis B and C, HIV and Influenza involves with
Sovaldi and Harvoni. The Headquarters of the company established in 1987 in Foster City of
California and Gilead is considered as the member of NASDAQ Biotechnology Index and the
S&P 500. The company has 10000 employees in January 2018 across six continents of the world
and company has become largest biopharmaceutical company of the world.
Gilead Sciences Inc has challenge to create or acquire more blockbuster medicines before
existing revenue producers and the expiry of patent protection. Company also gets benefits from
the vast expansion of Medicaid in ACA, the HIV drugs of company faces pressure of funds
under restructuring proposals where Gilead has $32 billion cash.
(Source: Capital cube, 2017)
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Question 2
In order to conduct the better functioning of Biopharmaceutical company which develops and do
trading of drugs. There are certain legislation is applied ion healthcare sector in United States
that applies on pharmaceutical organization. The accurate laws and principles are mandatory to
run the business in this competitive market at national and international level. The compliances
and laws enhance the goodwill and image of the company in all over market. As the organization
functions in various countries follow varied laws as per the legal system of specific country.
Some of the common legislation and regulatory frameworks are there which are as mentioned
here. The Corporations Act 2001 has an important place in running the business in the manner of
organizations. It involves the laws and provisions from incorporation to company and till the
winding up process, where duties and obligations of directors and employees are described
(Whish and Bailey, 2015). The Act provides that duties and responsibilities are conducted in fair
manner for the better growth and success of organization in this competitive marketplace. The
required documents are file to Australian Securities and investment commission relevant to
business functions and operations. The registration process of companies is similar as per the
legal system and applications of legislation are somehow different according to nature and
transactions. Gilead Sciences Inc follows and maintains the regulatory compliances of
healthcare sector on United States as the nationality of organization. There are number of
provisions are mentioned in Corporations Act 2001 that are applied and followed in acquisition
process as well (Regis, 2016). The Healthcare Quality Improvement Act of 1986 (HCQIA)
states immunity for institutions and medical professional for conducting the assessments. The
law is enacted to protect and help the medical professionals from high review based on lawsuits
and to increase physicians to file mandatory complaints after meeting dangerous and
unprofessional peer conduct. Health Insurance Portability and Accountability Act (HIPAA) of
1996 helps and supports American employees by permit them to carry the health policies of
insurance from one to another job. The policies also allow employees to apply for chosen group
of health plans for insurance to substitute lost coverage and manage for changes like births,
marriages and adoptions. Affordable Care Act of 2010, provides that most US residents to
comply for health insurance coverage and also applies penalty for person who fails to protect
insurance but developing exceptions for some protected teams (Sorenson and Drummond, 2014).
The Act also developed the American health exchange benefits where residents can compare and
4
In order to conduct the better functioning of Biopharmaceutical company which develops and do
trading of drugs. There are certain legislation is applied ion healthcare sector in United States
that applies on pharmaceutical organization. The accurate laws and principles are mandatory to
run the business in this competitive market at national and international level. The compliances
and laws enhance the goodwill and image of the company in all over market. As the organization
functions in various countries follow varied laws as per the legal system of specific country.
Some of the common legislation and regulatory frameworks are there which are as mentioned
here. The Corporations Act 2001 has an important place in running the business in the manner of
organizations. It involves the laws and provisions from incorporation to company and till the
winding up process, where duties and obligations of directors and employees are described
(Whish and Bailey, 2015). The Act provides that duties and responsibilities are conducted in fair
manner for the better growth and success of organization in this competitive marketplace. The
required documents are file to Australian Securities and investment commission relevant to
business functions and operations. The registration process of companies is similar as per the
legal system and applications of legislation are somehow different according to nature and
transactions. Gilead Sciences Inc follows and maintains the regulatory compliances of
healthcare sector on United States as the nationality of organization. There are number of
provisions are mentioned in Corporations Act 2001 that are applied and followed in acquisition
process as well (Regis, 2016). The Healthcare Quality Improvement Act of 1986 (HCQIA)
states immunity for institutions and medical professional for conducting the assessments. The
law is enacted to protect and help the medical professionals from high review based on lawsuits
and to increase physicians to file mandatory complaints after meeting dangerous and
unprofessional peer conduct. Health Insurance Portability and Accountability Act (HIPAA) of
1996 helps and supports American employees by permit them to carry the health policies of
insurance from one to another job. The policies also allow employees to apply for chosen group
of health plans for insurance to substitute lost coverage and manage for changes like births,
marriages and adoptions. Affordable Care Act of 2010, provides that most US residents to
comply for health insurance coverage and also applies penalty for person who fails to protect
insurance but developing exceptions for some protected teams (Sorenson and Drummond, 2014).
The Act also developed the American health exchange benefits where residents can compare and
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

review insurance plans. Patient safety and Quality Improvement Act (PSQIA) of 2005, helps
and preserve the healthcare employees who inform unsafe situations. Legislators developed the
law to increase the medical errors reporting and maintaining the confidentiality rights of patients.
Company prepares such drugs which are directly benefitted to patients and remove such diseases
with best effort. The regulations of Patent Act 1990 and the Patent Regulations 1991, is comply
as per receiving the patent. When the patent is gained on the services and products of company
creates use of same product and establishes same product, in that situations it is determined as
infringement and breach of patent. So the Gilead Sciences Inc follows these regulations to the
protection and safety of drugs from unauthorized use without the permission. So the company
has complete rights to make a suit against the defaulter who illegally use the drugs and medicines
produced by company. The next one is Trademarks Act, 1995whihc is also applicable on the
organization, trademark is a mark and symbol that is used in goods and services produced by
company. When the company is registered with the regulatory authority, then no other company
can use such similar symbol and sign to sell the goods and services of won in marketplace. It
gives the different identity and recognition of company goods and services from different similar
products in the marketplace. When any other organization is used the similar symbol and sign of
the Gilead Sciences Inc then strict legal penalties are there to protect the trademarks of Act. As
this company are biotechnology and biopharmaceutical sector then organization develops new
and various kinds of drugs for specific treatments and diseases such as Hepatitis B and c, HIV
and influenza etc. so it is necessary to protect the safety and security of new drugs manufactured
by company. When any other organization wants to use such drugs in their business then
permission is received in written manner with regulatory authorities (Kumari, et. al., 2016). As
per Gene Technology Act, 2000 is also complying on business organization and the objective of
this Act is giving a protection to the health and safety of people of the country and gives
environment protection. It can be done by determine the risk includes in gene techniques and
controlling the risk factors to regulate the deals with genetically changed organs. It is necessary
that relevant regulations of the Act are followed by the employees and other members
accountable for controlling the business activities and operations of the company. Further there is
Therapeutic Goods Act, 1989 which is established in giving permission for making the
therapeutic products and maintaining the safety, quality and efficacy and sufficient availability of
drugs and medicines described herewith are used and produced by Gilead Sciences Inc and
5
and preserve the healthcare employees who inform unsafe situations. Legislators developed the
law to increase the medical errors reporting and maintaining the confidentiality rights of patients.
Company prepares such drugs which are directly benefitted to patients and remove such diseases
with best effort. The regulations of Patent Act 1990 and the Patent Regulations 1991, is comply
as per receiving the patent. When the patent is gained on the services and products of company
creates use of same product and establishes same product, in that situations it is determined as
infringement and breach of patent. So the Gilead Sciences Inc follows these regulations to the
protection and safety of drugs from unauthorized use without the permission. So the company
has complete rights to make a suit against the defaulter who illegally use the drugs and medicines
produced by company. The next one is Trademarks Act, 1995whihc is also applicable on the
organization, trademark is a mark and symbol that is used in goods and services produced by
company. When the company is registered with the regulatory authority, then no other company
can use such similar symbol and sign to sell the goods and services of won in marketplace. It
gives the different identity and recognition of company goods and services from different similar
products in the marketplace. When any other organization is used the similar symbol and sign of
the Gilead Sciences Inc then strict legal penalties are there to protect the trademarks of Act. As
this company are biotechnology and biopharmaceutical sector then organization develops new
and various kinds of drugs for specific treatments and diseases such as Hepatitis B and c, HIV
and influenza etc. so it is necessary to protect the safety and security of new drugs manufactured
by company. When any other organization wants to use such drugs in their business then
permission is received in written manner with regulatory authorities (Kumari, et. al., 2016). As
per Gene Technology Act, 2000 is also complying on business organization and the objective of
this Act is giving a protection to the health and safety of people of the country and gives
environment protection. It can be done by determine the risk includes in gene techniques and
controlling the risk factors to regulate the deals with genetically changed organs. It is necessary
that relevant regulations of the Act are followed by the employees and other members
accountable for controlling the business activities and operations of the company. Further there is
Therapeutic Goods Act, 1989 which is established in giving permission for making the
therapeutic products and maintaining the safety, quality and efficacy and sufficient availability of
drugs and medicines described herewith are used and produced by Gilead Sciences Inc and
5

supplied to various countries. It also gives the states and territories to follow this approach and it
is perfect to regulate the availability and accessibility of poison and drugs in the country (Ivec,
2015).
6
is perfect to regulate the availability and accessibility of poison and drugs in the country (Ivec,
2015).
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Question 3
As per the discussion of Gilead sciences, it can be determined that it is the American based
biopharmaceutical that develops researches and commercializes the drugs. To operate the
business in different countries, it is necessary to analyse the different laws and legislation. The
agreement can be made by the company helps to understand the term of law with the necessary
terms and conditions. The agreements can be framed to resolve the issues among the different
countries. It also evaluates the conventions which can be defined as the pact, protocol, agreement
among the member nations in specific matter. It also discusses the treaties that can be defined as
the agreement among the different nations.
It is essential for the every company to make the agreements so that they can operate the number
of nations. Gilead makes the certain agreement for the purpose of research such as in 1990 he
frame the agreement with Glaxo for the development of blockers which can be recognized as
antisense. In 2007 he enters in to licensing agreement with parion for epithelial sodium channel
that can help in the treatment of diseases. With these agreement they can merge, acquire with the
other countries to perform their better function (Vandevelde, 2017).
In context to the biopharmaceutical sector there is convention on Biological Diversity that can be
considered as the Biodiversity convention and recognized as the multilateral treaty. The main
objective of the biodiversity convention is provide the fair and equitable share in the profit that
can be arises due to genetic resources. It can also state that the main aim of the convention is to
preserve and conserve the resources in relation to the biological diversity. It can also be
determined that the biological diversity was organized in Earth summit on 5th June for the
purpose of sustainable development and signed by the 168 nations . It comes in to existence on
29th December 1993. It includes the different species, genetic, ecosystems etc. This convention
provides the advantage to the organization by providing the better quality of services in the
market and that can be available to the company for the purpose of return (Mailloux, 2018).
There are different issues such as:
The convention of biological diversity measures the reassurances for satisfying and
nourishing the use of biological diversity.
7
As per the discussion of Gilead sciences, it can be determined that it is the American based
biopharmaceutical that develops researches and commercializes the drugs. To operate the
business in different countries, it is necessary to analyse the different laws and legislation. The
agreement can be made by the company helps to understand the term of law with the necessary
terms and conditions. The agreements can be framed to resolve the issues among the different
countries. It also evaluates the conventions which can be defined as the pact, protocol, agreement
among the member nations in specific matter. It also discusses the treaties that can be defined as
the agreement among the different nations.
It is essential for the every company to make the agreements so that they can operate the number
of nations. Gilead makes the certain agreement for the purpose of research such as in 1990 he
frame the agreement with Glaxo for the development of blockers which can be recognized as
antisense. In 2007 he enters in to licensing agreement with parion for epithelial sodium channel
that can help in the treatment of diseases. With these agreement they can merge, acquire with the
other countries to perform their better function (Vandevelde, 2017).
In context to the biopharmaceutical sector there is convention on Biological Diversity that can be
considered as the Biodiversity convention and recognized as the multilateral treaty. The main
objective of the biodiversity convention is provide the fair and equitable share in the profit that
can be arises due to genetic resources. It can also state that the main aim of the convention is to
preserve and conserve the resources in relation to the biological diversity. It can also be
determined that the biological diversity was organized in Earth summit on 5th June for the
purpose of sustainable development and signed by the 168 nations . It comes in to existence on
29th December 1993. It includes the different species, genetic, ecosystems etc. This convention
provides the advantage to the organization by providing the better quality of services in the
market and that can be available to the company for the purpose of return (Mailloux, 2018).
There are different issues such as:
The convention of biological diversity measures the reassurances for satisfying and
nourishing the use of biological diversity.
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

It also accesses the genetic resources and also helps to understand the knowledge of
resources that require the consent of parties who are providing services of resources.
It also provides the knowledge of diversity to public and also educates them in their
sectors.
With the help of convention, there can be transformation of technology to the government
of the state members.
It also discusses the influence power of the conserving and making the optimum
operation of resources.
The Gilead sciences acquire the Kite pharma that every organization exposed to CAR-T that can
be recognized as cancer therapy and allows the creation for drugs. It consist the companies such
as BLUE (bluebird bio) Juno Therapeutics (JUNO) and cellectis (CLLS). There is an agreement
with the Kite as the Gilead sciences want to purchase the Kite Pharma Inc. that can cost around
$12 billion. It replaces the sales from the hepatitis C with the expensive and emerging category
of cancer that generates the billions of dollars.
The Kite pharma and bluebird bio announce the strategic association to advance second-creation
TCR cell to treat the HPV- cancers. They entered in to collaboration agreement and co-develop
and co-commercialize the TCR product of second generation that can be directed against the
human papillomavirus type E6 .It also incorporates the gene editing and lent viral technologies.
Under this agreement both companies commercialize and develop the TCR products against the
co-protein. It also modifies the certain T-cell function and genes (Dörr & Schmalenbach, 2018).
There is landmark case in relation to treaty, case between the Ukraine’s Ministry of Health and
Gilead Sciences Inc. This states that when laws on the intellectual property rights and on the
exclusion of data does not perform then in that case the government and party find the legal
option to protect the investments. They can protect the treatment under the bilateral investment
treaties and reconciles the same to secure the access to the innovative technologies under the
value. It also uses in relation to the precedent to adopt the international practice of agreements
that can enter through the health institutions and manufacturers. This type of agreements divided
in to two classes:
8
resources that require the consent of parties who are providing services of resources.
It also provides the knowledge of diversity to public and also educates them in their
sectors.
With the help of convention, there can be transformation of technology to the government
of the state members.
It also discusses the influence power of the conserving and making the optimum
operation of resources.
The Gilead sciences acquire the Kite pharma that every organization exposed to CAR-T that can
be recognized as cancer therapy and allows the creation for drugs. It consist the companies such
as BLUE (bluebird bio) Juno Therapeutics (JUNO) and cellectis (CLLS). There is an agreement
with the Kite as the Gilead sciences want to purchase the Kite Pharma Inc. that can cost around
$12 billion. It replaces the sales from the hepatitis C with the expensive and emerging category
of cancer that generates the billions of dollars.
The Kite pharma and bluebird bio announce the strategic association to advance second-creation
TCR cell to treat the HPV- cancers. They entered in to collaboration agreement and co-develop
and co-commercialize the TCR product of second generation that can be directed against the
human papillomavirus type E6 .It also incorporates the gene editing and lent viral technologies.
Under this agreement both companies commercialize and develop the TCR products against the
co-protein. It also modifies the certain T-cell function and genes (Dörr & Schmalenbach, 2018).
There is landmark case in relation to treaty, case between the Ukraine’s Ministry of Health and
Gilead Sciences Inc. This states that when laws on the intellectual property rights and on the
exclusion of data does not perform then in that case the government and party find the legal
option to protect the investments. They can protect the treatment under the bilateral investment
treaties and reconciles the same to secure the access to the innovative technologies under the
value. It also uses in relation to the precedent to adopt the international practice of agreements
that can enter through the health institutions and manufacturers. This type of agreements divided
in to two classes:
8

Financial based agreements that can be based on the financial performance.
An agreement based on the outcomes that help to accelerate to access effective and safe
therapies. It can be analysed that the pharmaceutical is already in the market but there is
more need to collect the evidence.
To analyses the company, it can be determined that the potential agreements consist the market
access and the effective system of budget management (PharmaExec, 2017). From the above
analysis, it can be determined that these agreements, conventions provide the advantage to the
company and that leads to the development and success of organization. If Gilead sciences adopt
this agreement and conventions then there will be effective functioning among the different
nations. Through this agreement, they can attract number of legislation and also recognize in the
global market. By adopting all this regulations and treaties there will be an improvement in the
research and development of certain products such as Tamiflu, Harvoni , complera etc. that
provides the benefit to public.
9
An agreement based on the outcomes that help to accelerate to access effective and safe
therapies. It can be analysed that the pharmaceutical is already in the market but there is
more need to collect the evidence.
To analyses the company, it can be determined that the potential agreements consist the market
access and the effective system of budget management (PharmaExec, 2017). From the above
analysis, it can be determined that these agreements, conventions provide the advantage to the
company and that leads to the development and success of organization. If Gilead sciences adopt
this agreement and conventions then there will be effective functioning among the different
nations. Through this agreement, they can attract number of legislation and also recognize in the
global market. By adopting all this regulations and treaties there will be an improvement in the
research and development of certain products such as Tamiflu, Harvoni , complera etc. that
provides the benefit to public.
9
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

References
Capitalcube, (2017) Gilead Sciences, Inc. :GILD-US: Earnings Analysis: Q2, 2017 By
the Numbers : September 13, 2017. [Online] capitalcube.com. Available at:
http://www.capitalcube.com/blog/index.php/gilead-sciences-inc-gild-us-earnings-
analysis-q2-2017-by-the-numbers-september-13-2017/. [Accessed on 17 August, 2018]
Dörr, O., & Schmalenbach, K. (Eds.). (2018). Vienna Convention on the law of treaties.
Springer.
Ivec, M. (2015). Applications of Responsive Regulatory Theory in Australia and
Overseas. Occasional Paper.
Kumari, B. S., Hanuja, G. S., Nagabhushanam, M. V., Reddy, D. N., & Bonthagarala, B.
(2016). Current Regulatory Requirements for Registration of Medicines, Compilation and
Submission of Dossier in Australian Therapeutic goods Administration. International
Journal of Advanced Scientific and Technical Research, ISSN, 2249-9954
Mailloux, S. (2018). Interpretive conventions: The reader in the study of American
fiction. Cornell University Press.
PharmaExec, (2017). Ukraine: Protecting Investments through Entry Agreements
between Pharma and Government. [Online] PharmaExec. Available at:
http://www.pharmexec.com/ukraine-protecting-investments-through-entry-agreements-
between-pharma-and-government. Accessed on 17 August 2018]
Regis, (2016) 8 Important Regulations in United States Health Care [Online]
Online.regiscollege.edu. Available at: https://online.regiscollege.edu/blog/8-important-
regulations-united-states-health-care/. [Accessed on 17 August, 2018]
Sorenson, C. and Drummond, M. (2014). Improving medical device regulation: the
United States and Europe in perspective. The Milbank Quarterly, 92(1), pp.114-150.
Vandevelde, K. J. (2017). The First Bilateral Investment Treaties: US Postwar
Friendship, Commerce and Navigation Treaties. Oxford University Press.
Whish, R. and Bailey, D., 2015. Competition law. Oxford University Press, USA.
10
Capitalcube, (2017) Gilead Sciences, Inc. :GILD-US: Earnings Analysis: Q2, 2017 By
the Numbers : September 13, 2017. [Online] capitalcube.com. Available at:
http://www.capitalcube.com/blog/index.php/gilead-sciences-inc-gild-us-earnings-
analysis-q2-2017-by-the-numbers-september-13-2017/. [Accessed on 17 August, 2018]
Dörr, O., & Schmalenbach, K. (Eds.). (2018). Vienna Convention on the law of treaties.
Springer.
Ivec, M. (2015). Applications of Responsive Regulatory Theory in Australia and
Overseas. Occasional Paper.
Kumari, B. S., Hanuja, G. S., Nagabhushanam, M. V., Reddy, D. N., & Bonthagarala, B.
(2016). Current Regulatory Requirements for Registration of Medicines, Compilation and
Submission of Dossier in Australian Therapeutic goods Administration. International
Journal of Advanced Scientific and Technical Research, ISSN, 2249-9954
Mailloux, S. (2018). Interpretive conventions: The reader in the study of American
fiction. Cornell University Press.
PharmaExec, (2017). Ukraine: Protecting Investments through Entry Agreements
between Pharma and Government. [Online] PharmaExec. Available at:
http://www.pharmexec.com/ukraine-protecting-investments-through-entry-agreements-
between-pharma-and-government. Accessed on 17 August 2018]
Regis, (2016) 8 Important Regulations in United States Health Care [Online]
Online.regiscollege.edu. Available at: https://online.regiscollege.edu/blog/8-important-
regulations-united-states-health-care/. [Accessed on 17 August, 2018]
Sorenson, C. and Drummond, M. (2014). Improving medical device regulation: the
United States and Europe in perspective. The Milbank Quarterly, 92(1), pp.114-150.
Vandevelde, K. J. (2017). The First Bilateral Investment Treaties: US Postwar
Friendship, Commerce and Navigation Treaties. Oxford University Press.
Whish, R. and Bailey, D., 2015. Competition law. Oxford University Press, USA.
10
1 out of 10
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




