Federation University ITECH 5500 HREC Application Analysis
VerifiedAdded on 2023/06/04
|13
|3563
|88
Homework Assignment
AI Summary
This document presents an analysis of a Human Research Ethics Committee (HREC) application, likely for a research project involving human subjects. The assignment requires mapping the requirements of the Nuremberg Code to the sections of a standard ethics application form, demonstrating an understanding of ethical research principles. It also involves creating a plain language information statement, a crucial element of the ethical approval process, ensuring participants are fully informed about the research. The assignment covers research ethics, academic misconduct, and ethical frameworks. The document also touches upon the need for ethical standards in research, referencing historical examples such as the Nazi experiments and the Tuskegee Syphilis experiment. The assignment also includes a case study on an ethical dilemma and the application of ethical theories like utilitarianism and deontology. The assignment aims to develop the student's ability to apply an ethical decision-making model, analyze ethical situations, and discuss ethical dilemmas. The document is a comprehensive exploration of research ethics and its practical application.
Application for HREC
Approval (Standard)
Human Research Ethics Committee
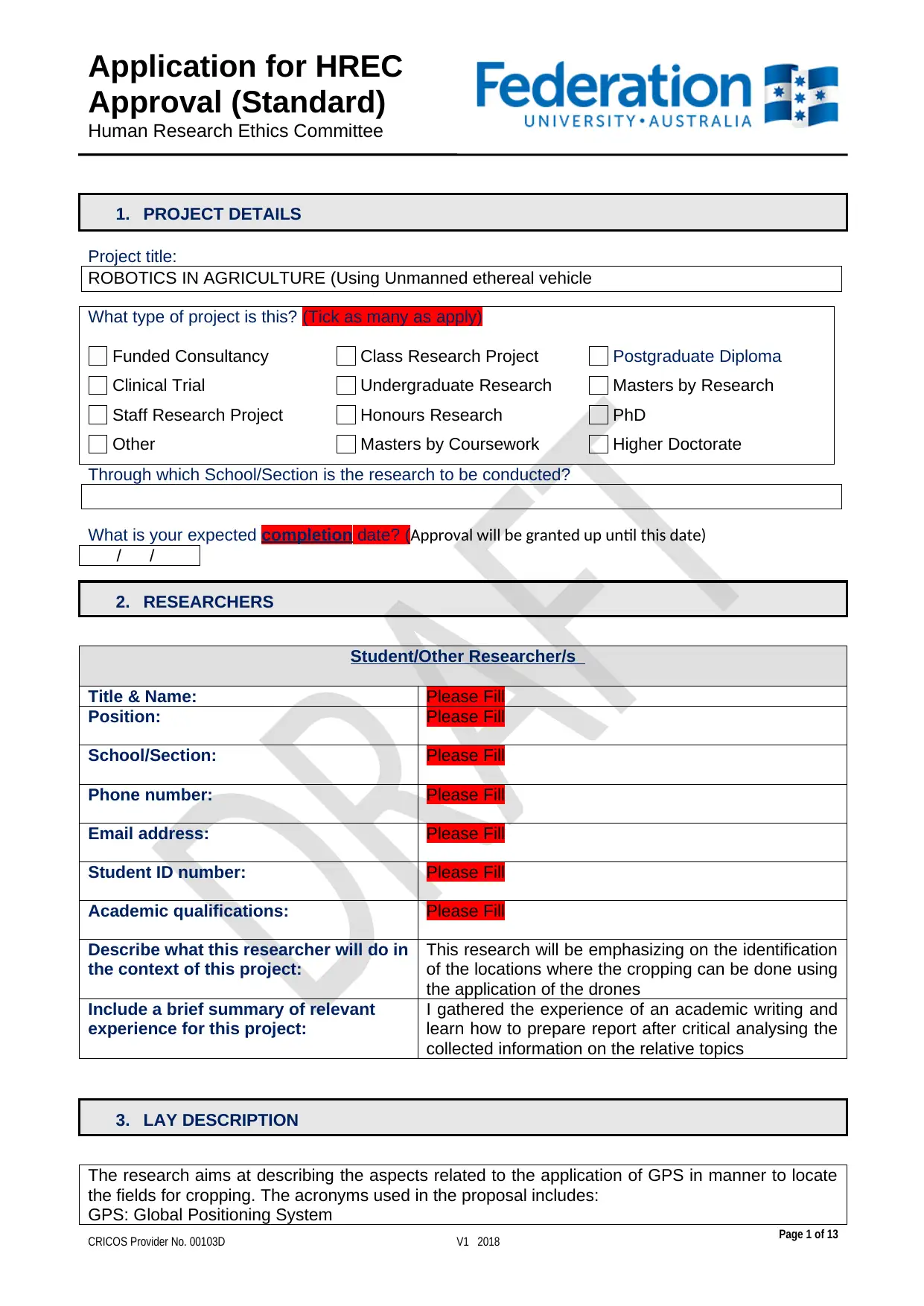
1. PROJECT DETAILS
Project title:
ROBOTICS IN AGRICULTURE (Using Unmanned ethereal vehicle
What type of project is this? (Tick as many as apply)
Funded Consultancy Class Research Project Postgraduate Diploma
Clinical Trial Undergraduate Research Masters by Research
Staff Research Project Honours Research PhD
Other Masters by Coursework Higher Doctorate
Through which School/Section is the research to be conducted?
What is your expected completion date? (Approval will be granted up until this date)
/ /
2. RESEARCHERS
Student/Other Researcher/s
Title & Name: Please Fill
Position: Please Fill
School/Section: Please Fill
Phone number: Please Fill
Email address: Please Fill
Student ID number: Please Fill
Academic qualifications: Please Fill
Describe what this researcher will do in
the context of this project:
This research will be emphasizing on the identification
of the locations where the cropping can be done using
the application of the drones
Include a brief summary of relevant
experience for this project:
I gathered the experience of an academic writing and
learn how to prepare report after critical analysing the
collected information on the relative topics
3. LAY DESCRIPTION
The research aims at describing the aspects related to the application of GPS in manner to locate
the fields for cropping. The acronyms used in the proposal includes:
GPS: Global Positioning System
CRICOS Provider No. 00103D V1 2018 Page 1 of 13
Approval (Standard)
Human Research Ethics Committee
1. PROJECT DETAILS
Project title:
ROBOTICS IN AGRICULTURE (Using Unmanned ethereal vehicle
What type of project is this? (Tick as many as apply)
Funded Consultancy Class Research Project Postgraduate Diploma
Clinical Trial Undergraduate Research Masters by Research
Staff Research Project Honours Research PhD
Other Masters by Coursework Higher Doctorate
Through which School/Section is the research to be conducted?
What is your expected completion date? (Approval will be granted up until this date)
/ /
2. RESEARCHERS
Student/Other Researcher/s
Title & Name: Please Fill
Position: Please Fill
School/Section: Please Fill
Phone number: Please Fill
Email address: Please Fill
Student ID number: Please Fill
Academic qualifications: Please Fill
Describe what this researcher will do in
the context of this project:
This research will be emphasizing on the identification
of the locations where the cropping can be done using
the application of the drones
Include a brief summary of relevant
experience for this project:
I gathered the experience of an academic writing and
learn how to prepare report after critical analysing the
collected information on the relative topics
3. LAY DESCRIPTION
The research aims at describing the aspects related to the application of GPS in manner to locate
the fields for cropping. The acronyms used in the proposal includes:
GPS: Global Positioning System
CRICOS Provider No. 00103D V1 2018 Page 1 of 13
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Application for HREC
Approval (Standard)
Human Research Ethics Committee
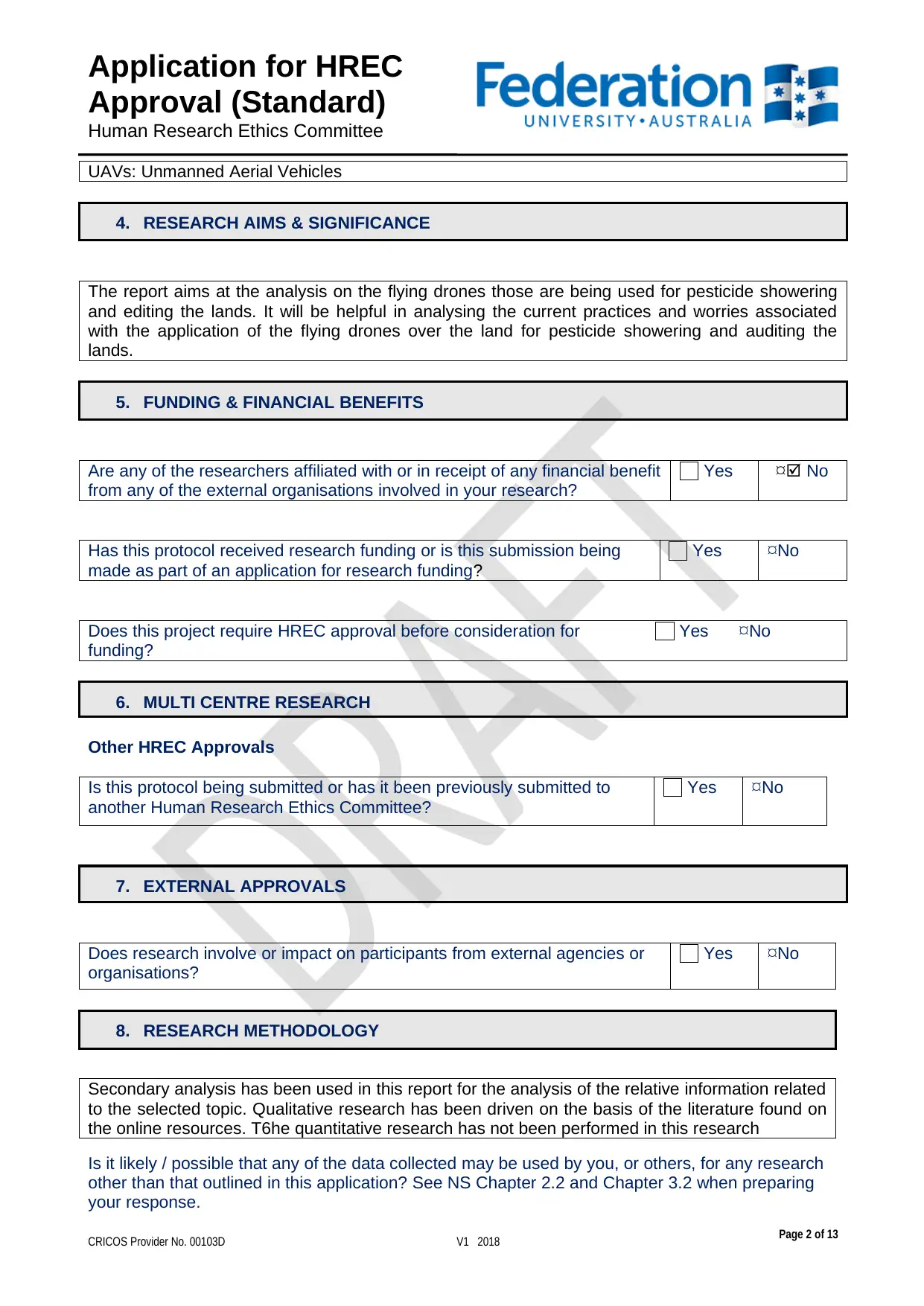
UAVs: Unmanned Aerial Vehicles
4. RESEARCH AIMS & SIGNIFICANCE
The report aims at the analysis on the flying drones those are being used for pesticide showering
and editing the lands. It will be helpful in analysing the current practices and worries associated
with the application of the flying drones over the land for pesticide showering and auditing the
lands.
5. FUNDING & FINANCIAL BENEFITS
Are any of the researchers affiliated with or in receipt of any financial benefit
from any of the external organisations involved in your research?
Yes No
Has this protocol received research funding or is this submission being
made as part of an application for research funding?
Yes No
Does this project require HREC approval before consideration for
funding?
Yes No
6. MULTI CENTRE RESEARCH
Other HREC Approvals
Is this protocol being submitted or has it been previously submitted to
another Human Research Ethics Committee?
Yes No
7. EXTERNAL APPROVALS
Does research involve or impact on participants from external agencies or
organisations?
Yes No
8. RESEARCH METHODOLOGY
Secondary analysis has been used in this report for the analysis of the relative information related
to the selected topic. Qualitative research has been driven on the basis of the literature found on
the online resources. T6he quantitative research has not been performed in this research
Is it likely / possible that any of the data collected may be used by you, or others, for any research
other than that outlined in this application? See NS Chapter 2.2 and Chapter 3.2 when preparing
your response.
CRICOS Provider No. 00103D V1 2018 Page 2 of 13
Approval (Standard)
Human Research Ethics Committee
UAVs: Unmanned Aerial Vehicles
4. RESEARCH AIMS & SIGNIFICANCE
The report aims at the analysis on the flying drones those are being used for pesticide showering
and editing the lands. It will be helpful in analysing the current practices and worries associated
with the application of the flying drones over the land for pesticide showering and auditing the
lands.
5. FUNDING & FINANCIAL BENEFITS
Are any of the researchers affiliated with or in receipt of any financial benefit
from any of the external organisations involved in your research?
Yes No
Has this protocol received research funding or is this submission being
made as part of an application for research funding?
Yes No
Does this project require HREC approval before consideration for
funding?
Yes No
6. MULTI CENTRE RESEARCH
Other HREC Approvals
Is this protocol being submitted or has it been previously submitted to
another Human Research Ethics Committee?
Yes No
7. EXTERNAL APPROVALS
Does research involve or impact on participants from external agencies or
organisations?
Yes No
8. RESEARCH METHODOLOGY
Secondary analysis has been used in this report for the analysis of the relative information related
to the selected topic. Qualitative research has been driven on the basis of the literature found on
the online resources. T6he quantitative research has not been performed in this research
Is it likely / possible that any of the data collected may be used by you, or others, for any research
other than that outlined in this application? See NS Chapter 2.2 and Chapter 3.2 when preparing
your response.
CRICOS Provider No. 00103D V1 2018 Page 2 of 13
Application for HREC
Approval (Standard)
Human Research Ethics Committee
Yes No
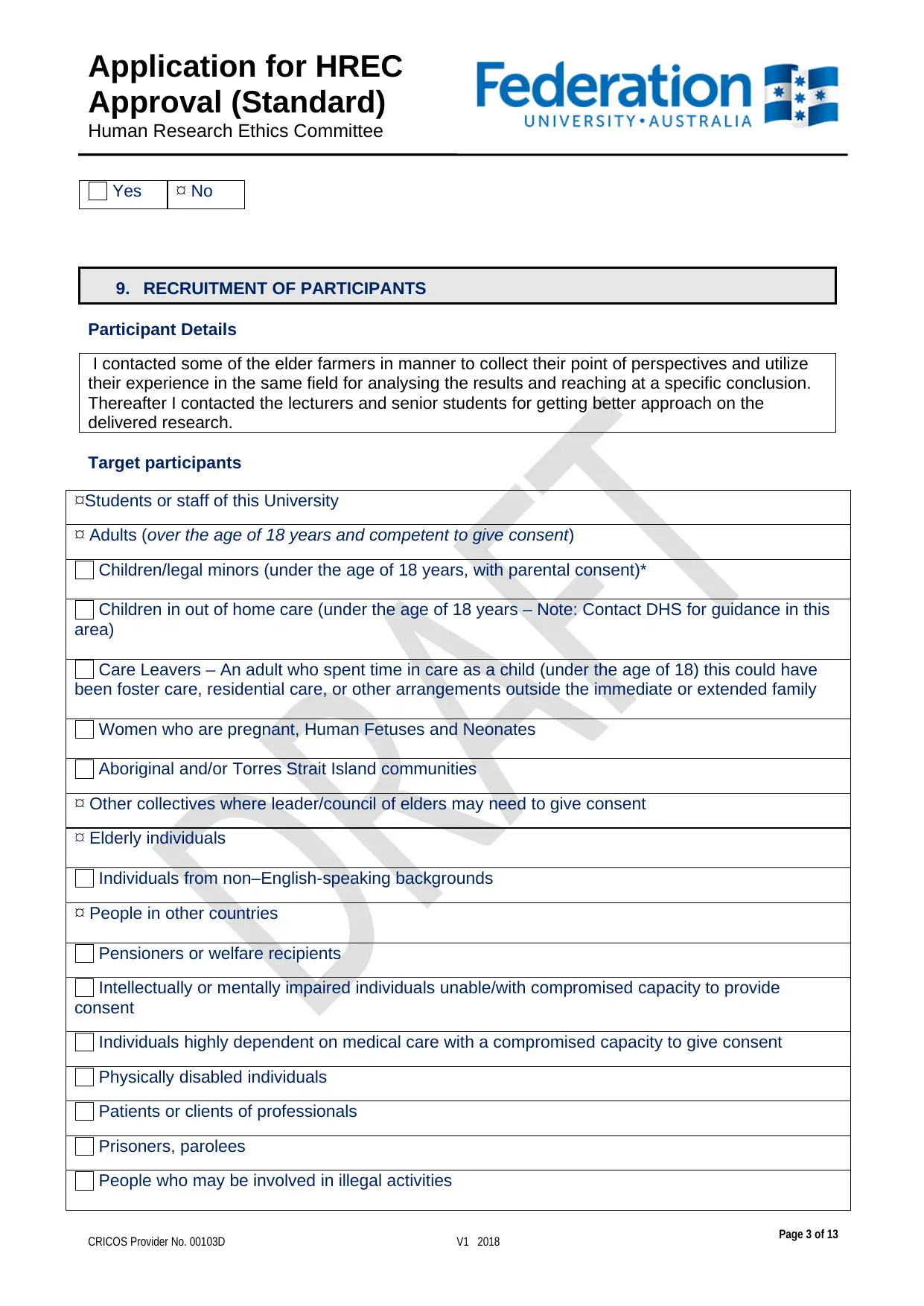
9. RECRUITMENT OF PARTICIPANTS
Participant Details
I contacted some of the elder farmers in manner to collect their point of perspectives and utilize
their experience in the same field for analysing the results and reaching at a specific conclusion.
Thereafter I contacted the lecturers and senior students for getting better approach on the
delivered research.
Target participants
Students or staff of this University
Adults (over the age of 18 years and competent to give consent)
Children/legal minors (under the age of 18 years, with parental consent)*
Children in out of home care (under the age of 18 years – Note: Contact DHS for guidance in this
area)
Care Leavers – An adult who spent time in care as a child (under the age of 18) this could have
been foster care, residential care, or other arrangements outside the immediate or extended family
Women who are pregnant, Human Fetuses and Neonates
Aboriginal and/or Torres Strait Island communities
Other collectives where leader/council of elders may need to give consent
Elderly individuals
Individuals from non–English-speaking backgrounds
People in other countries
Pensioners or welfare recipients
Intellectually or mentally impaired individuals unable/with compromised capacity to provide
consent
Individuals highly dependent on medical care with a compromised capacity to give consent
Physically disabled individuals
Patients or clients of professionals
Prisoners, parolees
People who may be involved in illegal activities
CRICOS Provider No. 00103D V1 2018 Page 3 of 13
Approval (Standard)
Human Research Ethics Committee
Yes No
9. RECRUITMENT OF PARTICIPANTS
Participant Details
I contacted some of the elder farmers in manner to collect their point of perspectives and utilize
their experience in the same field for analysing the results and reaching at a specific conclusion.
Thereafter I contacted the lecturers and senior students for getting better approach on the
delivered research.
Target participants
Students or staff of this University
Adults (over the age of 18 years and competent to give consent)
Children/legal minors (under the age of 18 years, with parental consent)*
Children in out of home care (under the age of 18 years – Note: Contact DHS for guidance in this
area)
Care Leavers – An adult who spent time in care as a child (under the age of 18) this could have
been foster care, residential care, or other arrangements outside the immediate or extended family
Women who are pregnant, Human Fetuses and Neonates
Aboriginal and/or Torres Strait Island communities
Other collectives where leader/council of elders may need to give consent
Elderly individuals
Individuals from non–English-speaking backgrounds
People in other countries
Pensioners or welfare recipients
Intellectually or mentally impaired individuals unable/with compromised capacity to provide
consent
Individuals highly dependent on medical care with a compromised capacity to give consent
Physically disabled individuals
Patients or clients of professionals
Prisoners, parolees
People who may be involved in illegal activities
CRICOS Provider No. 00103D V1 2018 Page 3 of 13
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Application for HREC
Approval (Standard)
Human Research Ethics Committee
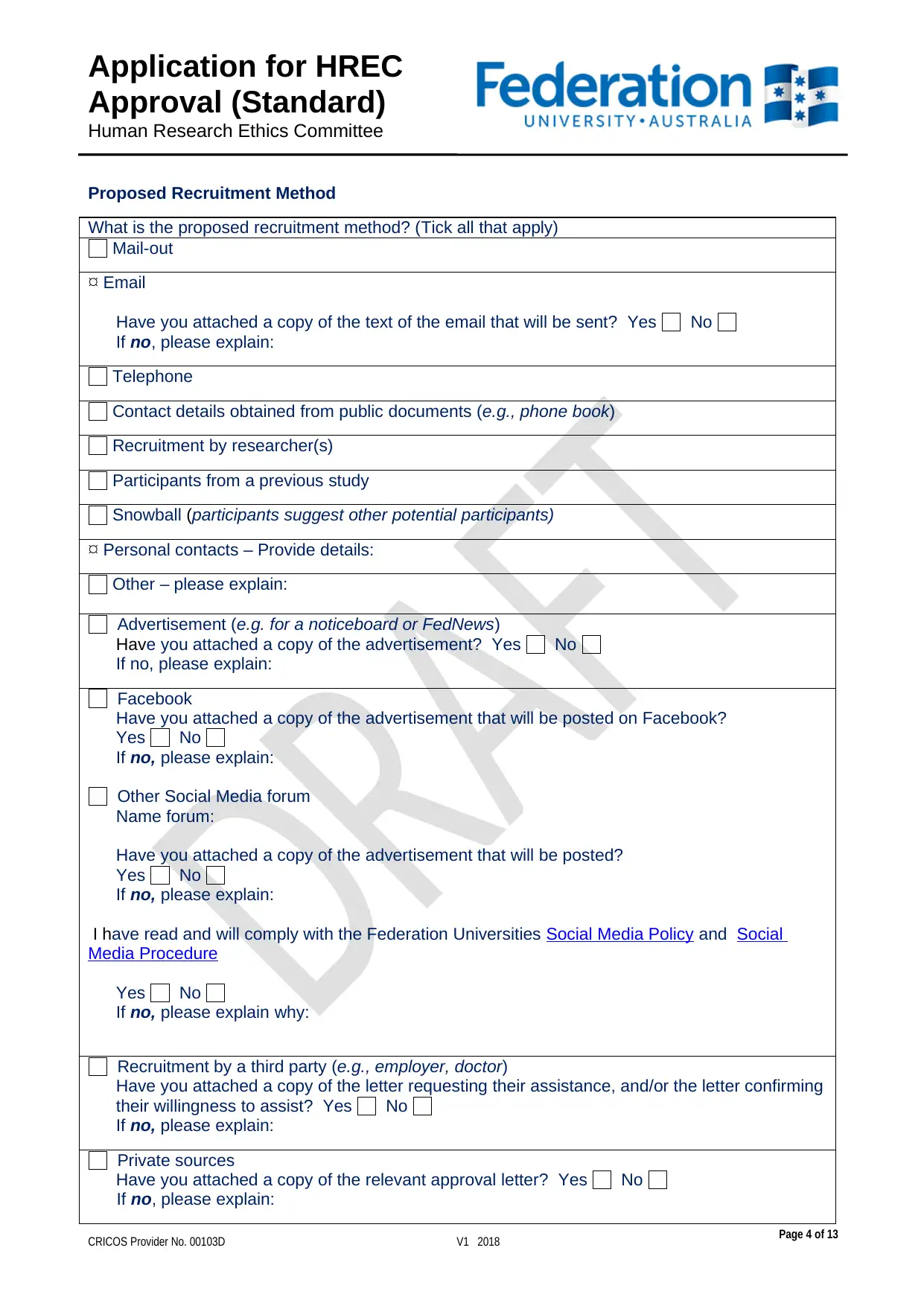
Proposed Recruitment Method
What is the proposed recruitment method? (Tick all that apply)
Mail-out
Email
Have you attached a copy of the text of the email that will be sent? Yes No
If no, please explain:
Telephone
Contact details obtained from public documents (e.g., phone book)
Recruitment by researcher(s)
Participants from a previous study
Snowball (participants suggest other potential participants)
Personal contacts – Provide details:
Other – please explain:
Advertisement (e.g. for a noticeboard or FedNews)
Have you attached a copy of the advertisement? Yes No
If no, please explain:
Facebook
Have you attached a copy of the advertisement that will be posted on Facebook?
Yes No
If no, please explain:
Other Social Media forum
Name forum:
Have you attached a copy of the advertisement that will be posted?
Yes No
If no, please explain:
I have read and will comply with the Federation Universities Social Media Policy and Social
Media Procedure
Yes No
If no, please explain why:
Recruitment by a third party (e.g., employer, doctor)
Have you attached a copy of the letter requesting their assistance, and/or the letter confirming
their willingness to assist? Yes No
If no, please explain:
Private sources
Have you attached a copy of the relevant approval letter? Yes No
If no, please explain:
CRICOS Provider No. 00103D V1 2018 Page 4 of 13
Approval (Standard)
Human Research Ethics Committee
Proposed Recruitment Method
What is the proposed recruitment method? (Tick all that apply)
Mail-out
Have you attached a copy of the text of the email that will be sent? Yes No
If no, please explain:
Telephone
Contact details obtained from public documents (e.g., phone book)
Recruitment by researcher(s)
Participants from a previous study
Snowball (participants suggest other potential participants)
Personal contacts – Provide details:
Other – please explain:
Advertisement (e.g. for a noticeboard or FedNews)
Have you attached a copy of the advertisement? Yes No
If no, please explain:
Have you attached a copy of the advertisement that will be posted on Facebook?
Yes No
If no, please explain:
Other Social Media forum
Name forum:
Have you attached a copy of the advertisement that will be posted?
Yes No
If no, please explain:
I have read and will comply with the Federation Universities Social Media Policy and Social
Media Procedure
Yes No
If no, please explain why:
Recruitment by a third party (e.g., employer, doctor)
Have you attached a copy of the letter requesting their assistance, and/or the letter confirming
their willingness to assist? Yes No
If no, please explain:
Private sources
Have you attached a copy of the relevant approval letter? Yes No
If no, please explain:
CRICOS Provider No. 00103D V1 2018 Page 4 of 13
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Application for HREC
Approval (Standard)
Human Research Ethics Committee
10. BURDENS OF RESEARCH (RISK & RISK MANAGEMENT)
Likely Benefits
Are participants likely to gain direct or indirect benefit from the research? Yes No
If yes, provide details
Provided the support to the farming industries through understanding the positive and negative
reflection of using automation
How will potential benefits to participants or community outweigh the risks?
There is not any risk
Research Activities
Use of a questionnaire (attach copy)
Interviews (attach interview questions)
Observation of participants without their knowledge
Participant observation
Audio- or video-taping of interviewees or events
Access to personal and/or confidential data (including student, patient or client data) without
participants’ specific consent
Administration of any stimuli, tasks, investigations or procedures which may be experienced by
participants as physically or mentally painful, stressful or unpleasant during or after the research
process
Performance of any acts which may diminish the self-esteem of participants or cause them to
experience embarrassment, regret or depression
Investigation of participants involved in illegal activities
Procedures that involve deception of participants
Administration of any substance or agent
Use of non-treatment of placebo control conditions
Collection of body tissues or fluid samples
Collection and/or testing of DNA samples
Participation in a clinical trial
CTN Trial CTX Trial Please provide Phase number, i.e., either 1, 2, 3 or 4
Testing a medical/diagnostic device
CRICOS Provider No. 00103D V1 2018 Page 5 of 13
Approval (Standard)
Human Research Ethics Committee
10. BURDENS OF RESEARCH (RISK & RISK MANAGEMENT)
Likely Benefits
Are participants likely to gain direct or indirect benefit from the research? Yes No
If yes, provide details
Provided the support to the farming industries through understanding the positive and negative
reflection of using automation
How will potential benefits to participants or community outweigh the risks?
There is not any risk
Research Activities
Use of a questionnaire (attach copy)
Interviews (attach interview questions)
Observation of participants without their knowledge
Participant observation
Audio- or video-taping of interviewees or events
Access to personal and/or confidential data (including student, patient or client data) without
participants’ specific consent
Administration of any stimuli, tasks, investigations or procedures which may be experienced by
participants as physically or mentally painful, stressful or unpleasant during or after the research
process
Performance of any acts which may diminish the self-esteem of participants or cause them to
experience embarrassment, regret or depression
Investigation of participants involved in illegal activities
Procedures that involve deception of participants
Administration of any substance or agent
Use of non-treatment of placebo control conditions
Collection of body tissues or fluid samples
Collection and/or testing of DNA samples
Participation in a clinical trial
CTN Trial CTX Trial Please provide Phase number, i.e., either 1, 2, 3 or 4
Testing a medical/diagnostic device
CRICOS Provider No. 00103D V1 2018 Page 5 of 13
Application for HREC
Approval (Standard)
Human Research Ethics Committee
11. RISK MANAGEMENT PROCEDURES
Are facilities at the research location appropriate for the scientific needs of
the research?
Yes No
Are the facilities appropriate to meet any physical, emotional or other
needs of participants that result from their participation?
Yes No
Are there any specific risks to researchers? Yes No
Will parts of this project be carried out by independent contractors? Yes No
If necessary, has the Principal Researcher ensured that the other
researchers have undergone a police check and a Working With
Children check?
Yes No N/A
12. INCENTIVES FOR PARTICIPATION
Are financial or other rewards proposed to be given to participants? Yes No
13. CONSENT
Dependent or Unequal Relationships
Does a dependent or unequal relationship exist between any participant
and researcher, particularly those involved in recruiting?
Yes No
Informing Participants – Plain Language Information Statement (PLIS)
Have you attached a copy of the PLIS for participants? Yes No
Does the PLIS comply with the following guidelines? YES N/A
It is presented on the Fed Uni HREC approved template, downloaded from the
website
*
It has clear identification of the University, the School(s) involved, the project *
CRICOS Provider No. 00103D V1 2018 Page 6 of 13
Approval (Standard)
Human Research Ethics Committee
11. RISK MANAGEMENT PROCEDURES
Are facilities at the research location appropriate for the scientific needs of
the research?
Yes No
Are the facilities appropriate to meet any physical, emotional or other
needs of participants that result from their participation?
Yes No
Are there any specific risks to researchers? Yes No
Will parts of this project be carried out by independent contractors? Yes No
If necessary, has the Principal Researcher ensured that the other
researchers have undergone a police check and a Working With
Children check?
Yes No N/A
12. INCENTIVES FOR PARTICIPATION
Are financial or other rewards proposed to be given to participants? Yes No
13. CONSENT
Dependent or Unequal Relationships
Does a dependent or unequal relationship exist between any participant
and researcher, particularly those involved in recruiting?
Yes No
Informing Participants – Plain Language Information Statement (PLIS)
Have you attached a copy of the PLIS for participants? Yes No
Does the PLIS comply with the following guidelines? YES N/A
It is presented on the Fed Uni HREC approved template, downloaded from the
website
*
It has clear identification of the University, the School(s) involved, the project *
CRICOS Provider No. 00103D V1 2018 Page 6 of 13
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Application for HREC
Approval (Standard)
Human Research Ethics Committee
title, the Principal and Other Researchers (including FedUni contact details).
It details what involvement in the project will require (e.g., involvement in
interviews, completion of questionnaire, audio/video-taping of events), estimated
time commitment, any risks involved.
*
It advises how participants’ contact details were obtained and/or how potential
participants were selected
If staff or students of the Federation University are to be involved as
participants, it advises that the project has received clearance by the HREC
It advises that if the sample size is small this may have implications for
privacy/anonymity.
It states clearly that if participants are in a dependent relationship with any of the
researchers involvement in the project will not affect ongoing assessment,
grades, employment, management or treatment of health (as relevant).
It states clearly that involvement in the project is voluntary and that participants
are free to withdraw their consent to participate at any time, and to withdraw any
unprocessed data previously supplied.
*
It states that arrangements will be made to protect confidentiality of data,
including that confidentiality of information provided is subject to legal limitations
(e.g., subpoena, freedom of information claim, or mandatory reporting in some
professions).
It advises whether or not data will be destroyed after a minimum period.
It advises the de-identified data collected may be used in future research
projects
It provides any other relevant information. *
* Required
Obtaining and Documenting Consent
How will informed consent be obtained/recorded?
Signed consent form
Recorded verbal consent
Implied by return of survey *NB If consent is to be implied by return of survey, all information
that would normally be presented on the consent form must be included in the PLIS
Other (Please specify):
Is a copy of the consent form attached to this application form? Yes No
Does the consent form comply with the following guidelines?
It is presented on the Fed Uni HREC approved template, downloaded from the website
It states the title of the project and names of the researchers
It confirms that the project is for research
It confirms that involvement in the project is voluntary and that participants are free to
withdraw at any time or withdraw any unprocessed data previously supplied
It details specific requirements of participants (e.g., interviews will be audio-/video-taped)
It advises of any legal limitations to data confidentiality
CRICOS Provider No. 00103D V1 2018 Page 7 of 13
Approval (Standard)
Human Research Ethics Committee
title, the Principal and Other Researchers (including FedUni contact details).
It details what involvement in the project will require (e.g., involvement in
interviews, completion of questionnaire, audio/video-taping of events), estimated
time commitment, any risks involved.
*
It advises how participants’ contact details were obtained and/or how potential
participants were selected
If staff or students of the Federation University are to be involved as
participants, it advises that the project has received clearance by the HREC
It advises that if the sample size is small this may have implications for
privacy/anonymity.
It states clearly that if participants are in a dependent relationship with any of the
researchers involvement in the project will not affect ongoing assessment,
grades, employment, management or treatment of health (as relevant).
It states clearly that involvement in the project is voluntary and that participants
are free to withdraw their consent to participate at any time, and to withdraw any
unprocessed data previously supplied.
*
It states that arrangements will be made to protect confidentiality of data,
including that confidentiality of information provided is subject to legal limitations
(e.g., subpoena, freedom of information claim, or mandatory reporting in some
professions).
It advises whether or not data will be destroyed after a minimum period.
It advises the de-identified data collected may be used in future research
projects
It provides any other relevant information. *
* Required
Obtaining and Documenting Consent
How will informed consent be obtained/recorded?
Signed consent form
Recorded verbal consent
Implied by return of survey *NB If consent is to be implied by return of survey, all information
that would normally be presented on the consent form must be included in the PLIS
Other (Please specify):
Is a copy of the consent form attached to this application form? Yes No
Does the consent form comply with the following guidelines?
It is presented on the Fed Uni HREC approved template, downloaded from the website
It states the title of the project and names of the researchers
It confirms that the project is for research
It confirms that involvement in the project is voluntary and that participants are free to
withdraw at any time or withdraw any unprocessed data previously supplied
It details specific requirements of participants (e.g., interviews will be audio-/video-taped)
It advises of any legal limitations to data confidentiality
CRICOS Provider No. 00103D V1 2018 Page 7 of 13
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Application for HREC
Approval (Standard)
Human Research Ethics Committee
It advises that if the sample size is small this may have implications for privacy/anonymity
It provides any other information relevant to obtaining participant consent
14. DISCONTINUING PARTICIPATION
Are participants advised as part of the informed consent process that they
have the right to withdraw at any time or withdraw any unprocessed data
previously supplied?
Yes No
IF YES, PLEASE DETAIL HOW PARTICIPANTS ARE INFORMED OF THIS RIGHT.
Through a written message using emails
15. INFORMATION PROTECTION (DATA; STORAGE; SECURITY)
Confidentiality
What are Data? (NS Ch3.2 Databanks)
Data are pieces of information, eg:
What people say in interviews, focus groups, questionnaires, personal histories and
biographies;
Analysis of existing information (clinical, social, observational or other);
Information derived from human tissue such as blood, bone, muscle and urine.
Individually identifiable data, where the identity of a specific individual can reasonably be
ascertained. Examples of identifiers include the individual’s name, image, and date of birth
or address.
Re-identifiable data, from which identifiers have been removed and replaced by a code,
but it remains possible to re-identify a specific individual by, for example, using the code or
linking different data sets.
Non-identifiable (anonymous) data, which have never been labelled with individual
identifiers or from which identifiers have been permanently removed, and by means of
which no specific individual can be identified. A subset of non-identifiable data are
those that can be linked with other data so it can be known that they are about the same
data subject, although the person’s identity remains unknown.
Tick all that apply from the boxes below:
Participants will have the option of being identified in publications arising from the research.
Participants will be referred to by pseudonym in publications arising from the research.
Personal information will be obtained from a Commonwealth department or agency? (If
yes, you may need to comply with the requirements of the Privacy Act 1988).
Any other method of protecting the privacy of participants (e.g., use of direct quotes with
specific, written permission only; use of real name with specific, written permission only).
Please describe:
Security and Storage
CRICOS Provider No. 00103D V1 2018 Page 8 of 13
Approval (Standard)
Human Research Ethics Committee
It advises that if the sample size is small this may have implications for privacy/anonymity
It provides any other information relevant to obtaining participant consent
14. DISCONTINUING PARTICIPATION
Are participants advised as part of the informed consent process that they
have the right to withdraw at any time or withdraw any unprocessed data
previously supplied?
Yes No
IF YES, PLEASE DETAIL HOW PARTICIPANTS ARE INFORMED OF THIS RIGHT.
Through a written message using emails
15. INFORMATION PROTECTION (DATA; STORAGE; SECURITY)
Confidentiality
What are Data? (NS Ch3.2 Databanks)
Data are pieces of information, eg:
What people say in interviews, focus groups, questionnaires, personal histories and
biographies;
Analysis of existing information (clinical, social, observational or other);
Information derived from human tissue such as blood, bone, muscle and urine.
Individually identifiable data, where the identity of a specific individual can reasonably be
ascertained. Examples of identifiers include the individual’s name, image, and date of birth
or address.
Re-identifiable data, from which identifiers have been removed and replaced by a code,
but it remains possible to re-identify a specific individual by, for example, using the code or
linking different data sets.
Non-identifiable (anonymous) data, which have never been labelled with individual
identifiers or from which identifiers have been permanently removed, and by means of
which no specific individual can be identified. A subset of non-identifiable data are
those that can be linked with other data so it can be known that they are about the same
data subject, although the person’s identity remains unknown.
Tick all that apply from the boxes below:
Participants will have the option of being identified in publications arising from the research.
Participants will be referred to by pseudonym in publications arising from the research.
Personal information will be obtained from a Commonwealth department or agency? (If
yes, you may need to comply with the requirements of the Privacy Act 1988).
Any other method of protecting the privacy of participants (e.g., use of direct quotes with
specific, written permission only; use of real name with specific, written permission only).
Please describe:
Security and Storage
CRICOS Provider No. 00103D V1 2018 Page 8 of 13
Application for HREC
Approval (Standard)
Human Research Ethics Committee
Does the Principal Researcher accept responsibility for the security of the data
collected?
Yes
Does data storage comply with the NHMRC/ARC Australian Code for the
Responsible Conduct of Research? See Section 2: Management of
research data and primary materials
Yes No
Please confirm that at the conclusion of the study, the data will be kept in locked
facilities in the School through which the project is being conducted
Yes
If data is to be kept elsewhere during fieldwork, please explain how and where data will be held, including
arrangements for data security
Please confirm that any data collected will be kept for a minimum of 5 years from date
of research publication.
Yes
Will the data be destroyed at some point after being kept for the minimum
5 year period? (Data may be kept indefinitely, but must be appropriately
secured)
Yes No
Please confirm that any data collected will be disposed of by the Principal Researcher,
who is responsible for the data.
Yes
16. DISSEMINATION OF RESULTS
How will results be made available to participants? (Tick as many as apply)
Written summary of results
Copy of final manuscript (thesis, article, etc.)
Verbal presentation (info session, debriefing, etc.)
Presented to all participants
Presented if requested
Presented to representative participants (e.g. CEO, school principal)
Other - Please explain:
None - Please explain:
How will results be made available to peers and colleagues: Tick as many as apply
Conference papers Journal article(s)
CRICOS Provider No. 00103D V1 2018 Page 9 of 13
Approval (Standard)
Human Research Ethics Committee
Does the Principal Researcher accept responsibility for the security of the data
collected?
Yes
Does data storage comply with the NHMRC/ARC Australian Code for the
Responsible Conduct of Research? See Section 2: Management of
research data and primary materials
Yes No
Please confirm that at the conclusion of the study, the data will be kept in locked
facilities in the School through which the project is being conducted
Yes
If data is to be kept elsewhere during fieldwork, please explain how and where data will be held, including
arrangements for data security
Please confirm that any data collected will be kept for a minimum of 5 years from date
of research publication.
Yes
Will the data be destroyed at some point after being kept for the minimum
5 year period? (Data may be kept indefinitely, but must be appropriately
secured)
Yes No
Please confirm that any data collected will be disposed of by the Principal Researcher,
who is responsible for the data.
Yes
16. DISSEMINATION OF RESULTS
How will results be made available to participants? (Tick as many as apply)
Written summary of results
Copy of final manuscript (thesis, article, etc.)
Verbal presentation (info session, debriefing, etc.)
Presented to all participants
Presented if requested
Presented to representative participants (e.g. CEO, school principal)
Other - Please explain:
None - Please explain:
How will results be made available to peers and colleagues: Tick as many as apply
Conference papers Journal article(s)
CRICOS Provider No. 00103D V1 2018 Page 9 of 13
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Application for HREC
Approval (Standard)
Human Research Ethics Committee
Thesis Book
Other - Please explain None - Please explain
17. LEGAL ISSUES
Does the project involve subject matter or conduct that may give rise to legal
vulnerability of participants or researchers?
Yes No
If yes, please give details
Are adequate precautions to be taken? Yes No N/A
If yes, please give details
Confidentiality of information provided can only be protected within
the limitations of the law. Depending on the research proposal, you
may need to state these limitations specifically (subpoena, freedom
of information claim, mandated reporting by some professions, etc.)
Have you included appropriate information on the legal limitations
of protecting confidentiality in the PLIS and consent form?
Yes No N/A
If no, please advise how participants will be advised
18. CHECKLIST OF ATTACHMENTS
Please check that the following documents are attached to your application. Applicants should note
that where questionnaire or interview questions are submitted in draft form, a copy of the final
documentation must be submitted for final approval when available.
Are the following documents attached? Yes No N/A
Recruitment advertisement (e.g. for noticeboard or FedNews)
Plain Language Information Statement *
Consent form
Evidence of external approvals related to the research Pending
Questionnaire Draft
Interview Schedule Draft
Debriefing material
Other
CRICOS Provider No. 00103D V1 2018 Page 10 of 13
Approval (Standard)
Human Research Ethics Committee
Thesis Book
Other - Please explain None - Please explain
17. LEGAL ISSUES
Does the project involve subject matter or conduct that may give rise to legal
vulnerability of participants or researchers?
Yes No
If yes, please give details
Are adequate precautions to be taken? Yes No N/A
If yes, please give details
Confidentiality of information provided can only be protected within
the limitations of the law. Depending on the research proposal, you
may need to state these limitations specifically (subpoena, freedom
of information claim, mandated reporting by some professions, etc.)
Have you included appropriate information on the legal limitations
of protecting confidentiality in the PLIS and consent form?
Yes No N/A
If no, please advise how participants will be advised
18. CHECKLIST OF ATTACHMENTS
Please check that the following documents are attached to your application. Applicants should note
that where questionnaire or interview questions are submitted in draft form, a copy of the final
documentation must be submitted for final approval when available.
Are the following documents attached? Yes No N/A
Recruitment advertisement (e.g. for noticeboard or FedNews)
Plain Language Information Statement *
Consent form
Evidence of external approvals related to the research Pending
Questionnaire Draft
Interview Schedule Draft
Debriefing material
Other
CRICOS Provider No. 00103D V1 2018 Page 10 of 13
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Application for HREC
Approval (Standard)
Human Research Ethics Committee
* Required
CRICOS Provider No. 00103D V1 2018 Page 11 of 13
Approval (Standard)
Human Research Ethics Committee
* Required
CRICOS Provider No. 00103D V1 2018 Page 11 of 13
Application for HREC
Approval (Standard)
Human Research Ethics Committee
19. DECLARATIONS <Please fill this section>
Researcher Declarations:
The information contained herein is, to the best of my knowledge and belief, accurate. I have read
the University’s current human ethics guidelines, and accept responsibility for the conduct of the
procedures set out in the attached application in accordance with the guidelines, the Australian
Government’s National Statement on Ethical Conduct in Human Research 2007 (Updated May
2015), The Australian Code for the responsible Conduct of Research, and any other condition laid
down by the Federation University’s Human Research Ethics Committee or its sub-committees. I
have attempted to identify all risks related to the research that may arise in conducting this
research and acknowledge my obligations and the rights of the participants. I and my co-
researchers and supporting staff have the appropriate qualifications, experience and facilities to
conduct the research set out in the attached application and to deal with any emergencies and
contingencies related to the research that may arise.
………………………………………………..
Principal Researcher
………………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………
Other Researcher
………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………………
Other Researcher
…………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………
Other Researcher
………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………………
Other Researcher
…………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………
Other Researcher
………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………………
Other Researcher
…………………………………………………
(Print name in block letters)
Date: …..../…...../….....
CRICOS Provider No. 00103D V1 2018 Page 12 of 13
Approval (Standard)
Human Research Ethics Committee
19. DECLARATIONS <Please fill this section>
Researcher Declarations:
The information contained herein is, to the best of my knowledge and belief, accurate. I have read
the University’s current human ethics guidelines, and accept responsibility for the conduct of the
procedures set out in the attached application in accordance with the guidelines, the Australian
Government’s National Statement on Ethical Conduct in Human Research 2007 (Updated May
2015), The Australian Code for the responsible Conduct of Research, and any other condition laid
down by the Federation University’s Human Research Ethics Committee or its sub-committees. I
have attempted to identify all risks related to the research that may arise in conducting this
research and acknowledge my obligations and the rights of the participants. I and my co-
researchers and supporting staff have the appropriate qualifications, experience and facilities to
conduct the research set out in the attached application and to deal with any emergencies and
contingencies related to the research that may arise.
………………………………………………..
Principal Researcher
………………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………
Other Researcher
………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………………
Other Researcher
…………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………
Other Researcher
………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………………
Other Researcher
…………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………
Other Researcher
………………………………………………
(Print name in block letters)
Date: …..../…...../….....
……………………………………………………
Other Researcher
…………………………………………………
(Print name in block letters)
Date: …..../…...../….....
CRICOS Provider No. 00103D V1 2018 Page 12 of 13
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 13
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


