NSC2500 - HIV-AIDS: Pathophysiology, Management, and Zidovudine
VerifiedAdded on 2023/01/18
|24
|1565
|82
Presentation
AI Summary
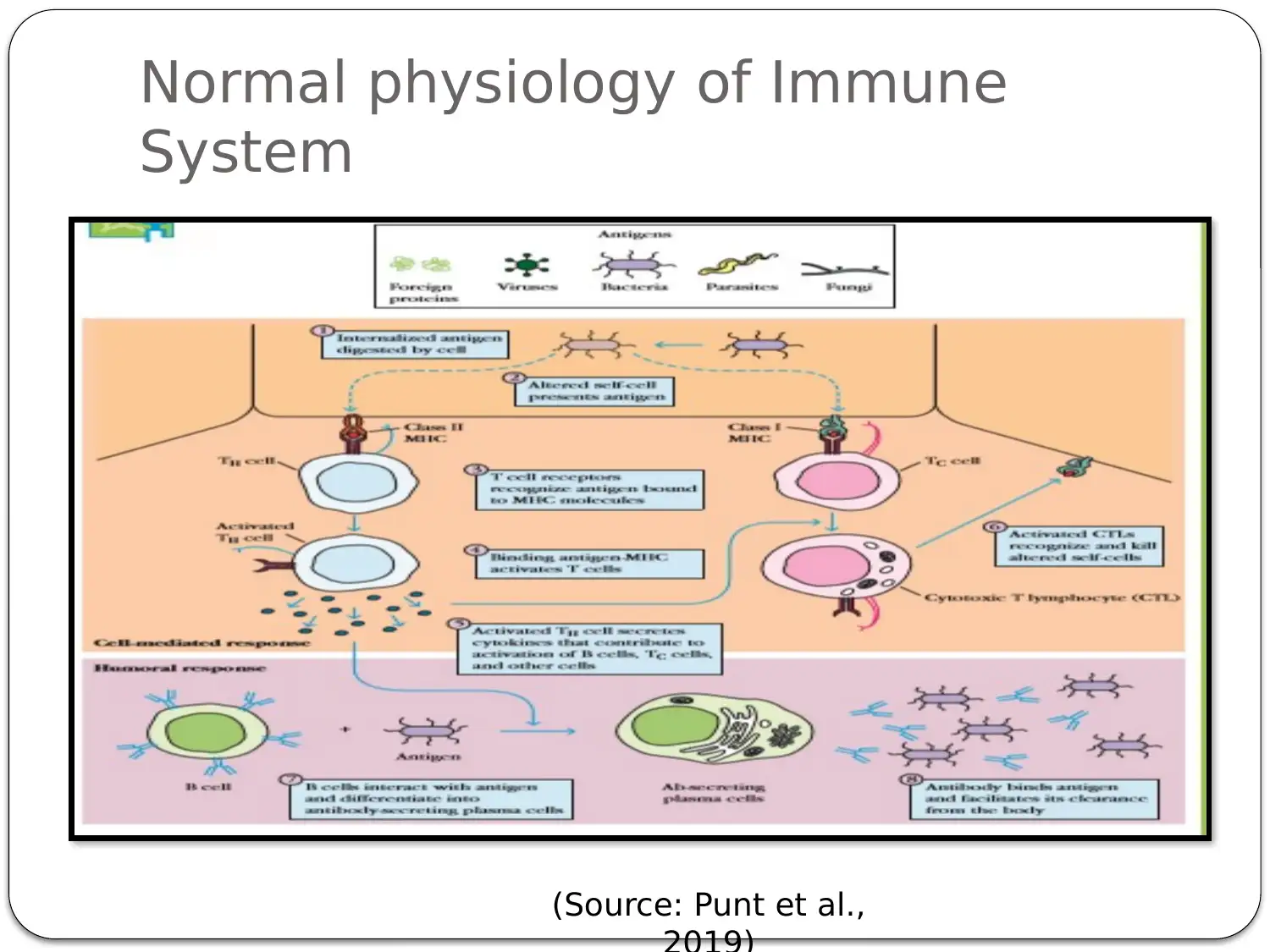
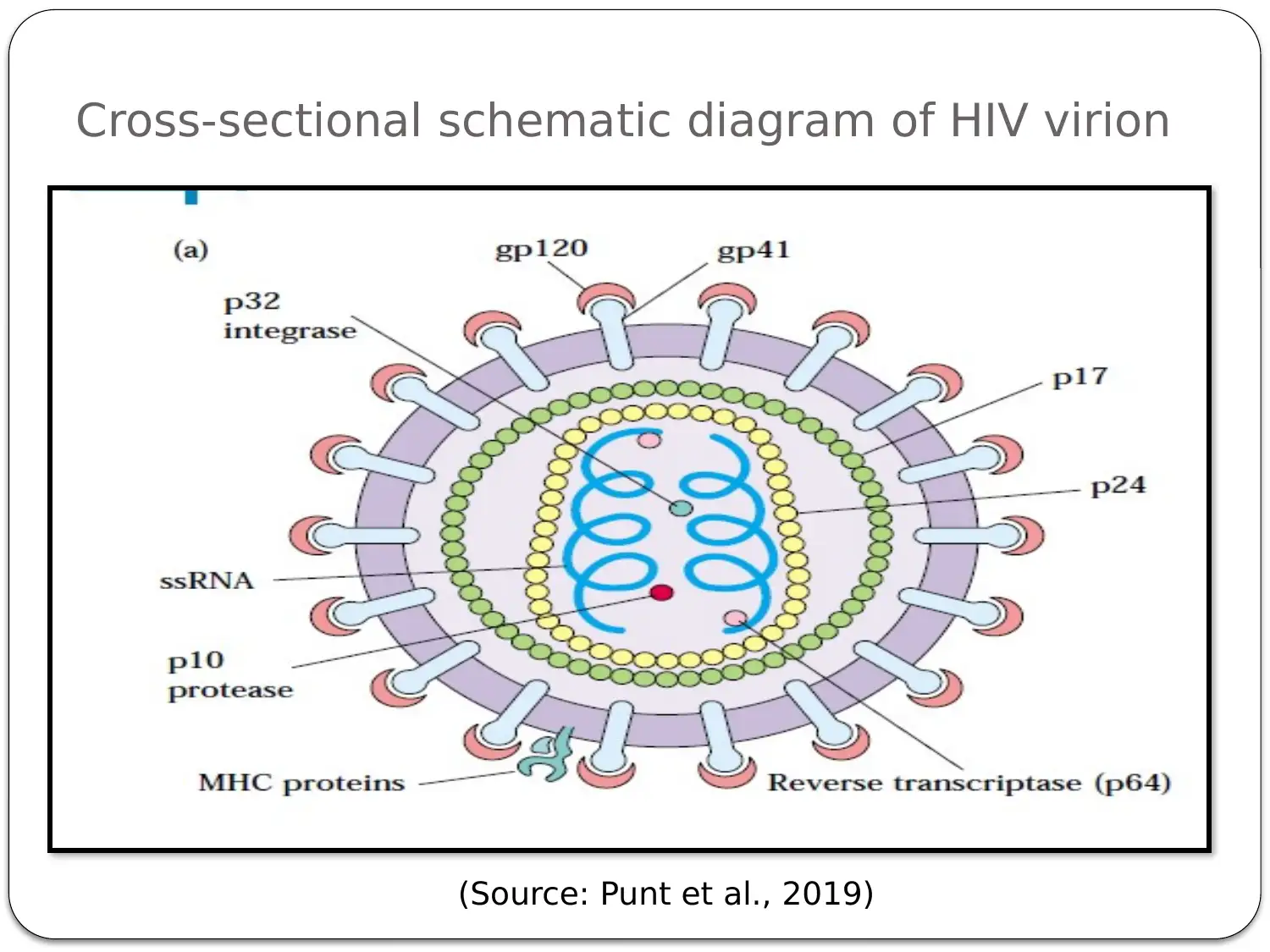
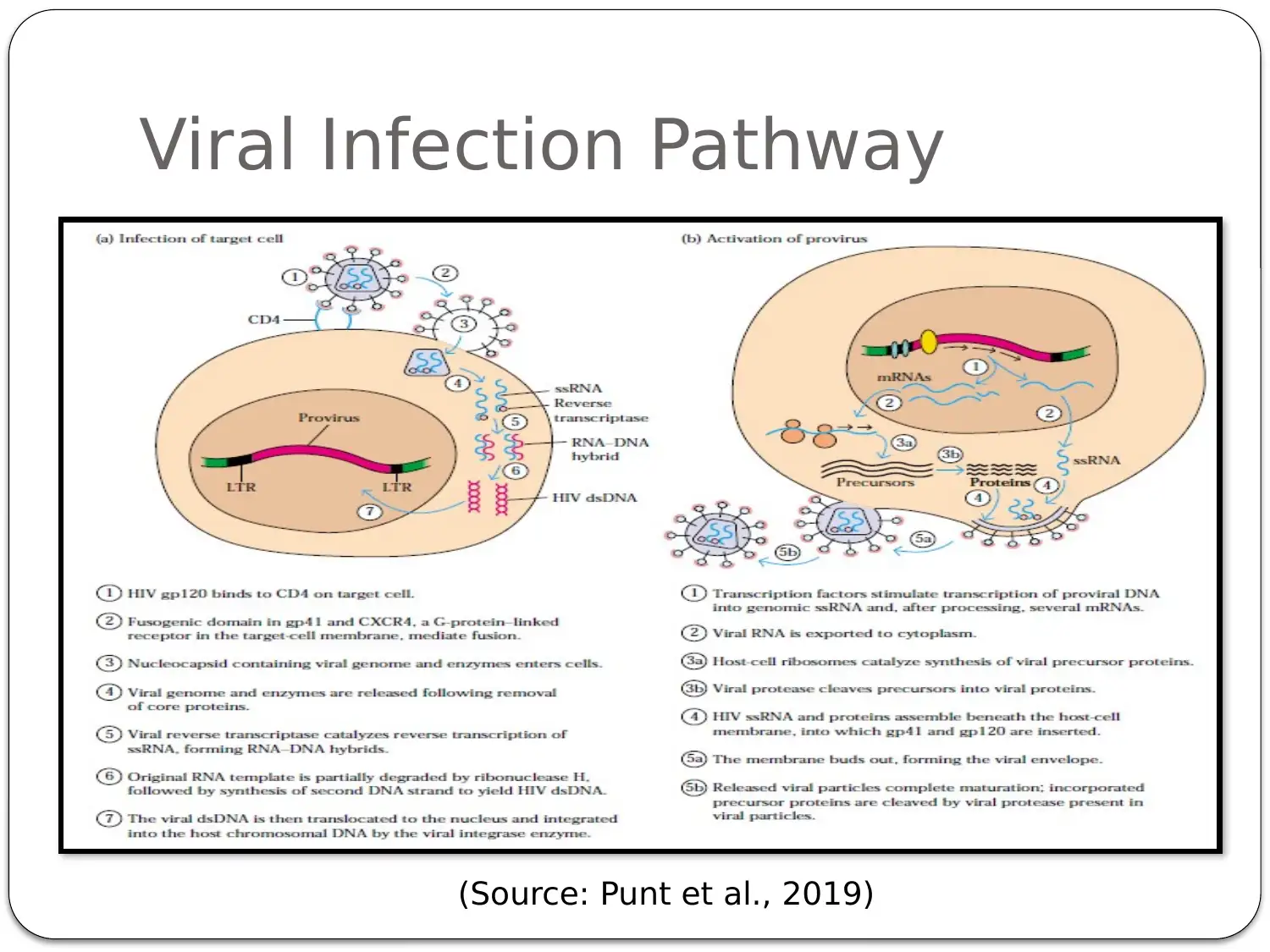
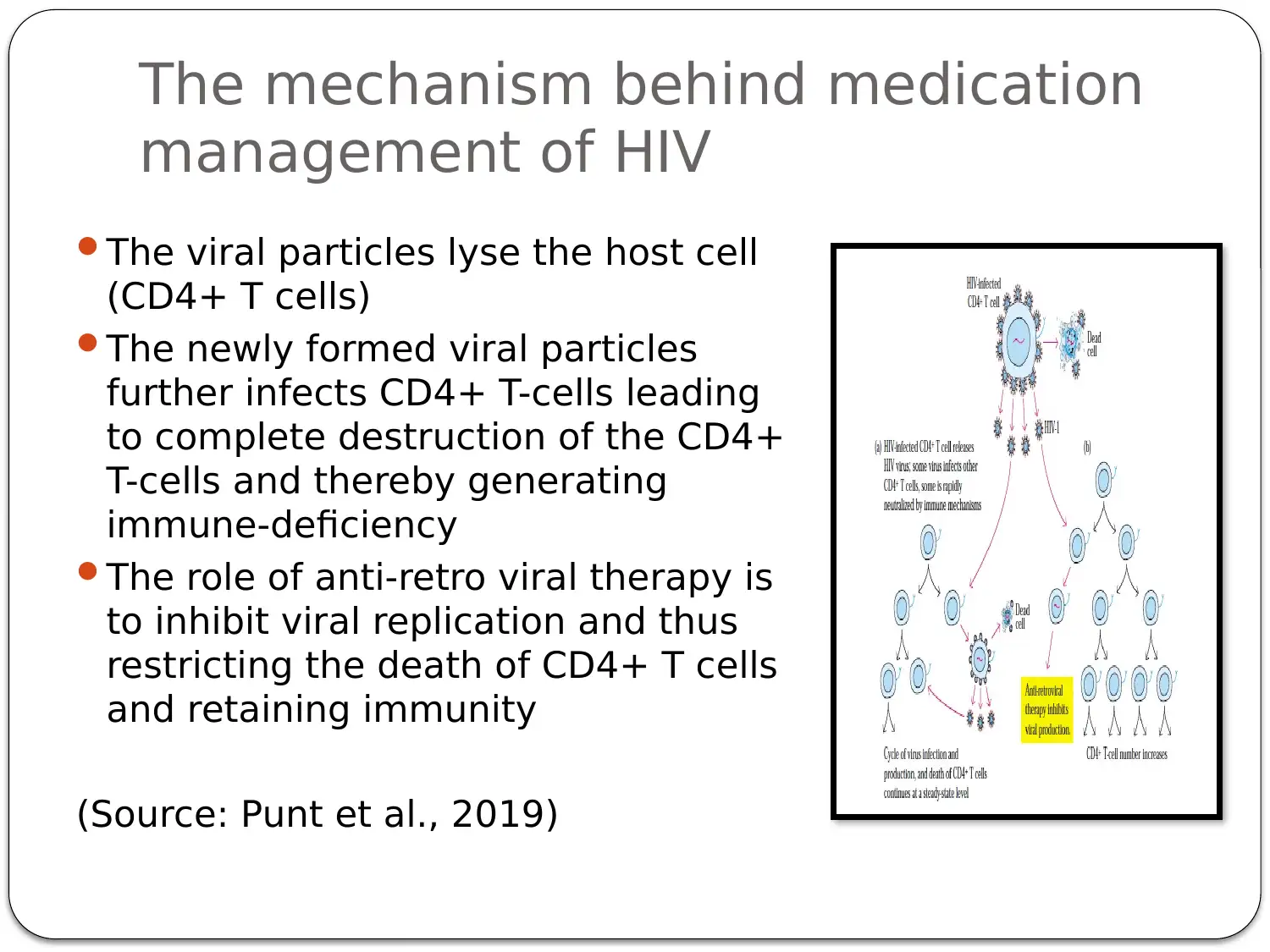
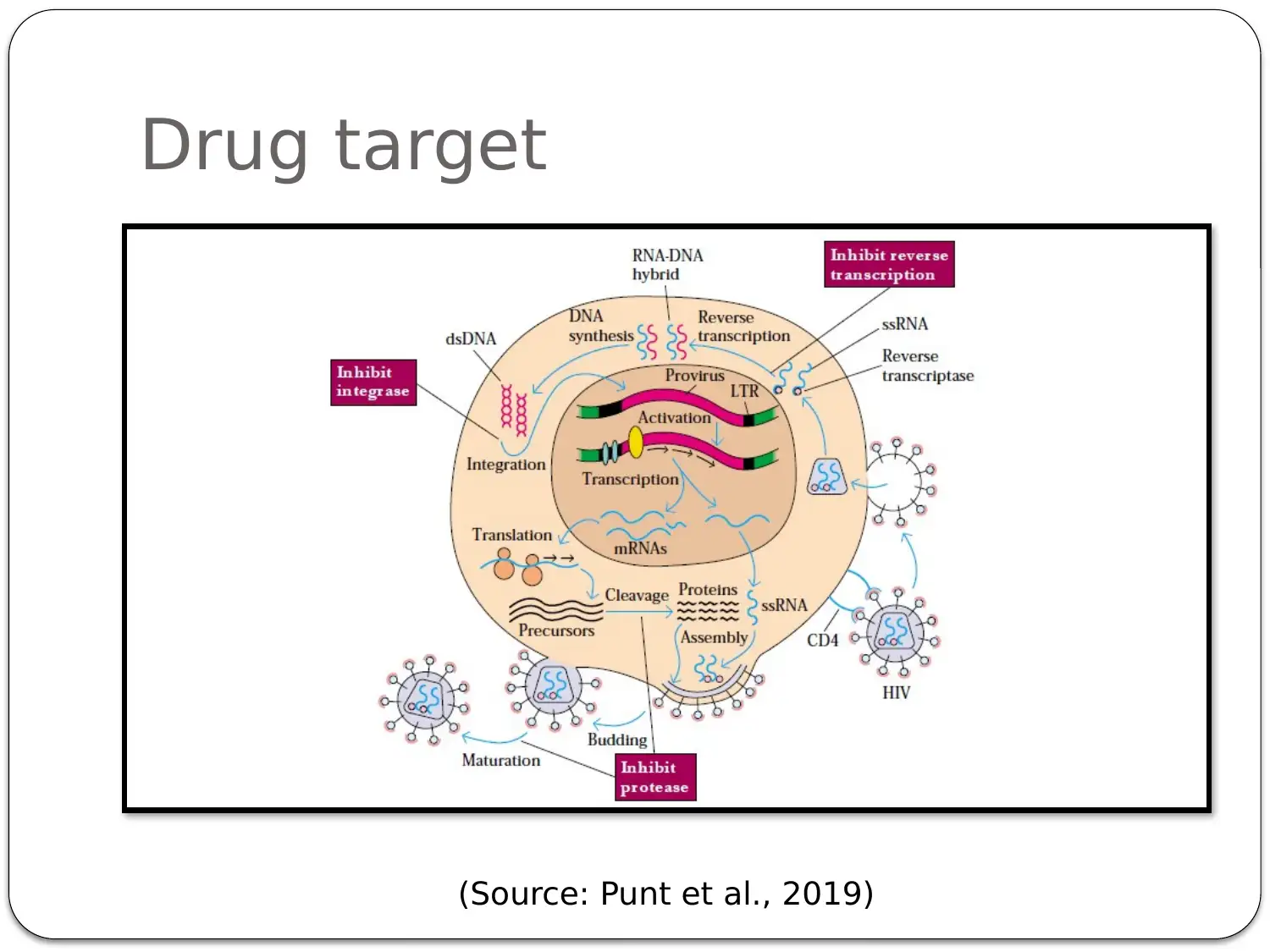
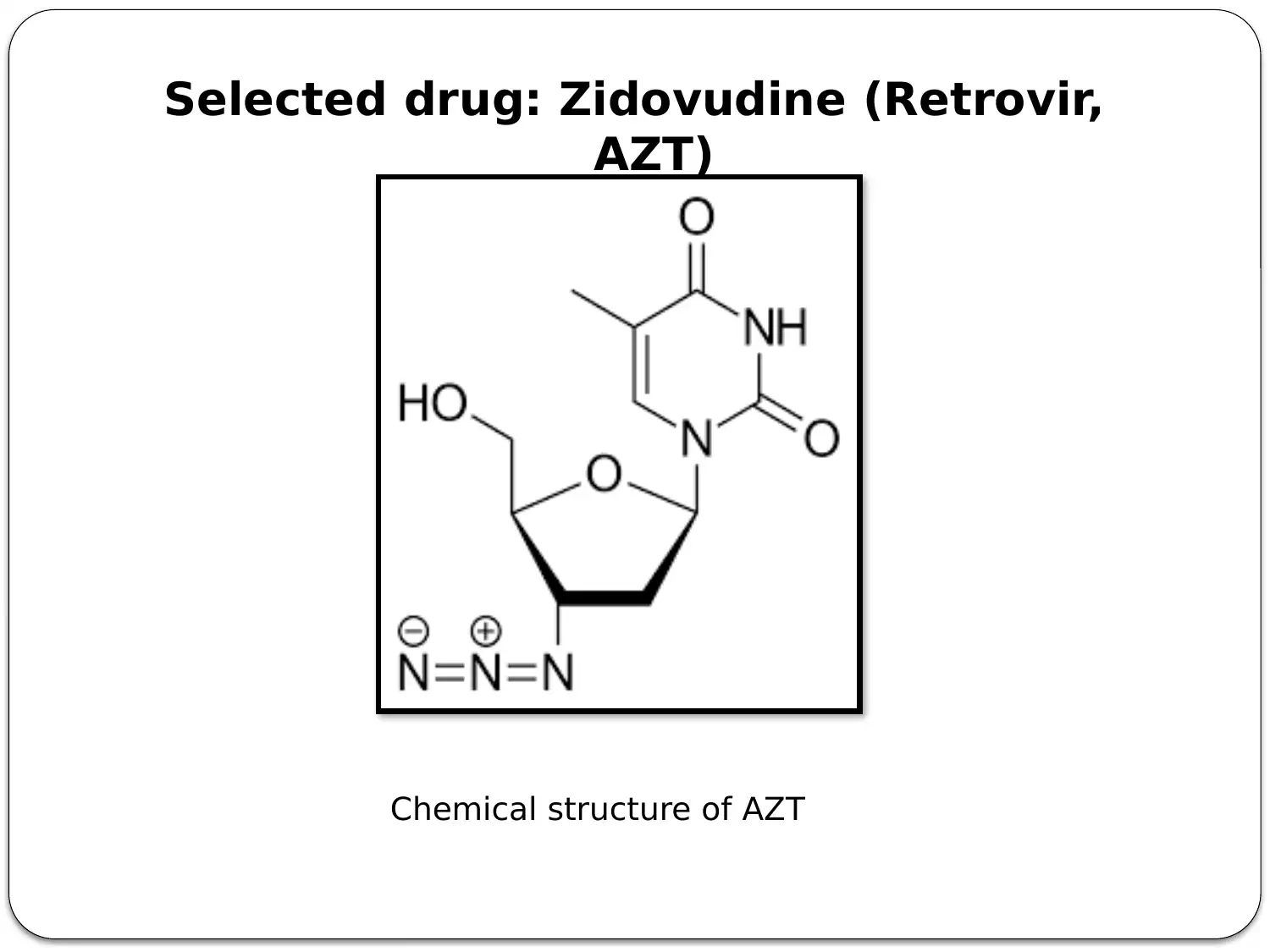
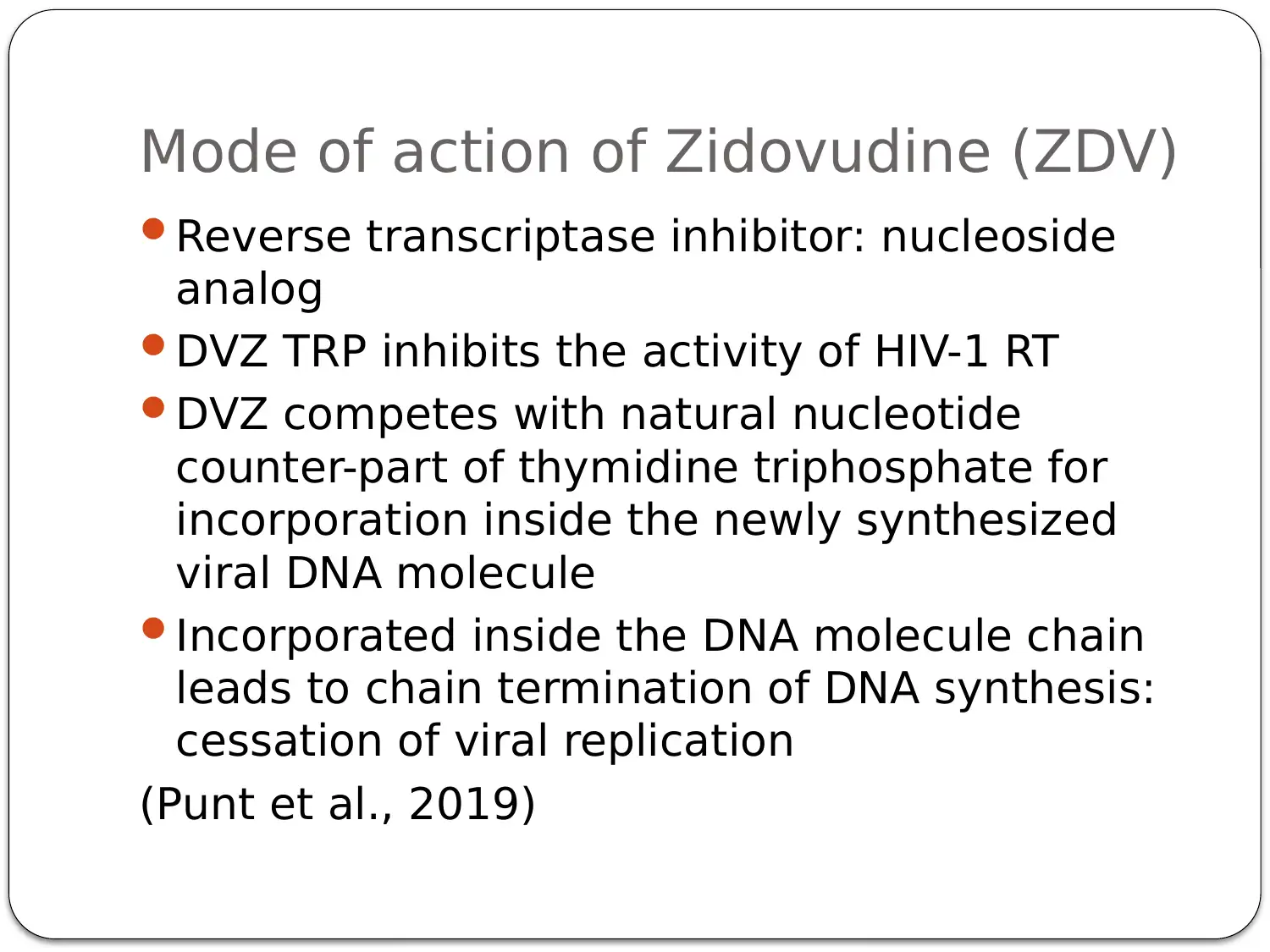
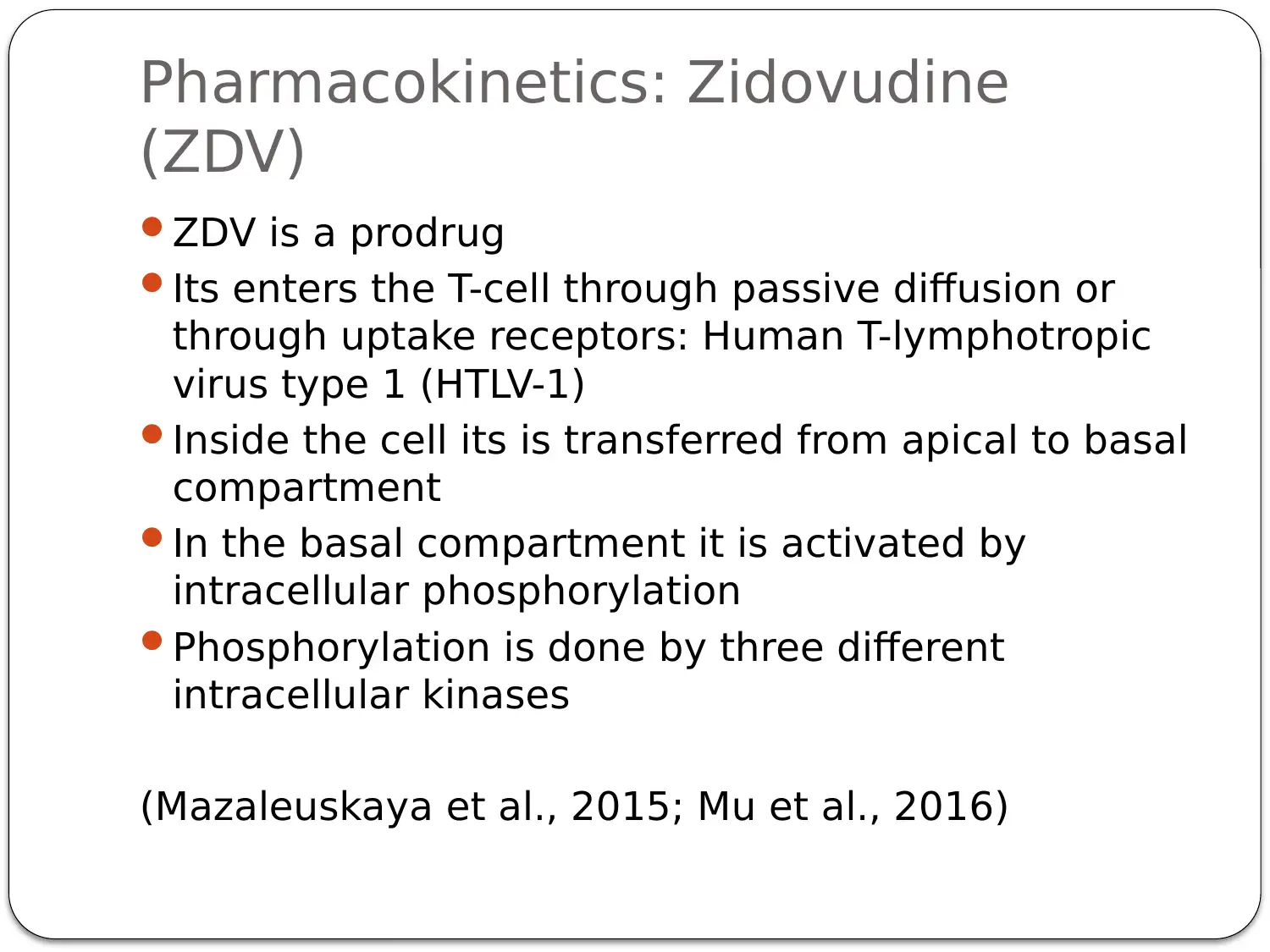
This presentation provides a comprehensive overview of HIV-AIDS, starting with an introduction to the virus as a retroviral disease. It details the normal physiology of the immune system and how HIV disrupts it, focusing on the inactivation of Th cells (CD4+ cells) and the resulting immune deficiency. The presentation explains the process of viral replication, including the role of gp41 and gp120 proteins in infecting host cells. It then delves into the mechanism of action of antiretroviral therapy, specifically highlighting Zidovudine (AZT) as a reverse transcriptase inhibitor. The pharmacokinetics of Zidovudine are discussed, including its prodrug nature, intracellular activation, and metabolic pathways. The presentation also covers the mode of administration, side effects, contraindications, and precautions associated with Zidovudine. Finally, it explores non-pharmacological interventions, such as patient education and safe sex practices, to promote comprehensive HIV/AIDS prevention. The presentation concludes with a summary of key points and a list of references.
1 out of 24








![[object Object]](/_next/static/media/star-bottom.7253800d.svg)