Does Warfarin Increase Subdural Haemorrhage Risk? A Systematic Review
VerifiedAdded on 2021/06/17
|30
|8039
|24
Report
AI Summary
This systematic review, conducted by a student at the University of Adelaide Nursing School, investigates the impact of Warfarin on the risk of subdural haemorrhage compared to other anticoagulants. The review follows the PRISMA flowchart, focusing on quantitative and longitudinal studies to assess the effects of Warfarin on clinical and functional outcomes in patients, considering factors like the internalized normalized ratio (INR) and optimal timing for Warfarin administration. The study aims to determine if Warfarin increases bleeding risk, mortality, and morbidity compared to other anti-platelet drugs, including adults and older adults. The review includes a detailed search strategy across multiple databases and employs the Joanna Briggs Institute (JBI) framework for critical appraisal and data extraction. The findings will address the major findings, implications for research and health, limitations, and recommendations based on the evidence collected, culminating in a conclusion about Warfarin's effects on intracranial haemorrhage.

Running head: SYSTEMATIC REVIEW
The University of Adelaide
Adelaide Nursing School
Does warfarin increase the risk of subdural haemorrhage when compared with other anti-
coagulant?
Name of the Student
Student Number
The University of Adelaide
Adelaide Nursing School
Does warfarin increase the risk of subdural haemorrhage when compared with other anti-
coagulant?
Name of the Student
Student Number
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2SYSTEMATIC REVIEW
Abstract
Subdural haematoma refers to collection of blood, below inner layers of the dura matter and
external to the arachnoid membrane, and is one of the most common kinds of traumatic
intracranial lesions. Accumulation of blood in the brain increases intracranial pressure and
results in conditions that can be life threatening. The systematic review aims to explore
impacts of warfarin on increasing the likelihood of intracranial hemorrhage, specifically
subdural haematoma, when compared to other anticoagulant therapy among patients. The
systematic review will focus on the PRISMA flowchart for extracting quantitative and
longitudinal articles that are relevant to the research objective, and will evaluate the
immediate and long term impacts of warfarin on the clinical and functional outcome of
subdural haematoma patients, their internalized normalised rates and optimal timing for
reception of warfarin, in comparison to other anticoagulant. This will be followed by drawing
a conclusion that demonstrates the effects of warfarin on intracranial hemorrhage.
Keywords- intracranial, subdural, haematoma, warfarin. Anticoagulant
Abstract
Subdural haematoma refers to collection of blood, below inner layers of the dura matter and
external to the arachnoid membrane, and is one of the most common kinds of traumatic
intracranial lesions. Accumulation of blood in the brain increases intracranial pressure and
results in conditions that can be life threatening. The systematic review aims to explore
impacts of warfarin on increasing the likelihood of intracranial hemorrhage, specifically
subdural haematoma, when compared to other anticoagulant therapy among patients. The
systematic review will focus on the PRISMA flowchart for extracting quantitative and
longitudinal articles that are relevant to the research objective, and will evaluate the
immediate and long term impacts of warfarin on the clinical and functional outcome of
subdural haematoma patients, their internalized normalised rates and optimal timing for
reception of warfarin, in comparison to other anticoagulant. This will be followed by drawing
a conclusion that demonstrates the effects of warfarin on intracranial hemorrhage.
Keywords- intracranial, subdural, haematoma, warfarin. Anticoagulant

3SYSTEMATIC REVIEW
Table of Contents
1. Introduction........................................................................................................................5
2. Background........................................................................................................................5
3. Systematic review question/objectives..............................................................................7
4. Search strategy...................................................................................................................9
5. Method of review...............................................................................................................9
6. Results..............................................................................................................................11
Articles identified.................................................................................................................11
Included and excluded studies.............................................................................................13
Summary of characteristics of the included studies.............................................................16
Analysis................................................................................................................................17
Clinical and functional outcomes.....................................................................................17
Internalised normalised ratio............................................................................................18
Resumption of anticoagulation agents.............................................................................19
7. Discussion........................................................................................................................21
Major findings......................................................................................................................21
Implications for research......................................................................................................23
Implications to nursing or to health.....................................................................................23
Recommendations................................................................................................................23
Limitations...........................................................................................................................24
8. Conclusion........................................................................................................................25
Table of Contents
1. Introduction........................................................................................................................5
2. Background........................................................................................................................5
3. Systematic review question/objectives..............................................................................7
4. Search strategy...................................................................................................................9
5. Method of review...............................................................................................................9
6. Results..............................................................................................................................11
Articles identified.................................................................................................................11
Included and excluded studies.............................................................................................13
Summary of characteristics of the included studies.............................................................16
Analysis................................................................................................................................17
Clinical and functional outcomes.....................................................................................17
Internalised normalised ratio............................................................................................18
Resumption of anticoagulation agents.............................................................................19
7. Discussion........................................................................................................................21
Major findings......................................................................................................................21
Implications for research......................................................................................................23
Implications to nursing or to health.....................................................................................23
Recommendations................................................................................................................23
Limitations...........................................................................................................................24
8. Conclusion........................................................................................................................25
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

4SYSTEMATIC REVIEW
References................................................................................................................................27
References................................................................................................................................27
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5SYSTEMATIC REVIEW
1. Introduction
Subdural hematoma refers to the localised accumulation of blood, outside the vessels, and
is generally associated with different forms of traumatic brain injury. Blood is found to gather
between the inner layers of the dura mater and the arachnoid. This usually results as a direct
manifestation of tars in the bridging veins that cross the subdural spaces. The condition
eventually leads to an increase in the intracranial pressure, thereby leading to subsequent
damage and compression of delicate nervous tissues of the brain (Almenawer et al. 2014).
Subdural haemorrhages are also life-threatening in an acute condition. Depending upon the
rate of onset, the condition is generally classified as chronic, subacute or acute (Ohba et al.
2013). Anticoagulants are chemical substances, frequently referred to as blood thinners,
which reduce or eliminate chances of blood clotting. Warfarin is one such blood thinner,
commonly used for treating pulmonary embolism and deep vein thrombosis. There lies a
perception that patients who have developed chronic subdural haemorrhages, while taking
warfarin medications, do less well than patients not under warfarin medication (Hankey et al.
2014). Subdural haemorrhages are more common among patients, under the prescribed
medication of warfarin and aspirin like anticoagulants. The systematic review will explore
the effects of warfarin on increasing the likelihood of subdural haemorrhages, in comparison
to other anticoagulants.
2. Background
Blood thinning chemicals have been in medical use since ages. The use of these
anticoagulants is generally based upon decisions that take into consideration the benefits and
associated risks of anticoagulation. Some of the greatest risks associated with this
anticoagulation therapy involve the increased risk of bleeding (Dewilde et al. 2013). The
most common benefits of anticoagulation are reduction or prevention of disease progression.
1. Introduction
Subdural hematoma refers to the localised accumulation of blood, outside the vessels, and
is generally associated with different forms of traumatic brain injury. Blood is found to gather
between the inner layers of the dura mater and the arachnoid. This usually results as a direct
manifestation of tars in the bridging veins that cross the subdural spaces. The condition
eventually leads to an increase in the intracranial pressure, thereby leading to subsequent
damage and compression of delicate nervous tissues of the brain (Almenawer et al. 2014).
Subdural haemorrhages are also life-threatening in an acute condition. Depending upon the
rate of onset, the condition is generally classified as chronic, subacute or acute (Ohba et al.
2013). Anticoagulants are chemical substances, frequently referred to as blood thinners,
which reduce or eliminate chances of blood clotting. Warfarin is one such blood thinner,
commonly used for treating pulmonary embolism and deep vein thrombosis. There lies a
perception that patients who have developed chronic subdural haemorrhages, while taking
warfarin medications, do less well than patients not under warfarin medication (Hankey et al.
2014). Subdural haemorrhages are more common among patients, under the prescribed
medication of warfarin and aspirin like anticoagulants. The systematic review will explore
the effects of warfarin on increasing the likelihood of subdural haemorrhages, in comparison
to other anticoagulants.
2. Background
Blood thinning chemicals have been in medical use since ages. The use of these
anticoagulants is generally based upon decisions that take into consideration the benefits and
associated risks of anticoagulation. Some of the greatest risks associated with this
anticoagulation therapy involve the increased risk of bleeding (Dewilde et al. 2013). The
most common benefits of anticoagulation are reduction or prevention of disease progression.

6SYSTEMATIC REVIEW
Some of the anticoagulant therapy indications, known to pose direct benefits include deep
vein thrombosis, atrial fibrillation, coronary artery disease, myocardial infarction, restenosis,
and pulmonary embolism. Recent reports suggest an increase in the number of patients
getting admitted to healthcare facilities due to intracranial haemorrhages, which occur due to
the administration of anticoagulants, used for the purpose of treating previous instances of
cardiovascular abnormalities such as, stroke, and pulmonary embolism (Roldán et al. 2013).
These anticoagulants inhibit synthesis of the calcium dependent clotting factors that are
dependent on vitamin K. The UK Committee on Safety of Medicines had received huge
number of reports regarding increased risks of haemorrhages and INR among patients under
warfarin treatment (Larsen et al. 2016).
Effects of anticoagulant son haemorrhages were also found in cattle, as early as in the
1920s, when those that fed on moldy silage, which functioned as a potent anticoagulant,
developed haemorrhaging symptoms. An estimated 1.7 million traumatic brain injury cases
have been found to occur on an annual basis in the United States. 275,000 patients require
hospitalisation, of which 52,000 fail to survive. Thus, significant changes have been observed
in the rates of hospital admissions due to subdural and intracranial haemorrhages, following
use of anticoagulation agents, like warfarin. Approximately 5-25% of patients suffering
severe head injuries develop acute subdural haemorrhages (Fountain et al. 2017). Oral
anticoagulation therapy has been associated with higher risks of intracranial haemorrhages,
following minor head trauma. Thus, traumatic brain injury patients under coagulotherapy
treatment are often included in high risk groups, regardless of the clinical presentation of the
symptoms. Some of the major risk factors that result in development of subdural
haemorrhages include health conditions such as, hypertension, vascular complications, and
anticoagulant consumption (Son et al. 2013).
Some of the anticoagulant therapy indications, known to pose direct benefits include deep
vein thrombosis, atrial fibrillation, coronary artery disease, myocardial infarction, restenosis,
and pulmonary embolism. Recent reports suggest an increase in the number of patients
getting admitted to healthcare facilities due to intracranial haemorrhages, which occur due to
the administration of anticoagulants, used for the purpose of treating previous instances of
cardiovascular abnormalities such as, stroke, and pulmonary embolism (Roldán et al. 2013).
These anticoagulants inhibit synthesis of the calcium dependent clotting factors that are
dependent on vitamin K. The UK Committee on Safety of Medicines had received huge
number of reports regarding increased risks of haemorrhages and INR among patients under
warfarin treatment (Larsen et al. 2016).
Effects of anticoagulant son haemorrhages were also found in cattle, as early as in the
1920s, when those that fed on moldy silage, which functioned as a potent anticoagulant,
developed haemorrhaging symptoms. An estimated 1.7 million traumatic brain injury cases
have been found to occur on an annual basis in the United States. 275,000 patients require
hospitalisation, of which 52,000 fail to survive. Thus, significant changes have been observed
in the rates of hospital admissions due to subdural and intracranial haemorrhages, following
use of anticoagulation agents, like warfarin. Approximately 5-25% of patients suffering
severe head injuries develop acute subdural haemorrhages (Fountain et al. 2017). Oral
anticoagulation therapy has been associated with higher risks of intracranial haemorrhages,
following minor head trauma. Thus, traumatic brain injury patients under coagulotherapy
treatment are often included in high risk groups, regardless of the clinical presentation of the
symptoms. Some of the major risk factors that result in development of subdural
haemorrhages include health conditions such as, hypertension, vascular complications, and
anticoagulant consumption (Son et al. 2013).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

7SYSTEMATIC REVIEW
Warfarin is the mainstay treatment for peripheral vascular diseases and associated
arteriopathic conditions. Pharmacological therapies that have been linked with development
of acute subdural haemorrhages particularly include warfarin, heparin and aspirin. An INR
above 3.0 are most commonly found in cases of hypertension, hepatic disorder, bleeding
lesions and noncompliance to medications (Elliott 2014). Subdural haemorrhages occur as a
direct result of two major mechanisms namely, vasoconstriction and pressure on the cortical
blood vessels. The growth in the size of the haemorrhage makes the intracranial pressure to
rise and squeezes the intracranial blood to the dural venous sinuses, thereby elevating the
dural venous pressure. This leads to increased bleeding from the ruptured bridging veins
(Anderson et al. 2013). The anticoagulant warfarin promotes a reduction in the levels of
protein C in the first 36 hours, thereby reducing its activity and lowering the degradation of
factor VIIIa and Va. This creates a bias in the haemostasis system towards formation of
thrombus, subsequently forming a prothrombic state (Woo et al. 2014). Although warfarin
has some advantages such as, long half-life, the fact that it increases risks of thrombosis, if
administered without anticoagulant cover, forms the basis of the research.
3. Systematic review question/objectives
Does Warfarin compared with other anti-platelet; increase the risk of subdural
haemorrhage?
The systematic review aims to obtain the understanding of the cause-and-effect
associations between warfarin as well as other anti-platelets when used for people with a risk
of subdural haemorrhage.
The objectives of the systematic review are-
To determine if anticoagulant Warfarin increases the risk of bleeding in
comparison to other anti-platelet drugs
Warfarin is the mainstay treatment for peripheral vascular diseases and associated
arteriopathic conditions. Pharmacological therapies that have been linked with development
of acute subdural haemorrhages particularly include warfarin, heparin and aspirin. An INR
above 3.0 are most commonly found in cases of hypertension, hepatic disorder, bleeding
lesions and noncompliance to medications (Elliott 2014). Subdural haemorrhages occur as a
direct result of two major mechanisms namely, vasoconstriction and pressure on the cortical
blood vessels. The growth in the size of the haemorrhage makes the intracranial pressure to
rise and squeezes the intracranial blood to the dural venous sinuses, thereby elevating the
dural venous pressure. This leads to increased bleeding from the ruptured bridging veins
(Anderson et al. 2013). The anticoagulant warfarin promotes a reduction in the levels of
protein C in the first 36 hours, thereby reducing its activity and lowering the degradation of
factor VIIIa and Va. This creates a bias in the haemostasis system towards formation of
thrombus, subsequently forming a prothrombic state (Woo et al. 2014). Although warfarin
has some advantages such as, long half-life, the fact that it increases risks of thrombosis, if
administered without anticoagulant cover, forms the basis of the research.
3. Systematic review question/objectives
Does Warfarin compared with other anti-platelet; increase the risk of subdural
haemorrhage?
The systematic review aims to obtain the understanding of the cause-and-effect
associations between warfarin as well as other anti-platelets when used for people with a risk
of subdural haemorrhage.
The objectives of the systematic review are-
To determine if anticoagulant Warfarin increases the risk of bleeding in
comparison to other anti-platelet drugs
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8SYSTEMATIC REVIEW
To determine the mortality and morbidity due to warfarin in adult and older
adult population when compared to other anti-platelet irrespective of gender
and ethnicity
Inclusion and exclusion criteria
PICO method will be used to assess the inclusion criteria. In this regard the
population considered is the people on anticoagulants, the intervention used is warfarin, and
the comparator is anti-platelets. The outcomes to be measured may include mortality,
morbidity and subdural haemorrhage.
Types of participants
The systematic review will extract studies that considered participants who acquired
intracranial blood taking anti-platelet and anticoagulant drugs. Quantitative studies are
included as they address the effectiveness of the anticoagulants. The longitudinal
observational research will contribute to the knowledge of both short term and long term
effect of the anticoagulants. Further, the studies that address the risk factors associated with
the anticoagulants are case-control and cohort studies. The population relevant to this review
are adults and older adults taking anticoagulants.
Types of intervention(s)/phenomena of interest
Studies evaluating the use of Warfarin in participants with the subdural haemorrhage
and compared with other anti-platelet are included
Types of studies
For inclusion, the systematic review will consider the case-control studies,
prospective and retrospective studies, and epidemiological study.
Types of outcomes
To determine the mortality and morbidity due to warfarin in adult and older
adult population when compared to other anti-platelet irrespective of gender
and ethnicity
Inclusion and exclusion criteria
PICO method will be used to assess the inclusion criteria. In this regard the
population considered is the people on anticoagulants, the intervention used is warfarin, and
the comparator is anti-platelets. The outcomes to be measured may include mortality,
morbidity and subdural haemorrhage.
Types of participants
The systematic review will extract studies that considered participants who acquired
intracranial blood taking anti-platelet and anticoagulant drugs. Quantitative studies are
included as they address the effectiveness of the anticoagulants. The longitudinal
observational research will contribute to the knowledge of both short term and long term
effect of the anticoagulants. Further, the studies that address the risk factors associated with
the anticoagulants are case-control and cohort studies. The population relevant to this review
are adults and older adults taking anticoagulants.
Types of intervention(s)/phenomena of interest
Studies evaluating the use of Warfarin in participants with the subdural haemorrhage
and compared with other anti-platelet are included
Types of studies
For inclusion, the systematic review will consider the case-control studies,
prospective and retrospective studies, and epidemiological study.
Types of outcomes

9SYSTEMATIC REVIEW
Studies with outcome measure such as subdural haemorrhage, mortality due to pre-
existing use of antiplatelets, and morbidity are considered.
4. Search strategy
The search strategy with a three-step search approach will be used in this review. Initially,
the search will be restricted to the MEDLINE and CINAHL databases. Initially, all the
published and unpublished data will be extracted. The search is based on keywords present in
the title, and abstract, of the paper as well as the index terms that describe the article. In the
second search, all the recognised key terms and index terms are used to carry the search in all
the other selected databases. It includes Cochrane Library, Embase, Clinical Key for Nursing,
Web of Science, Health Source: Nursing/Academic Edition, PubMed, Scopus, Joanna Briggs
Institute EBP database, and CINAHL with Full Text. In the third step, the all the relevant
articles and reports that have been found are screened for a reference list. It will help
determine other studies pertinent to the area of investigation. Studies published in English
and all the observational studies will be considered for the systematic review. The keywords
used for searching the database are Anti-coagulants, Intracranial haemorrhage, Warfarin,
Subdural hematoma, Subdural haemorrhage, chronic subdural haemorrhage, bleeding and
anticoagulants.
5. Method of review
The Joanna Briggs Institute user guideline version has been taken as a framework for
appraisal of both qualitative and quantitative evidence. It will help maintain the
methodological rigour. The critical appraisal aims to establish the validity. After searching
each paper, it is assessed if it should be retrieved. The next step is a critical appraisal. It will
help determine if the study can be included in the review. During appraisal the hierarchy of
quantitative evidence followed is the RCT with concealed allocation, experimental study
Studies with outcome measure such as subdural haemorrhage, mortality due to pre-
existing use of antiplatelets, and morbidity are considered.
4. Search strategy
The search strategy with a three-step search approach will be used in this review. Initially,
the search will be restricted to the MEDLINE and CINAHL databases. Initially, all the
published and unpublished data will be extracted. The search is based on keywords present in
the title, and abstract, of the paper as well as the index terms that describe the article. In the
second search, all the recognised key terms and index terms are used to carry the search in all
the other selected databases. It includes Cochrane Library, Embase, Clinical Key for Nursing,
Web of Science, Health Source: Nursing/Academic Edition, PubMed, Scopus, Joanna Briggs
Institute EBP database, and CINAHL with Full Text. In the third step, the all the relevant
articles and reports that have been found are screened for a reference list. It will help
determine other studies pertinent to the area of investigation. Studies published in English
and all the observational studies will be considered for the systematic review. The keywords
used for searching the database are Anti-coagulants, Intracranial haemorrhage, Warfarin,
Subdural hematoma, Subdural haemorrhage, chronic subdural haemorrhage, bleeding and
anticoagulants.
5. Method of review
The Joanna Briggs Institute user guideline version has been taken as a framework for
appraisal of both qualitative and quantitative evidence. It will help maintain the
methodological rigour. The critical appraisal aims to establish the validity. After searching
each paper, it is assessed if it should be retrieved. The next step is a critical appraisal. It will
help determine if the study can be included in the review. During appraisal the hierarchy of
quantitative evidence followed is the RCT with concealed allocation, experimental study
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

10SYSTEMATIC REVIEW
without randomisation, controlled observational study (both case-control and cohort) and
lastly observational studies without control. RCTs are critiqued based on the random
allocation of the follow up participants to intervention and control group. It will be checked if
there is a comparison of the outcome rates within time. Randomisation method avoids bias
(performance bias, selection bias, measurement bias and attrition bias) and the same will be
ensured during critical appraisal.
If the study is quasi-experimental, it will be assessed if the participants are allocated to
the different intervention and if the investigator controls the process. In case of observational
studies, the papers will be critically appraised to determine if there is the positive or negative
effect of the intervention on the health outcomes. In case of experimental study, it is assessed
to which degree the potential bias is addressed by the investigator. The quantitative papers
retrieved will be assessed by two independent reviewers to ensure the methodological
validity. Any disagreement will be resolved by discussing with third reviewer.
The next step in the review process is to collect data. This step includes obtaining
necessary information from the findings of the relevant studies obtained. Using the “JBI Data
Extraction Form for observational and experimental studies, comparable/cohort/case-control
studies”, quantitative data will be extracted for the systematic review. This data may
comprise of study methods, population, interventions, and outcomes in significance to the
objectives. The aspects of study for which data will be collected are setting, methodology,
outcome measures, subjects, treatment and comparison, report citation and results. For the
JBI Data Extraction Form, the factors to be calculated and documented are a number of
events, mean, standard deviation. In the comments column of the form, the new information
obtained is documented. Data extraction form will be tested on the sample of included studies
to avoid missing any necessary information. Appropriate measures will be taken to ensure
without randomisation, controlled observational study (both case-control and cohort) and
lastly observational studies without control. RCTs are critiqued based on the random
allocation of the follow up participants to intervention and control group. It will be checked if
there is a comparison of the outcome rates within time. Randomisation method avoids bias
(performance bias, selection bias, measurement bias and attrition bias) and the same will be
ensured during critical appraisal.
If the study is quasi-experimental, it will be assessed if the participants are allocated to
the different intervention and if the investigator controls the process. In case of observational
studies, the papers will be critically appraised to determine if there is the positive or negative
effect of the intervention on the health outcomes. In case of experimental study, it is assessed
to which degree the potential bias is addressed by the investigator. The quantitative papers
retrieved will be assessed by two independent reviewers to ensure the methodological
validity. Any disagreement will be resolved by discussing with third reviewer.
The next step in the review process is to collect data. This step includes obtaining
necessary information from the findings of the relevant studies obtained. Using the “JBI Data
Extraction Form for observational and experimental studies, comparable/cohort/case-control
studies”, quantitative data will be extracted for the systematic review. This data may
comprise of study methods, population, interventions, and outcomes in significance to the
objectives. The aspects of study for which data will be collected are setting, methodology,
outcome measures, subjects, treatment and comparison, report citation and results. For the
JBI Data Extraction Form, the factors to be calculated and documented are a number of
events, mean, standard deviation. In the comments column of the form, the new information
obtained is documented. Data extraction form will be tested on the sample of included studies
to avoid missing any necessary information. Appropriate measures will be taken to ensure
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

11SYSTEMATIC REVIEW
consistency of the data extraction and to remain reliable and unbiased. Consistency will help
in the effective interpretation of results.
The results of the quantitative review will be compiled to develop a conclusion about the
effectiveness of warfarin and anti-platelets and its effect in different studies, participants and
settings. It will include summarising the study purpose for each evidence, the total number of
participants, how the outcomes are measured, and the time period. The final report is based
on the synopsis table. Based on this all the findings will be summarised and discuss its
relevance as per the JBI final report template. The findings will be circulated to the review
team, and common themes will be generated for further discussion in the form of narrative.
6. Results
Articles identified
Inclusion of articles in the systematic review were done with the use of PRISMA
(Preferred Reporting Items for Systematic Reviews and Meta-Analyses), an evidence-based
tool that helped in improving the quality of the systematic review, in addition to providing
substantial transparency in the selection of the articles. The figure provided below illustrates
the process that was involved in selection and extraction of the articles (Fleming, Koletsi and
Pandis 2014). A thorough search of the databases assisted in the extraction of 340 articles,
which were later on thoroughly reviewed to assess their reliability. The titles and abstracts
were screened, followed by elimination of articles that appeared irrelevant. Following
removal of duplicates from the initial hits, 101 articles were obtained, reviewing the titles and
abstracts of which resulted in 43 articles. Upon evaluation of the full text eligibility, only 9
articles were extracted. The Joanna Briggs Institute Critical Appraisal tools were used for
critically determining the methodological quality of the retrieved studies and for evaluating
consistency of the data extraction and to remain reliable and unbiased. Consistency will help
in the effective interpretation of results.
The results of the quantitative review will be compiled to develop a conclusion about the
effectiveness of warfarin and anti-platelets and its effect in different studies, participants and
settings. It will include summarising the study purpose for each evidence, the total number of
participants, how the outcomes are measured, and the time period. The final report is based
on the synopsis table. Based on this all the findings will be summarised and discuss its
relevance as per the JBI final report template. The findings will be circulated to the review
team, and common themes will be generated for further discussion in the form of narrative.
6. Results
Articles identified
Inclusion of articles in the systematic review were done with the use of PRISMA
(Preferred Reporting Items for Systematic Reviews and Meta-Analyses), an evidence-based
tool that helped in improving the quality of the systematic review, in addition to providing
substantial transparency in the selection of the articles. The figure provided below illustrates
the process that was involved in selection and extraction of the articles (Fleming, Koletsi and
Pandis 2014). A thorough search of the databases assisted in the extraction of 340 articles,
which were later on thoroughly reviewed to assess their reliability. The titles and abstracts
were screened, followed by elimination of articles that appeared irrelevant. Following
removal of duplicates from the initial hits, 101 articles were obtained, reviewing the titles and
abstracts of which resulted in 43 articles. Upon evaluation of the full text eligibility, only 9
articles were extracted. The Joanna Briggs Institute Critical Appraisal tools were used for
critically determining the methodological quality of the retrieved studies and for evaluating
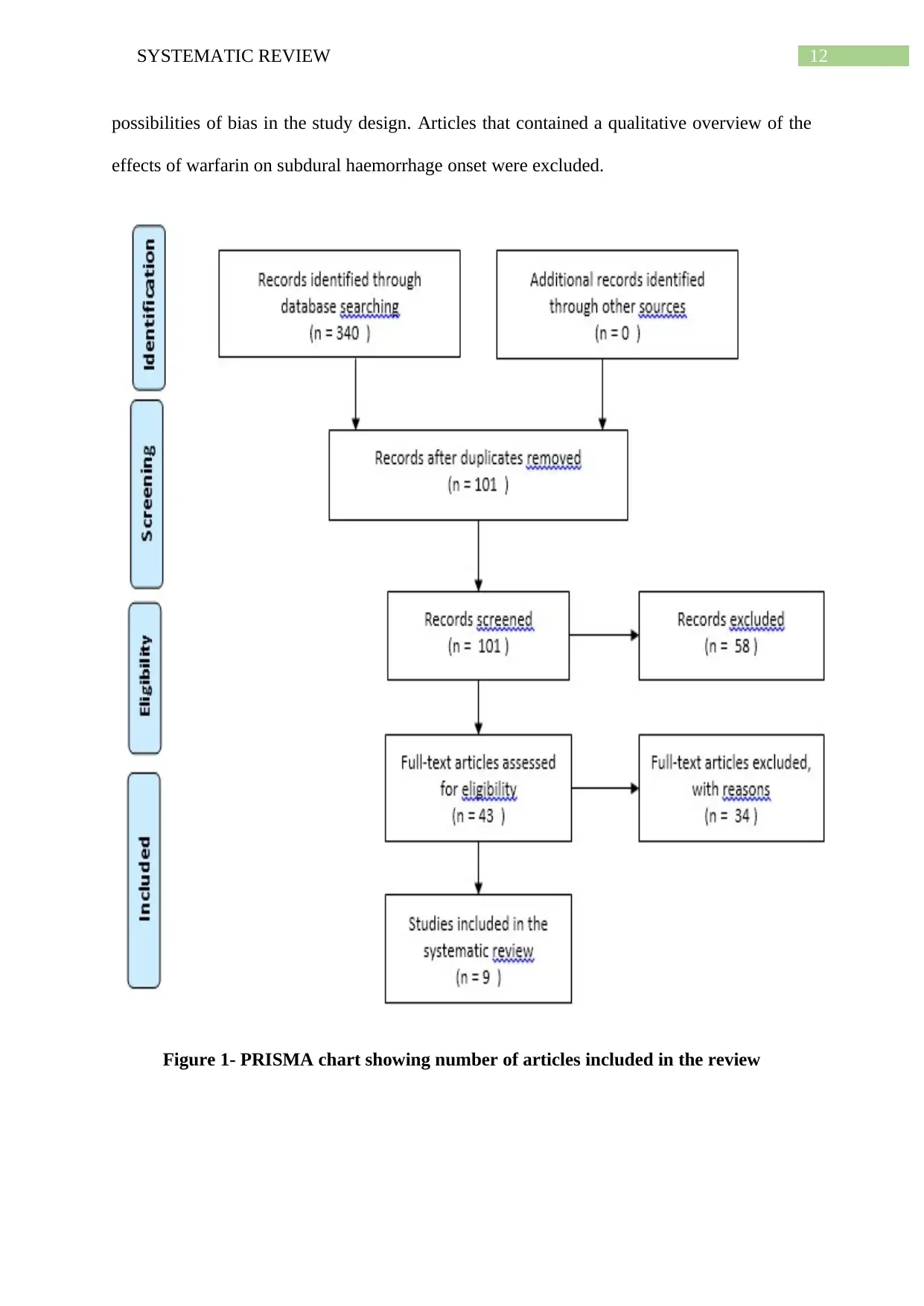
12SYSTEMATIC REVIEW
possibilities of bias in the study design. Articles that contained a qualitative overview of the
effects of warfarin on subdural haemorrhage onset were excluded.
Figure 1- PRISMA chart showing number of articles included in the review
possibilities of bias in the study design. Articles that contained a qualitative overview of the
effects of warfarin on subdural haemorrhage onset were excluded.
Figure 1- PRISMA chart showing number of articles included in the review
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 30
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

