Renewable Energy in Domestic Hot Water Systems & Specific Heat
VerifiedAdded on 2023/06/12
|21
|5125
|162
Report
AI Summary
This report provides a comprehensive analysis of domestic hot water systems, focusing on the integration of renewable energy sources and the scientific principles governing their design. It begins by examining the factors influencing the design of unvented domestic hot water systems, including user needs, safety, energy efficiency, water efficiency, durability, cost, sustainability, materials, and technology. The report details the use of various safety devices such as expansion vessels, twin thermostats, and pressure relief valves to ensure the safe and efficient operation of these systems. It further explores current renewable technologies for hot water systems, including solar thermal, ground and air source heat pumps, biomass boilers, direct and deep geothermal systems, wind turbines, and hydroelectric technology, evaluating their installation, practicality, long-term cost, maintenance, benefits, limitations, and opportunities. The second part of the report presents an experiment on determining the specific heat of various metals, outlining the introduction, aims and objectives, apparatus, procedure, results, analysis, and conclusions. The report highlights the importance of renewable energy in reducing environmental impact and lowering long-term costs associated with domestic hot water systems, emphasizing the need for sustainable and efficient designs.

Hot Water Systems and Specific Heat Experiment 1
DOMESTIC HOT WATER SYSTEMS AND SPECIFIC HEAT EXPERIMENT
Name
Professor
Course
University
City/state
Date
DOMESTIC HOT WATER SYSTEMS AND SPECIFIC HEAT EXPERIMENT
Name
Professor
Course
University
City/state
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Hot Water Systems and Specific Heat Experiment 2
Table of Contents
Part One.....................................................................................................................................................3
1. Scientific principles affecting the design of unvented domestic water systems.........................3
2. Use of safety devices for hot water systems..................................................................................5
3. Current technologies available for renewable hot water systems..............................................6
3.1. Solar thermal technology.........................................................................................................7
3.2. Ground and air source heat pumps...........................................................................................8
3.3. Biomass boilers........................................................................................................................8
3.4. Direct geothermal and deep geothermal systems.....................................................................8
3.5. Wind turbine............................................................................................................................9
3.6. Hydroelectric technology.........................................................................................................9
4. Analysis of use of renewable methods in domestic hot water systems.......................................9
4.1. Installation.............................................................................................................................10
4.2. Practicability..........................................................................................................................10
4.3. Long term cost.......................................................................................................................10
4.4. Maintenance...........................................................................................................................11
4.5. Benefits..................................................................................................................................11
4.6. Limitations.............................................................................................................................11
4.7. Opportunities.........................................................................................................................12
Part Two: Experiment: Determination of specific heat of various metals..........................................12
1. Introduction.................................................................................................................................12
2. Aims and Objectives....................................................................................................................13
3. Apparatus.....................................................................................................................................13
4. Procedure.....................................................................................................................................13
5. Results..........................................................................................................................................14
6. Analysis and Discussion...............................................................................................................18
7. Conclusions..................................................................................................................................19
References................................................................................................................................................19
Table of Contents
Part One.....................................................................................................................................................3
1. Scientific principles affecting the design of unvented domestic water systems.........................3
2. Use of safety devices for hot water systems..................................................................................5
3. Current technologies available for renewable hot water systems..............................................6
3.1. Solar thermal technology.........................................................................................................7
3.2. Ground and air source heat pumps...........................................................................................8
3.3. Biomass boilers........................................................................................................................8
3.4. Direct geothermal and deep geothermal systems.....................................................................8
3.5. Wind turbine............................................................................................................................9
3.6. Hydroelectric technology.........................................................................................................9
4. Analysis of use of renewable methods in domestic hot water systems.......................................9
4.1. Installation.............................................................................................................................10
4.2. Practicability..........................................................................................................................10
4.3. Long term cost.......................................................................................................................10
4.4. Maintenance...........................................................................................................................11
4.5. Benefits..................................................................................................................................11
4.6. Limitations.............................................................................................................................11
4.7. Opportunities.........................................................................................................................12
Part Two: Experiment: Determination of specific heat of various metals..........................................12
1. Introduction.................................................................................................................................12
2. Aims and Objectives....................................................................................................................13
3. Apparatus.....................................................................................................................................13
4. Procedure.....................................................................................................................................13
5. Results..........................................................................................................................................14
6. Analysis and Discussion...............................................................................................................18
7. Conclusions..................................................................................................................................19
References................................................................................................................................................19

Hot Water Systems and Specific Heat Experiment 3
Part One
1. Scientific principles affecting the design of unvented domestic water systems
Hot water is a basic need in many domestic buildings. Hot water systems are installed in
these buildings to supply hot water, depending on the occupants’ needs. The hot water is usually
used for space heating, cooking, bathing or cleaning. The main focus in this report is unvented
hot water systems used for space heating in domestic buildings. An unvented hot water system is
a pressurized system that is fed directly from water feed in the cold water mains, as shown in
Figure 1 below. This system does not need a cold water storage tank. The water in unvented hot
water system can be heated indirectly through central heating system, biomass, heat pumps or
solar thermal technology, or directly through an immersion heater. For an unvented domestic hot
water systems to perform their intended functions effectively, they must be designed by
considering several factors. Some of the scientific principles that affect the design of unvented
domestic hot water systems are:
User’s needs – this is the first scientific principle guiding design of unvented domestic
hot water systems. The system should be designed to meet the hot water demands of the
building’s occupants. This requires consideration of factors such as the number of persons living
in the building (which affect the size and flow capacity of the hot water system) (Zhang, et al.,
2014), local temperatures, orientation and layout of the building and indoor thermal comfort
requirements.
Safety – the hot water system should be designed so as to meet certain safety
requirements and standards. These safety requirements are mainly concerned with temperature
and pressure control that reduces the risk of exploding or scalding.
Part One
1. Scientific principles affecting the design of unvented domestic water systems
Hot water is a basic need in many domestic buildings. Hot water systems are installed in
these buildings to supply hot water, depending on the occupants’ needs. The hot water is usually
used for space heating, cooking, bathing or cleaning. The main focus in this report is unvented
hot water systems used for space heating in domestic buildings. An unvented hot water system is
a pressurized system that is fed directly from water feed in the cold water mains, as shown in
Figure 1 below. This system does not need a cold water storage tank. The water in unvented hot
water system can be heated indirectly through central heating system, biomass, heat pumps or
solar thermal technology, or directly through an immersion heater. For an unvented domestic hot
water systems to perform their intended functions effectively, they must be designed by
considering several factors. Some of the scientific principles that affect the design of unvented
domestic hot water systems are:
User’s needs – this is the first scientific principle guiding design of unvented domestic
hot water systems. The system should be designed to meet the hot water demands of the
building’s occupants. This requires consideration of factors such as the number of persons living
in the building (which affect the size and flow capacity of the hot water system) (Zhang, et al.,
2014), local temperatures, orientation and layout of the building and indoor thermal comfort
requirements.
Safety – the hot water system should be designed so as to meet certain safety
requirements and standards. These safety requirements are mainly concerned with temperature
and pressure control that reduces the risk of exploding or scalding.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Hot Water Systems and Specific Heat Experiment 4
Energy efficiency – the system should be designed to consume as less energy as possible
throughout its lifecycle (Crawford & Treloar, 2012). The source of energy must also be
considered. Today, there is need to design unvented domestic hot water systems that use
renewable energy.
Water efficiency – the system must also be designed to minimize wastage of water. It has
to be watertight so as to avoid or minimize leakages, and should only operate when needed. It
should also heat and supply water at exact quantities to avoid wastage.
Performance efficiency – the system should be designed with the ability to operate
properly and achieve its objectives within set conditions and limits, with minimal variations.
Durability – the system should also be durable so as to avoid frequent repair or
replacement. This prevents wastage of natural resources and also saves users money.
Cost – the system should also be designed such that the cost of purchasing, installing,
operating and maintaining is reasonable.
Sustainability – the design of a domestic hot water system should also be sustainable so
as to increase resource efficiency throughout the lifecycle of the system. Nevertheless, this
should not compromise the performance of the system.
Materials – the materials used to make the hot water system also affect the design of the
system. These materials should be environmentally friendly (Martinopoulos, et al., 2013), cost-
effective and able to be used within anticipated conditions (temperature and pressure).
Technology – modern technology has become a very important scientific principle
guiding the design of unvented domestic hot water systems. Technology should be used to
Energy efficiency – the system should be designed to consume as less energy as possible
throughout its lifecycle (Crawford & Treloar, 2012). The source of energy must also be
considered. Today, there is need to design unvented domestic hot water systems that use
renewable energy.
Water efficiency – the system must also be designed to minimize wastage of water. It has
to be watertight so as to avoid or minimize leakages, and should only operate when needed. It
should also heat and supply water at exact quantities to avoid wastage.
Performance efficiency – the system should be designed with the ability to operate
properly and achieve its objectives within set conditions and limits, with minimal variations.
Durability – the system should also be durable so as to avoid frequent repair or
replacement. This prevents wastage of natural resources and also saves users money.
Cost – the system should also be designed such that the cost of purchasing, installing,
operating and maintaining is reasonable.
Sustainability – the design of a domestic hot water system should also be sustainable so
as to increase resource efficiency throughout the lifecycle of the system. Nevertheless, this
should not compromise the performance of the system.
Materials – the materials used to make the hot water system also affect the design of the
system. These materials should be environmentally friendly (Martinopoulos, et al., 2013), cost-
effective and able to be used within anticipated conditions (temperature and pressure).
Technology – modern technology has become a very important scientific principle
guiding the design of unvented domestic hot water systems. Technology should be used to
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Hot Water Systems and Specific Heat Experiment 5
achieve all the above mentioned principles. Most importantly is that the technology is used to
improve the efficiency of the system, reduce cost, improve sustainability and reduce wastage.
One of such technologies is nanotechnology and use of integrated renewable energy.
Figure 1: Schematic diagram of an unvented domestic hot water system (McDonald Engineers
UK, 2018)
2. Use of safety devices for hot water systems
Unvented hot water systems have different safety mechanisms, including expansion
vessels, twin thermostats, pressure relief valves, etc. These devices ensure efficient and safe
operation of the systems. To ensure safety of hot water systems at all times, the safety devices
should be inspected and tested frequently by well-trained technicians. Some of the safety devices
include the following:
Expansion vessels – these safety devices are used to allow expansion of water as it gets
heated up (McDonald Engineers UK, 2018) thus preventing the system from scalding.
achieve all the above mentioned principles. Most importantly is that the technology is used to
improve the efficiency of the system, reduce cost, improve sustainability and reduce wastage.
One of such technologies is nanotechnology and use of integrated renewable energy.
Figure 1: Schematic diagram of an unvented domestic hot water system (McDonald Engineers
UK, 2018)
2. Use of safety devices for hot water systems
Unvented hot water systems have different safety mechanisms, including expansion
vessels, twin thermostats, pressure relief valves, etc. These devices ensure efficient and safe
operation of the systems. To ensure safety of hot water systems at all times, the safety devices
should be inspected and tested frequently by well-trained technicians. Some of the safety devices
include the following:
Expansion vessels – these safety devices are used to allow expansion of water as it gets
heated up (McDonald Engineers UK, 2018) thus preventing the system from scalding.

Hot Water Systems and Specific Heat Experiment 6
Twin thermostats – these safety devices ensure that the system operates within pre-
determined temperature and pressure thus avoiding damage of the system. Most importantly is
that the safety devices ensures that the hot water supplied by the system is at the right
temperature and does not burn end users.
Pressure relief valves – they are used to prevent excess pressure in the hot water systems.
These valves ensure that pressure inside the domestic hot water system is within designed limits.
Anti-scald valve – this device ensures that water released from the hot water system is at
the desired temperature. If the water is too hot, the device mixes it with some cold water to
prevent scolding of end users.
Water-level control – this device is designed to ensure that water level in a hot water
system does not fall below a certain or programmed volume.
Low-water level fuel cutoff – this device is used to shut down the system by cutting off
fuel in case water level falls below a programmed volume (Piper, 2010).
Discharge tube or drain pipe – this device is used to release excess water from the system
in case it passes a predetermined level.
Fuel line fire safety valve – this device is used to disconnect the system from power in
case of a fire event (InspectAPedia, 2015).
3. Current technologies available for renewable hot water systems
Energy efficiency is very important when designing or selecting a hot water system. The
main objective should be to reduce the cost of energy used by the hot water system (De Oliveira,
et al., 2016) and the environmental impacts associated with the hot water system. For many
Twin thermostats – these safety devices ensure that the system operates within pre-
determined temperature and pressure thus avoiding damage of the system. Most importantly is
that the safety devices ensures that the hot water supplied by the system is at the right
temperature and does not burn end users.
Pressure relief valves – they are used to prevent excess pressure in the hot water systems.
These valves ensure that pressure inside the domestic hot water system is within designed limits.
Anti-scald valve – this device ensures that water released from the hot water system is at
the desired temperature. If the water is too hot, the device mixes it with some cold water to
prevent scolding of end users.
Water-level control – this device is designed to ensure that water level in a hot water
system does not fall below a certain or programmed volume.
Low-water level fuel cutoff – this device is used to shut down the system by cutting off
fuel in case water level falls below a programmed volume (Piper, 2010).
Discharge tube or drain pipe – this device is used to release excess water from the system
in case it passes a predetermined level.
Fuel line fire safety valve – this device is used to disconnect the system from power in
case of a fire event (InspectAPedia, 2015).
3. Current technologies available for renewable hot water systems
Energy efficiency is very important when designing or selecting a hot water system. The
main objective should be to reduce the cost of energy used by the hot water system (De Oliveira,
et al., 2016) and the environmental impacts associated with the hot water system. For many
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Hot Water Systems and Specific Heat Experiment 7
years, hot water systems have been using non-renewable energy. However, the current global
problem of climate change has prompted design of hot water systems that use renewable energy.
Today, there are several hot water systems that can be powered by solar, wind, biomass,
hydropower or geothermal energy, or a combination of different types of energy (Palizban &
Kauhaniemi, 2016). Some of the current renewable technologies that can be used in domestic hot
water systems are:
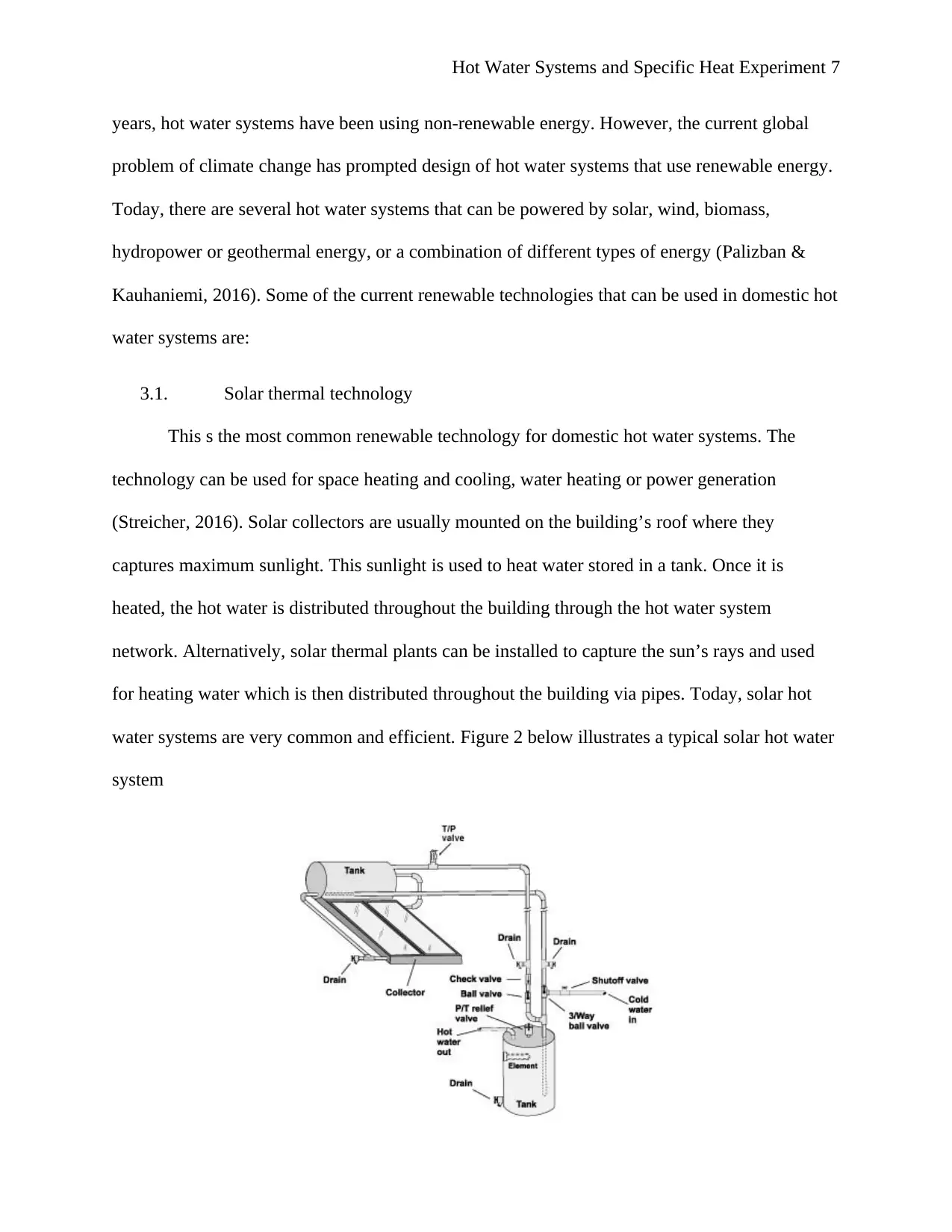
3.1. Solar thermal technology
This s the most common renewable technology for domestic hot water systems. The
technology can be used for space heating and cooling, water heating or power generation
(Streicher, 2016). Solar collectors are usually mounted on the building’s roof where they
captures maximum sunlight. This sunlight is used to heat water stored in a tank. Once it is
heated, the hot water is distributed throughout the building through the hot water system
network. Alternatively, solar thermal plants can be installed to capture the sun’s rays and used
for heating water which is then distributed throughout the building via pipes. Today, solar hot
water systems are very common and efficient. Figure 2 below illustrates a typical solar hot water
system
years, hot water systems have been using non-renewable energy. However, the current global
problem of climate change has prompted design of hot water systems that use renewable energy.
Today, there are several hot water systems that can be powered by solar, wind, biomass,
hydropower or geothermal energy, or a combination of different types of energy (Palizban &
Kauhaniemi, 2016). Some of the current renewable technologies that can be used in domestic hot
water systems are:
3.1. Solar thermal technology
This s the most common renewable technology for domestic hot water systems. The
technology can be used for space heating and cooling, water heating or power generation
(Streicher, 2016). Solar collectors are usually mounted on the building’s roof where they
captures maximum sunlight. This sunlight is used to heat water stored in a tank. Once it is
heated, the hot water is distributed throughout the building through the hot water system
network. Alternatively, solar thermal plants can be installed to capture the sun’s rays and used
for heating water which is then distributed throughout the building via pipes. Today, solar hot
water systems are very common and efficient. Figure 2 below illustrates a typical solar hot water
system
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Hot Water Systems and Specific Heat Experiment 8
Figure 2: Typical solar hot water system (Union of Concerned Scientists, 2015)
3.2. Ground and air source heat pumps
These pumps extract heat from either the ground or outside air and transfers it to a heat
exchanger that heats water and distributes it for various uses. For an air source heat pump, heat is
absorbed from air into a fluid that is then passed via a compressor where its temperature gets
increased. The higher temperature heat is transferred to the heating system where water is heated
and distributed in the building (Energy Saving Trust, 2018). For a ground source heat pump, the
pump taps energy stored underground using a long, coiled fluid-filled pipe. This energy is
transferred to a heat exchanger that heats water to the desired temperature (Birch, 2011).
3.3. Biomass boilers
This technology involves heating water by burning biomass instead of fossil fuels
(Saidur, et al., 2011), which reduces carbon dioxide emissions (Berlanga & Ruiz, 2013).
Examples of biomass include forest residues or wood remains (tree trumps, branches and dead
trees), wood chips, yard chippings, municipal solid waste, dry/wet animal manure and
agricultural wastes, among others (Liu, et al., 2010). The burning of biomass produces heat that
is used to heat water (Skinner, 2012); (The Green Age, 2015).
3.4. Direct geothermal and deep geothermal systems
This technology is only practical if the building is located in a place with geothermal
source. The technology harnesses heat from the ground. Direct geothermal system works by
collecting hot underground water and supplying it to the building for the intended use. This is
achieved by drilling a well, installing a pumping system, and delivering the water to the final
destination in the building or passing it through a heat exchanger. Deep geothermal system
involves drilling two wells deep into the ground. One well is used to inject cold water into the
Figure 2: Typical solar hot water system (Union of Concerned Scientists, 2015)
3.2. Ground and air source heat pumps
These pumps extract heat from either the ground or outside air and transfers it to a heat
exchanger that heats water and distributes it for various uses. For an air source heat pump, heat is
absorbed from air into a fluid that is then passed via a compressor where its temperature gets
increased. The higher temperature heat is transferred to the heating system where water is heated
and distributed in the building (Energy Saving Trust, 2018). For a ground source heat pump, the
pump taps energy stored underground using a long, coiled fluid-filled pipe. This energy is
transferred to a heat exchanger that heats water to the desired temperature (Birch, 2011).
3.3. Biomass boilers
This technology involves heating water by burning biomass instead of fossil fuels
(Saidur, et al., 2011), which reduces carbon dioxide emissions (Berlanga & Ruiz, 2013).
Examples of biomass include forest residues or wood remains (tree trumps, branches and dead
trees), wood chips, yard chippings, municipal solid waste, dry/wet animal manure and
agricultural wastes, among others (Liu, et al., 2010). The burning of biomass produces heat that
is used to heat water (Skinner, 2012); (The Green Age, 2015).
3.4. Direct geothermal and deep geothermal systems
This technology is only practical if the building is located in a place with geothermal
source. The technology harnesses heat from the ground. Direct geothermal system works by
collecting hot underground water and supplying it to the building for the intended use. This is
achieved by drilling a well, installing a pumping system, and delivering the water to the final
destination in the building or passing it through a heat exchanger. Deep geothermal system
involves drilling two wells deep into the ground. One well is used to inject cold water into the

Hot Water Systems and Specific Heat Experiment 9
ground and the other is used for bringing superheated steam or hot water to the surface. The hot
water can be used directly by transferring it where it is needed or the superheated steam can be
used for heating a secondary fluid. Once the hot water has been used, it is dispersed or injected
back into the ground, and the process continues (U.S. Environmental Protection Agency, 2016).
3.5. Wind turbine
If there is enough space, wind turbines can be installed to generate wind energy that is
then used to heat water for domestic use.
3.6. Hydroelectric technology
If the building is located near a river or stream, water can be harnessed and used to spin a
turbine that activates a generator. The generator then produces electricity that is used to heat
water in the building.
The most common, effective, reliable, efficient and environmentally friendly technology
today is solar thermal technology.
4. Analysis of use of renewable methods in domestic hot water systems
Today, most domestic buildings use electricity, oil or natural gas generated from non-
resources resources for heating water. The amount of energy used to meet domestic hot water
needs is very huge. In the U.S., for example, heating water accounts for up to 14% of average
energy used by most households, and about 4% of total U.S. energy use (Union of Concerned
Scientists, 2015). This is a significant amount, which translates into an equivalent amount of
carbon emissions. Considering the negative environmental impacts of electricity, oil and natural
gas produced from fossil fuels, use of renewable methods in domestic hot water systems is
worthwhile. This makes use of renewable hot water systems, such as solar water heating systems
ground and the other is used for bringing superheated steam or hot water to the surface. The hot
water can be used directly by transferring it where it is needed or the superheated steam can be
used for heating a secondary fluid. Once the hot water has been used, it is dispersed or injected
back into the ground, and the process continues (U.S. Environmental Protection Agency, 2016).
3.5. Wind turbine
If there is enough space, wind turbines can be installed to generate wind energy that is
then used to heat water for domestic use.
3.6. Hydroelectric technology
If the building is located near a river or stream, water can be harnessed and used to spin a
turbine that activates a generator. The generator then produces electricity that is used to heat
water in the building.
The most common, effective, reliable, efficient and environmentally friendly technology
today is solar thermal technology.
4. Analysis of use of renewable methods in domestic hot water systems
Today, most domestic buildings use electricity, oil or natural gas generated from non-
resources resources for heating water. The amount of energy used to meet domestic hot water
needs is very huge. In the U.S., for example, heating water accounts for up to 14% of average
energy used by most households, and about 4% of total U.S. energy use (Union of Concerned
Scientists, 2015). This is a significant amount, which translates into an equivalent amount of
carbon emissions. Considering the negative environmental impacts of electricity, oil and natural
gas produced from fossil fuels, use of renewable methods in domestic hot water systems is
worthwhile. This makes use of renewable hot water systems, such as solar water heating systems
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Hot Water Systems and Specific Heat Experiment 10
very essential because of their reduced environmental impacts and lower long-term cost benefits
(Greening & Azapagic, 2014). These systems heat water using renewable energy, which is
natural, free, clean and unlimited.
4.1. Installation
Installation of renewable hot water systems is relatively changing because most of these
systems are still new and there are few contractors, consultants and specialists. This increases the
overall installation cost. Installation of some systems such as wind turbines also require land to
be set aside for this purpose. But with adequate resources (technical personnel, land,
tools/equipment, land and money), the system can be installed within a few days. This challenge
can be overcome through increases awareness and training of renewable hot water systems
engineers, technicians, contractors and specialists.
4.2. Practicability
There are a wide range of renewable methods that can be used for heating water in
domestic buildings. This allows users to choose the method that suits them best in terms of hot
water needs, geographical location, capital requirements, etc. Most importantly is that these
domestic hot water systems can be customized to meet the specific needs of the users. Since they
are powered by renewable energy, they can be used in any part of the world including in the
remotest locations. Therefore use of renewable methods to heat water in domestic buildings is
very practical.
4.3. Long term cost
The capital costs of most renewable methods for heating water in domestic buildings are
relatively high. The upfront expenses used to purchase and install renewable domestic hot water
systems are still high because demand for these systems is higher than supply. But once the
very essential because of their reduced environmental impacts and lower long-term cost benefits
(Greening & Azapagic, 2014). These systems heat water using renewable energy, which is
natural, free, clean and unlimited.
4.1. Installation
Installation of renewable hot water systems is relatively changing because most of these
systems are still new and there are few contractors, consultants and specialists. This increases the
overall installation cost. Installation of some systems such as wind turbines also require land to
be set aside for this purpose. But with adequate resources (technical personnel, land,
tools/equipment, land and money), the system can be installed within a few days. This challenge
can be overcome through increases awareness and training of renewable hot water systems
engineers, technicians, contractors and specialists.
4.2. Practicability
There are a wide range of renewable methods that can be used for heating water in
domestic buildings. This allows users to choose the method that suits them best in terms of hot
water needs, geographical location, capital requirements, etc. Most importantly is that these
domestic hot water systems can be customized to meet the specific needs of the users. Since they
are powered by renewable energy, they can be used in any part of the world including in the
remotest locations. Therefore use of renewable methods to heat water in domestic buildings is
very practical.
4.3. Long term cost
The capital costs of most renewable methods for heating water in domestic buildings are
relatively high. The upfront expenses used to purchase and install renewable domestic hot water
systems are still high because demand for these systems is higher than supply. But once the
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Hot Water Systems and Specific Heat Experiment 11
system is installed, operating cost is very low. In the long-term, the cost of heating hot water
using renewable methods is very low in comparison with conventional methods.
4.4. Maintenance
Renewable hot water systems have very low maintenance requirements. This translates
into low maintenance costs. Once the system has been installed and is operated/used as
designed, it can last for several years without requiring any major maintenance.
4.5. Benefits
The benefits of renewable hot water systems for domestic buildings cannot be
overemphasized. Some of them include: reduced energy costs, reduced environmental impacts
(low carbon footprint), greater savings, adequate supply of hot water, reliable supply of hot water
throughout the year, quick installation (for smaller buildings), increased energy independence,
and increased home value. In general, these renewable methods have economic, environmental
and social benefits. Therefore they not only benefit the building owner but also the society as a
whole. Considering these benefits, investing in renewable methods of heating water in domestic
buildings is a worthwhile decision.
4.6. Limitations
Despite the numerous potential benefits of heating water in domestic buildings using
renewable methods, there are also several limitations of these methods. As mentioned before,
upfront expenses (i.e. initial costs) are the first limitation. Others include: lack of adequate
awareness about available methods, technical barriers, reliability misconceptions, social-cultural
barriers, political barriers, regulatory barriers, and ecological and geographical barriers.
system is installed, operating cost is very low. In the long-term, the cost of heating hot water
using renewable methods is very low in comparison with conventional methods.
4.4. Maintenance
Renewable hot water systems have very low maintenance requirements. This translates
into low maintenance costs. Once the system has been installed and is operated/used as
designed, it can last for several years without requiring any major maintenance.
4.5. Benefits
The benefits of renewable hot water systems for domestic buildings cannot be
overemphasized. Some of them include: reduced energy costs, reduced environmental impacts
(low carbon footprint), greater savings, adequate supply of hot water, reliable supply of hot water
throughout the year, quick installation (for smaller buildings), increased energy independence,
and increased home value. In general, these renewable methods have economic, environmental
and social benefits. Therefore they not only benefit the building owner but also the society as a
whole. Considering these benefits, investing in renewable methods of heating water in domestic
buildings is a worthwhile decision.
4.6. Limitations
Despite the numerous potential benefits of heating water in domestic buildings using
renewable methods, there are also several limitations of these methods. As mentioned before,
upfront expenses (i.e. initial costs) are the first limitation. Others include: lack of adequate
awareness about available methods, technical barriers, reliability misconceptions, social-cultural
barriers, political barriers, regulatory barriers, and ecological and geographical barriers.

Hot Water Systems and Specific Heat Experiment 12
4.7. Opportunities
Modern technology can be used to solve most of the challenges or limitations mentioned
above. With modern technology, renewable domestic hot water systems can become easier to
install, affordable to own and more efficient to operate. Governments have to continue providing
incentives to building owners who install renewable hot water systems. More technicians have to
be trained on how to install, repair and maintain these systems. The entire world should be
encouraged to use renewable technologies for heating water for domestic uses so as to enjoy
their benefits and also contribute towards solving the problem of global climate change.
Considering all these opportunities, this is the best time for everyone to install and use renewable
methods for heating water in domestic buildings.
Part Two: Experiment: Determination of specific heat of various metals
1. Introduction
Specific heat is a very important property of a material because it shows the capability of a
material to transfer heat. Different metals have varied capabilities to transfer heat because they
have different specific heat capacities (Barth & Moran, 2014). This is due to the arrangement of
atoms in the metal (quantum effect). In the context of this report, specific heat refers to the
amount of heat needed to raise temperature of one gram of metal by one degree centigrade. The
specific heat capacity of metals is lower than that of water. This means that metals require less
heat to get hot than water. Raising a body temperature depends on several factors including the
body’s mass, temperature difference and specific heat of the body’s material. Specific heat of
water is 4.184 J/g°C, which means that when 1 gram of water absorbs 4.184 joules of heat, the
4.7. Opportunities
Modern technology can be used to solve most of the challenges or limitations mentioned
above. With modern technology, renewable domestic hot water systems can become easier to
install, affordable to own and more efficient to operate. Governments have to continue providing
incentives to building owners who install renewable hot water systems. More technicians have to
be trained on how to install, repair and maintain these systems. The entire world should be
encouraged to use renewable technologies for heating water for domestic uses so as to enjoy
their benefits and also contribute towards solving the problem of global climate change.
Considering all these opportunities, this is the best time for everyone to install and use renewable
methods for heating water in domestic buildings.
Part Two: Experiment: Determination of specific heat of various metals
1. Introduction
Specific heat is a very important property of a material because it shows the capability of a
material to transfer heat. Different metals have varied capabilities to transfer heat because they
have different specific heat capacities (Barth & Moran, 2014). This is due to the arrangement of
atoms in the metal (quantum effect). In the context of this report, specific heat refers to the
amount of heat needed to raise temperature of one gram of metal by one degree centigrade. The
specific heat capacity of metals is lower than that of water. This means that metals require less
heat to get hot than water. Raising a body temperature depends on several factors including the
body’s mass, temperature difference and specific heat of the body’s material. Specific heat of
water is 4.184 J/g°C, which means that when 1 gram of water absorbs 4.184 joules of heat, the
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 21
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





