Tropospheric Chlorofluorocarbon (CFC) Concentrations
VerifiedAdded on 2023/01/05
|9
|1600
|77
AI Summary
This document discusses the decline in atmospheric CFC levels and the reasons behind it. It also calculates the CO2 emission intensity for different fuels and recommends the best fuel based on emissions intensity. Additionally, it provides noise control strategies for a construction site and estimates sound pressure levels.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Homework 1
BLOCK 6 HOMEWORK
By (Student’s Name)
Course Name
Professor’s Name
School Name
City and State location
Date
BLOCK 6 HOMEWORK
By (Student’s Name)
Course Name
Professor’s Name
School Name
City and State location
Date
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Homework 2
BLOCK 6 HOMEWORK
Problem 1. Tropospheric chlorofluorocarbon (CFC) concentrations.
There has been a significant decline in the emission of the ozone-depleting substances
(ODS) release into the atmosphere by the humans on the earth surface. The ozone loss intensified
and reached a staggering point the early 1980s. This resulted in the signing of the treaty intended
to protect the ozone layer, dubbed The Montreal Protocol on Substances that Deplete the Ozone
layer. There has been a recovery of the ozone layer in Antarctica and is projected to hit its
original levels between 2050 and 2070, thanks to this treaty signed in Montreal in August 26,
1987.
State at least two (2) reasons for the very slow decline in atmospheric CFC levels.
CFCs in the troposphere are partially dormant, meaning they are passive and hardly react.
Due to this relative stability, they can remain in the troposphere for a relatively long time to
breakdown.
There is still continued release of CFCs into the troposphere. There are countries like
Russia, which currently not in compliance with the Montreal protocol, and still manufactures
CFCs for export and domestic markets.
CFCs have a long life span of up to 200 years, hence can persevere in the troposphere for
a long time before rising into the stratosphere where they can be broken down by the ultraviolet
rays. Hence undergoing photolytic breakdown
Problem 2.
Calculate the CO2 emission intensity (kg CO2/kg fuel burnt) for the following three fuels:
BLOCK 6 HOMEWORK
Problem 1. Tropospheric chlorofluorocarbon (CFC) concentrations.
There has been a significant decline in the emission of the ozone-depleting substances
(ODS) release into the atmosphere by the humans on the earth surface. The ozone loss intensified
and reached a staggering point the early 1980s. This resulted in the signing of the treaty intended
to protect the ozone layer, dubbed The Montreal Protocol on Substances that Deplete the Ozone
layer. There has been a recovery of the ozone layer in Antarctica and is projected to hit its
original levels between 2050 and 2070, thanks to this treaty signed in Montreal in August 26,
1987.
State at least two (2) reasons for the very slow decline in atmospheric CFC levels.
CFCs in the troposphere are partially dormant, meaning they are passive and hardly react.
Due to this relative stability, they can remain in the troposphere for a relatively long time to
breakdown.
There is still continued release of CFCs into the troposphere. There are countries like
Russia, which currently not in compliance with the Montreal protocol, and still manufactures
CFCs for export and domestic markets.
CFCs have a long life span of up to 200 years, hence can persevere in the troposphere for
a long time before rising into the stratosphere where they can be broken down by the ultraviolet
rays. Hence undergoing photolytic breakdown
Problem 2.
Calculate the CO2 emission intensity (kg CO2/kg fuel burnt) for the following three fuels:

Homework 3
The molecular masses of the following compounds are; carbon=12 grams, oxygen=16
grams, hydrogen=1 gram.
1. Coal (C)
C (s) + O2 (g) CO2 (g)
Molecular weights in grams;
C=12, O2 = (16*2) = 32, CO2 = (12+16) =44
12 grams of Coal (C) 44 grams of carbon (IV) oxide gas (CO2)
1000 grams of Coal (C) ?? Grams of carbon (IV) oxide gas (CO2)
1000 grams (C) x 44 grams (CO2)
12 grams (C)
12 grams of carbon burns in oxygen to produce 44 grams of carbon (IV) oxide gas.
Therefore, 1000 grams of carbon (1 kg) burns in oxygen to give out 3,666.67 grams (3.66667 kg)
of carbon (IV) oxide.
Therefore the CO2 emission intensity of coal is, (kg CO2/kg fuel burnt) = (3.66667 kg of
CO2/1 kg of coal (C)) =3.67
2. Natural Gas (methane)
CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (g)
Molecular weights in grams;
CH4=16, 2O2 = (16*4) = 64, CO2 = (12+16) =44, 2H2O= (2*18) =32
=3666.67 grams of (CO2) = 3.67 kg
The molecular masses of the following compounds are; carbon=12 grams, oxygen=16
grams, hydrogen=1 gram.
1. Coal (C)
C (s) + O2 (g) CO2 (g)
Molecular weights in grams;
C=12, O2 = (16*2) = 32, CO2 = (12+16) =44
12 grams of Coal (C) 44 grams of carbon (IV) oxide gas (CO2)
1000 grams of Coal (C) ?? Grams of carbon (IV) oxide gas (CO2)
1000 grams (C) x 44 grams (CO2)
12 grams (C)
12 grams of carbon burns in oxygen to produce 44 grams of carbon (IV) oxide gas.
Therefore, 1000 grams of carbon (1 kg) burns in oxygen to give out 3,666.67 grams (3.66667 kg)
of carbon (IV) oxide.
Therefore the CO2 emission intensity of coal is, (kg CO2/kg fuel burnt) = (3.66667 kg of
CO2/1 kg of coal (C)) =3.67
2. Natural Gas (methane)
CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (g)
Molecular weights in grams;
CH4=16, 2O2 = (16*4) = 64, CO2 = (12+16) =44, 2H2O= (2*18) =32
=3666.67 grams of (CO2) = 3.67 kg

Homework 4
16 grams of CH4 (methane) 44 grams of carbon (IV) oxide gas (CO2)
1000 grams of CH4 (methane) ?? Grams of carbon (IV) oxide gas (CO2)
1000 grams (CH4) x 44 grams (CH4)
16 grams (CH4)
16 grams of CH4 (methane) burns in the presence of oxygen to produce 44 grams of
carbon (IV) oxide gas and steam. Therefore, 1000 grams of CH4 (1 kg) burns in oxygen to give
out 2, 750 grams (2.75 kg) of carbon (IV) oxide, and steam.
Therefore the CO2 emission intensity of methane (CH4) is, (kg CO2/kg fuel burnt) = (2.75
kg of CO2/1 kg of methane (CH4)) =2.75
3. Ethanol (C2H5OH)
C2H5OH (l) + 3O2 (g) 2CO2 (g) + 3H2O (g)
Molecular weights in grams;
C2H5OH =46, 3O2 = (3*32) = 96, 2CO2 = (2*44) =88, 3H2O= (3*18) =54
46 grams of (C2H5OH) 88 grams of carbon (IV) oxide gas (CO2)
1000 grams of (C2H5OH) ?? Grams of carbon (IV) oxide gas (CO2)
1000 grams (C2H5OH) x 88 grams (CO2)
46 grams (C2H5OH)
=2750 grams of (CO2) =2.75 kg
=1913.04 grams of (CO2) =1.913 kg
16 grams of CH4 (methane) 44 grams of carbon (IV) oxide gas (CO2)
1000 grams of CH4 (methane) ?? Grams of carbon (IV) oxide gas (CO2)
1000 grams (CH4) x 44 grams (CH4)
16 grams (CH4)
16 grams of CH4 (methane) burns in the presence of oxygen to produce 44 grams of
carbon (IV) oxide gas and steam. Therefore, 1000 grams of CH4 (1 kg) burns in oxygen to give
out 2, 750 grams (2.75 kg) of carbon (IV) oxide, and steam.
Therefore the CO2 emission intensity of methane (CH4) is, (kg CO2/kg fuel burnt) = (2.75
kg of CO2/1 kg of methane (CH4)) =2.75
3. Ethanol (C2H5OH)
C2H5OH (l) + 3O2 (g) 2CO2 (g) + 3H2O (g)
Molecular weights in grams;
C2H5OH =46, 3O2 = (3*32) = 96, 2CO2 = (2*44) =88, 3H2O= (3*18) =54
46 grams of (C2H5OH) 88 grams of carbon (IV) oxide gas (CO2)
1000 grams of (C2H5OH) ?? Grams of carbon (IV) oxide gas (CO2)
1000 grams (C2H5OH) x 88 grams (CO2)
46 grams (C2H5OH)
=2750 grams of (CO2) =2.75 kg
=1913.04 grams of (CO2) =1.913 kg
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Homework 5
46 grams of C2H5OH (ethanol) reacts with oxygen to produce 88 grams of carbon (IV)
oxide gas and steam. Therefore, 1000 grams of C2H5OH (1 kg) burns in oxygen to give out 1,
913.04 grams (1.913 kg) of carbon (IV) oxide, and steam.
Therefore the CO2 emission intensity of C2H5OH (ethanol) is, (kg CO2/kg fuel burnt) = (1.913 kg
of CO2/1 kg of ethanol (C2H5OH) =1.913
What fuel do you recommend based on its emissions intensity?
Ethanol with a CO2 emission intensity of 1.913 will be the best fuel. Ethanol is the least
air pollutant compared to coal and methane having CO2 emission intensities of 3.67 and 2.75
respectively.
Problem 3.
A capstone student of mine measured A-weighted sound pressure levels of 127 dB (A)
from a jackhammer on a construction site averaged over a 15 minute interval. What noise control
strategies would you recommend at a construction site like this?
Personal protective equipment.
Personal protective equipment will be the best safety protection for the workers at the
construction site since a sound pressure 127 (A) dB is far higher than recommended safety
levels. Earplugs are recommended for the user of the equipment (jackhammer). Rules stipulate
that earplugs should be used whenever noise levels exceed 85 dB (A).
46 grams of C2H5OH (ethanol) reacts with oxygen to produce 88 grams of carbon (IV)
oxide gas and steam. Therefore, 1000 grams of C2H5OH (1 kg) burns in oxygen to give out 1,
913.04 grams (1.913 kg) of carbon (IV) oxide, and steam.
Therefore the CO2 emission intensity of C2H5OH (ethanol) is, (kg CO2/kg fuel burnt) = (1.913 kg
of CO2/1 kg of ethanol (C2H5OH) =1.913
What fuel do you recommend based on its emissions intensity?
Ethanol with a CO2 emission intensity of 1.913 will be the best fuel. Ethanol is the least
air pollutant compared to coal and methane having CO2 emission intensities of 3.67 and 2.75
respectively.
Problem 3.
A capstone student of mine measured A-weighted sound pressure levels of 127 dB (A)
from a jackhammer on a construction site averaged over a 15 minute interval. What noise control
strategies would you recommend at a construction site like this?
Personal protective equipment.
Personal protective equipment will be the best safety protection for the workers at the
construction site since a sound pressure 127 (A) dB is far higher than recommended safety
levels. Earplugs are recommended for the user of the equipment (jackhammer). Rules stipulate
that earplugs should be used whenever noise levels exceed 85 dB (A).

Homework 6
Administrative controls
These are the adopted management decisions on work undertakings, work duration and
work replacement or taking turns so as to limit worker exposure to high levels of noise for long
durations. These management decisions involve taking out employees from noise sources,
workers rotation more so those performing noisy tasks and limiting access to noisy areas and
switching off noisy machinery when not in use.
Engineering control.
Modifying the equipment (jackhammer) to make it less noisy or substituting it with less
noisy and quieter equipment. Barriers too can be erected to limit sound movement to unwanted
premises.
Problem 4.
During the noise laboratory session we visited the construction site outside the new
Building 2 that is adjacent to Building 11. In this session, we measured noise levels adjacent to
the sound barrier. Estimate the Fresnel number for a sound wave generated by a vehicle on the
construction site that reaches your measurement site on the other side of the sound barrier. What
level of sound attenuation do you expect from the sound barrier? If you measure a sound
pressure level of 80 dB (A) behind the sound barrier, what sound pressure level do you expect on
the construction site?
Administrative controls
These are the adopted management decisions on work undertakings, work duration and
work replacement or taking turns so as to limit worker exposure to high levels of noise for long
durations. These management decisions involve taking out employees from noise sources,
workers rotation more so those performing noisy tasks and limiting access to noisy areas and
switching off noisy machinery when not in use.
Engineering control.
Modifying the equipment (jackhammer) to make it less noisy or substituting it with less
noisy and quieter equipment. Barriers too can be erected to limit sound movement to unwanted
premises.
Problem 4.
During the noise laboratory session we visited the construction site outside the new
Building 2 that is adjacent to Building 11. In this session, we measured noise levels adjacent to
the sound barrier. Estimate the Fresnel number for a sound wave generated by a vehicle on the
construction site that reaches your measurement site on the other side of the sound barrier. What
level of sound attenuation do you expect from the sound barrier? If you measure a sound
pressure level of 80 dB (A) behind the sound barrier, what sound pressure level do you expect on
the construction site?
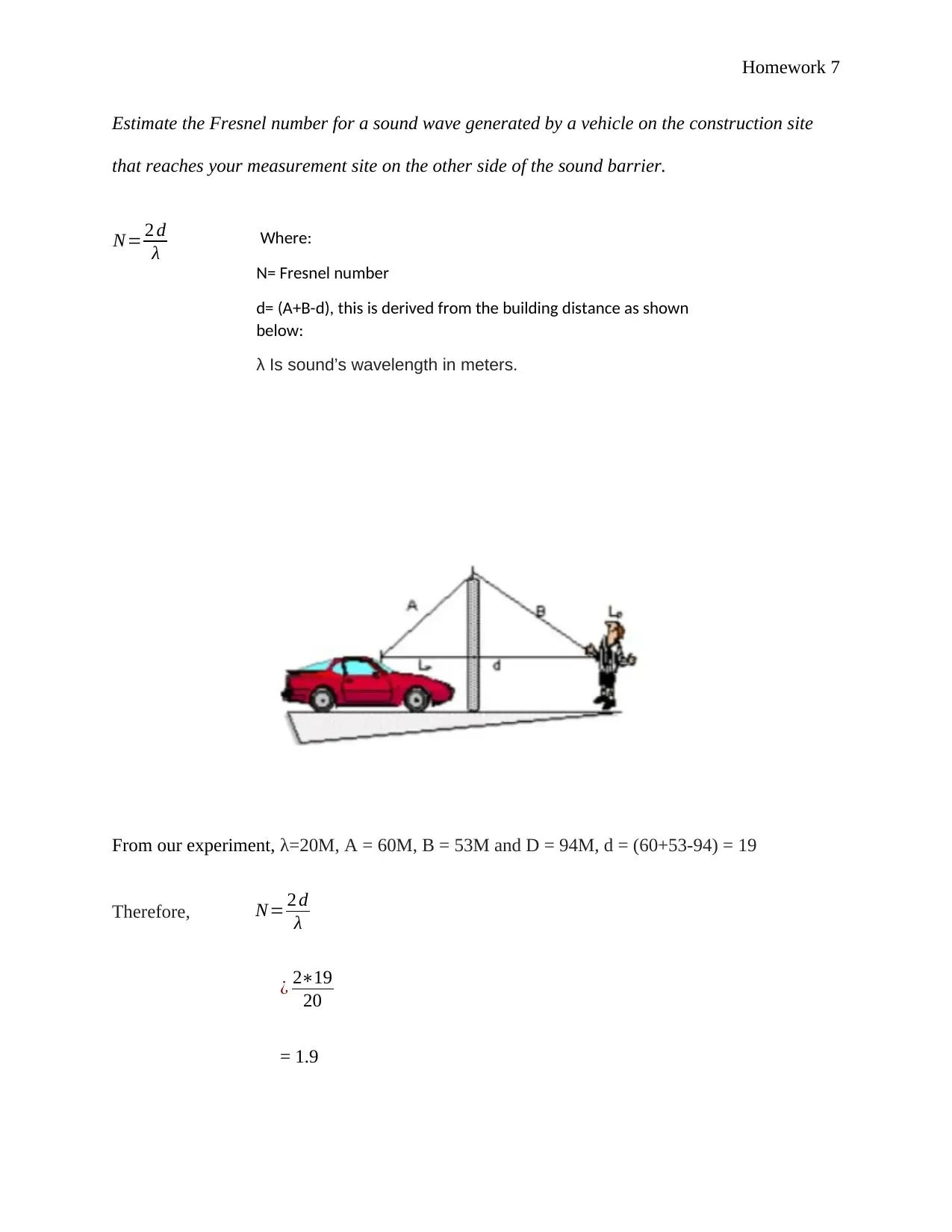
Homework 7
Estimate the Fresnel number for a sound wave generated by a vehicle on the construction site
that reaches your measurement site on the other side of the sound barrier.
N= 2 d
λ
From our experiment, λ=20M, A = 60M, B = 53M and D = 94M, d = (60+53-94) = 19
Therefore, N= 2 d
λ
¿ 2∗19
20
= 1.9
Where:
N= Fresnel number
d= (A+B-d), this is derived from the building distance as shown
below:
λ Is sound’s wavelength in meters.
Estimate the Fresnel number for a sound wave generated by a vehicle on the construction site
that reaches your measurement site on the other side of the sound barrier.
N= 2 d
λ
From our experiment, λ=20M, A = 60M, B = 53M and D = 94M, d = (60+53-94) = 19
Therefore, N= 2 d
λ
¿ 2∗19
20
= 1.9
Where:
N= Fresnel number
d= (A+B-d), this is derived from the building distance as shown
below:
λ Is sound’s wavelength in meters.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
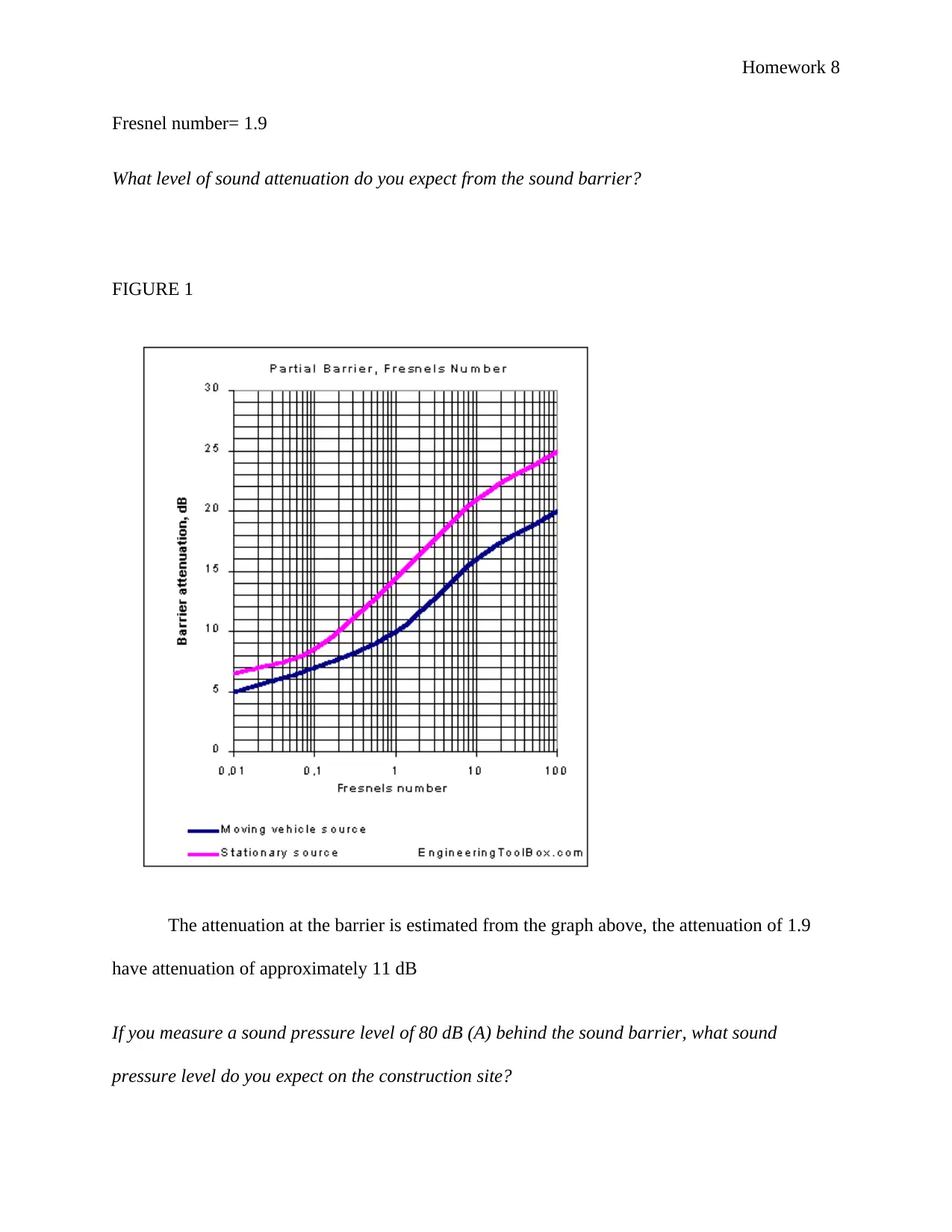
Homework 8
Fresnel number= 1.9
What level of sound attenuation do you expect from the sound barrier?
FIGURE 1
The attenuation at the barrier is estimated from the graph above, the attenuation of 1.9
have attenuation of approximately 11 dB
If you measure a sound pressure level of 80 dB (A) behind the sound barrier, what sound
pressure level do you expect on the construction site?
Fresnel number= 1.9
What level of sound attenuation do you expect from the sound barrier?
FIGURE 1
The attenuation at the barrier is estimated from the graph above, the attenuation of 1.9
have attenuation of approximately 11 dB
If you measure a sound pressure level of 80 dB (A) behind the sound barrier, what sound
pressure level do you expect on the construction site?

Homework 9
Lp(R1) = Lp(R2) + 20·Log10(R2/R1)
Where:
Lp (R1) = Sound Pressure at pressure level of 80 dB (A)
Lp (R2) = Sound Pressure Level at construction site
R1 = Distance at construction site which we put at 1 meter.
R2 = Distance from construction site to point A
Therefore, Lp(R1) = Lp(R2) + 20·Log10(R2/R1)
=80+20*log 10 (94/1)
= 119.46 dB
Lp(R1) = Lp(R2) + 20·Log10(R2/R1)
Where:
Lp (R1) = Sound Pressure at pressure level of 80 dB (A)
Lp (R2) = Sound Pressure Level at construction site
R1 = Distance at construction site which we put at 1 meter.
R2 = Distance from construction site to point A
Therefore, Lp(R1) = Lp(R2) + 20·Log10(R2/R1)
=80+20*log 10 (94/1)
= 119.46 dB
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.