Human Resource Regulatory Act of United Kingdom
VerifiedAdded on 2020/05/01
|10
|2166
|146
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: HUMAN TISSUE REGULATORY ACT OF UNITED KINGDOM
UK REGULATIONS RELATING TO THE USE OF HUMAN TISSUE IN RESEARCH
Name of the Student
Name of the University
Author note
UK REGULATIONS RELATING TO THE USE OF HUMAN TISSUE IN RESEARCH
Name of the Student
Name of the University
Author note
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1HUMAN TISSUE REGULATORY ACT OF UNITED KINGDOM
Human tissue is the mass of cells that represents an internal environment of the human
body and researchers uses this human environment to find out different facts about diseases, their
treatment and their signs and symptoms (Lonsdale et al. 2013). The first human cell line used in
the research is HeLa in 1951 and it was derived from Henrietta Lacks, a patient suffering from
cancer. Biologist George Otto Gey found her cell line appropriate for research purpose because
of its immortal nature(malignant cell property) and this way the invention of immortal cell line
was created (Adey et al. 2013).
Usage of human tissue for medical research and development raises many ethical and law
related problems around the state of European Union. The procurement, storage, and allocation
of human tissue for research purposes have posed noteworthy questions over recent years, and a
different high profile scandal in the UK encouraged the publication of the Madden Report on
Post Mortem Exercise and Trials in Irish hospitals in 2006. Moreover, tissue-related
investigation tends to be most encouraging if samples and material are shared through domestic
borders, but the heterogeneity of present rules and strategies within the member states of the
European Union and the United Kingdom calls all the more for explanation (Best and Kahn
2016). This essay will be reviewing the different rules and regulations of the Human Tissue Act
(HT) 2004 of United Kingdom and the human tissue quality and safety regulation (2007) in
detail.
This HT act 2004 covers the entire England, Northern Ireland and Wales with the
exclusion of the provisions regarding the use of DNA and this applies to the Scotland as well.
This act establishes the authority of Human Tissue (HTA) to regulate different concerns about
the storage, removal and disposal of the human tissue. However, Scotland has a separate
legislation for that and it is termed as the Human Tissue (Scotland) Act 2006 (Human Tissue
Human tissue is the mass of cells that represents an internal environment of the human
body and researchers uses this human environment to find out different facts about diseases, their
treatment and their signs and symptoms (Lonsdale et al. 2013). The first human cell line used in
the research is HeLa in 1951 and it was derived from Henrietta Lacks, a patient suffering from
cancer. Biologist George Otto Gey found her cell line appropriate for research purpose because
of its immortal nature(malignant cell property) and this way the invention of immortal cell line
was created (Adey et al. 2013).
Usage of human tissue for medical research and development raises many ethical and law
related problems around the state of European Union. The procurement, storage, and allocation
of human tissue for research purposes have posed noteworthy questions over recent years, and a
different high profile scandal in the UK encouraged the publication of the Madden Report on
Post Mortem Exercise and Trials in Irish hospitals in 2006. Moreover, tissue-related
investigation tends to be most encouraging if samples and material are shared through domestic
borders, but the heterogeneity of present rules and strategies within the member states of the
European Union and the United Kingdom calls all the more for explanation (Best and Kahn
2016). This essay will be reviewing the different rules and regulations of the Human Tissue Act
(HT) 2004 of United Kingdom and the human tissue quality and safety regulation (2007) in
detail.
This HT act 2004 covers the entire England, Northern Ireland and Wales with the
exclusion of the provisions regarding the use of DNA and this applies to the Scotland as well.
This act establishes the authority of Human Tissue (HTA) to regulate different concerns about
the storage, removal and disposal of the human tissue. However, Scotland has a separate
legislation for that and it is termed as the Human Tissue (Scotland) Act 2006 (Human Tissue

2HUMAN TISSUE REGULATORY ACT OF UNITED KINGDOM
Authority 2017) because it is a part of Scottish parliament. The European Union Tissue and Cell
Directives (EUTCD) have implemented another act for the Human Tissue (Quality and Safety
for human application) Regulation 2007 (The Human Tissue Q and S Regulations, 2017).
Therefore, HTA is termed as the competent authority in the UK regarding the use of human
tissue in research and development. The strength of this human tissue act is its integrity and
widespread nature. It also allows the honest and ethical distribution of organs around the UK and
the chances of corruption are less because of its ability to crosscheck the donation procedure
(Whitburn, Marsden and Sooriakumaran 2017).
The HTA is also the Capable Authority in the UK for the application of the European
Union Directive 2010/53/EU on the ethics of quality and safety of human organs envisioned for.
These requirements of the directive are transferred into the UK law through the regulations of the
Quality and Safety of those organs, which are intended, to relocation Regulation 2012. This act is
generally termed as Quality and Safety of Organs Intended for Transplantation Regulations
2012. These ethics for use of human tissue in research falls under twenty-nine code of ethics
(The Human Tissue Q and S Transplantation Regulations, 2017). The important points of these
codes are- Consent. donation of solid organs for transplantation, examination for post mortem,
anatomical examination, human tissue disposal, donation of allogeneic bone marrow and
peripheral blood stem cells for organ transplantation, public display, export import of human
bodies, parts and tissues and finally research.
There are several strengths and weaknesses, which are associated with the human tissue
and many more acts that deals with human organ or tissue transplantation in UK. The strengths
are decrease in the number of human organ trafficking. Patients in need are easily applying for
the organ requirement after the formation of the transplantation act 2012. Finally, the major
Authority 2017) because it is a part of Scottish parliament. The European Union Tissue and Cell
Directives (EUTCD) have implemented another act for the Human Tissue (Quality and Safety
for human application) Regulation 2007 (The Human Tissue Q and S Regulations, 2017).
Therefore, HTA is termed as the competent authority in the UK regarding the use of human
tissue in research and development. The strength of this human tissue act is its integrity and
widespread nature. It also allows the honest and ethical distribution of organs around the UK and
the chances of corruption are less because of its ability to crosscheck the donation procedure
(Whitburn, Marsden and Sooriakumaran 2017).
The HTA is also the Capable Authority in the UK for the application of the European
Union Directive 2010/53/EU on the ethics of quality and safety of human organs envisioned for.
These requirements of the directive are transferred into the UK law through the regulations of the
Quality and Safety of those organs, which are intended, to relocation Regulation 2012. This act is
generally termed as Quality and Safety of Organs Intended for Transplantation Regulations
2012. These ethics for use of human tissue in research falls under twenty-nine code of ethics
(The Human Tissue Q and S Transplantation Regulations, 2017). The important points of these
codes are- Consent. donation of solid organs for transplantation, examination for post mortem,
anatomical examination, human tissue disposal, donation of allogeneic bone marrow and
peripheral blood stem cells for organ transplantation, public display, export import of human
bodies, parts and tissues and finally research.
There are several strengths and weaknesses, which are associated with the human tissue
and many more acts that deals with human organ or tissue transplantation in UK. The strengths
are decrease in the number of human organ trafficking. Patients in need are easily applying for
the organ requirement after the formation of the transplantation act 2012. Finally, the major

3HUMAN TISSUE REGULATORY ACT OF UNITED KINGDOM
strength is the research sector is able to conduct their research on cell lines, which are with
complete details and consent letter. The weaknesses are also associated with it and people are
complaining that surgeons are extracting the organs of their loved ones who are on deathbeds
and are providing fake consent letter in return. The Organ transplant act 2012 need to be more
strong and harsh on such medical institutions where such practices are followed.
Procedure
The first procedure of using human tissue for research is taking consent of the patient
before using his bone marrow or tissue and it falls under the code of ethics by the Human Tissue
act 2004. However, it is not important to collect the consent letter of the donor always, if the
tissue is existing holding or is kept with the researcher before September 2006. In this case, the
researcher do not need any consent to continue with his research (The Human Tissue Q and S
Regulations, 2017). The reason is the law was implemented after the researcher collected the
tissue and therefore, consent is not required. Furthermore, if the tissue is imported or the
researcher is not been able to identify the donor of the tissue and the recognized Research Ethics
Committee (REC) approved the research, then the consent is not necessary as per the code of
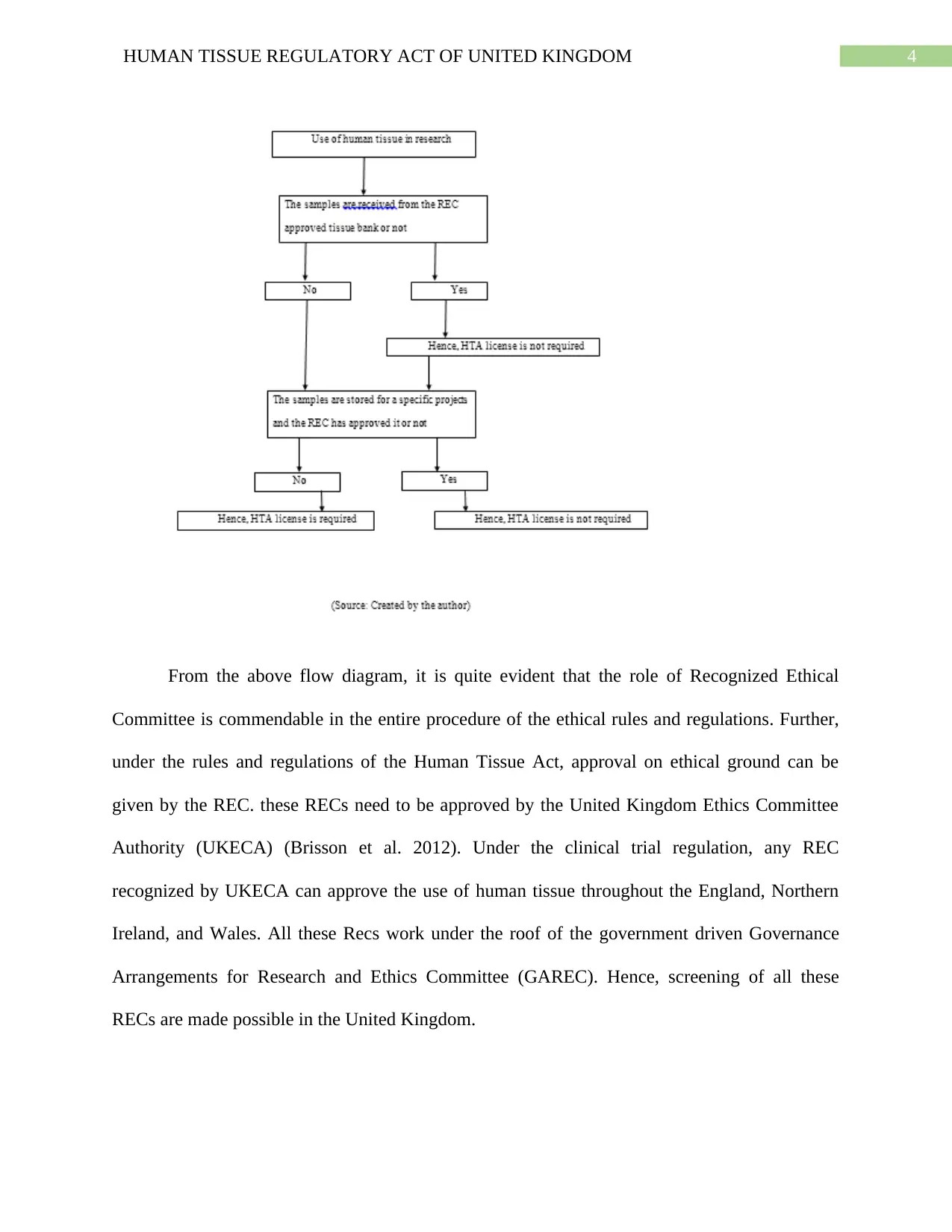
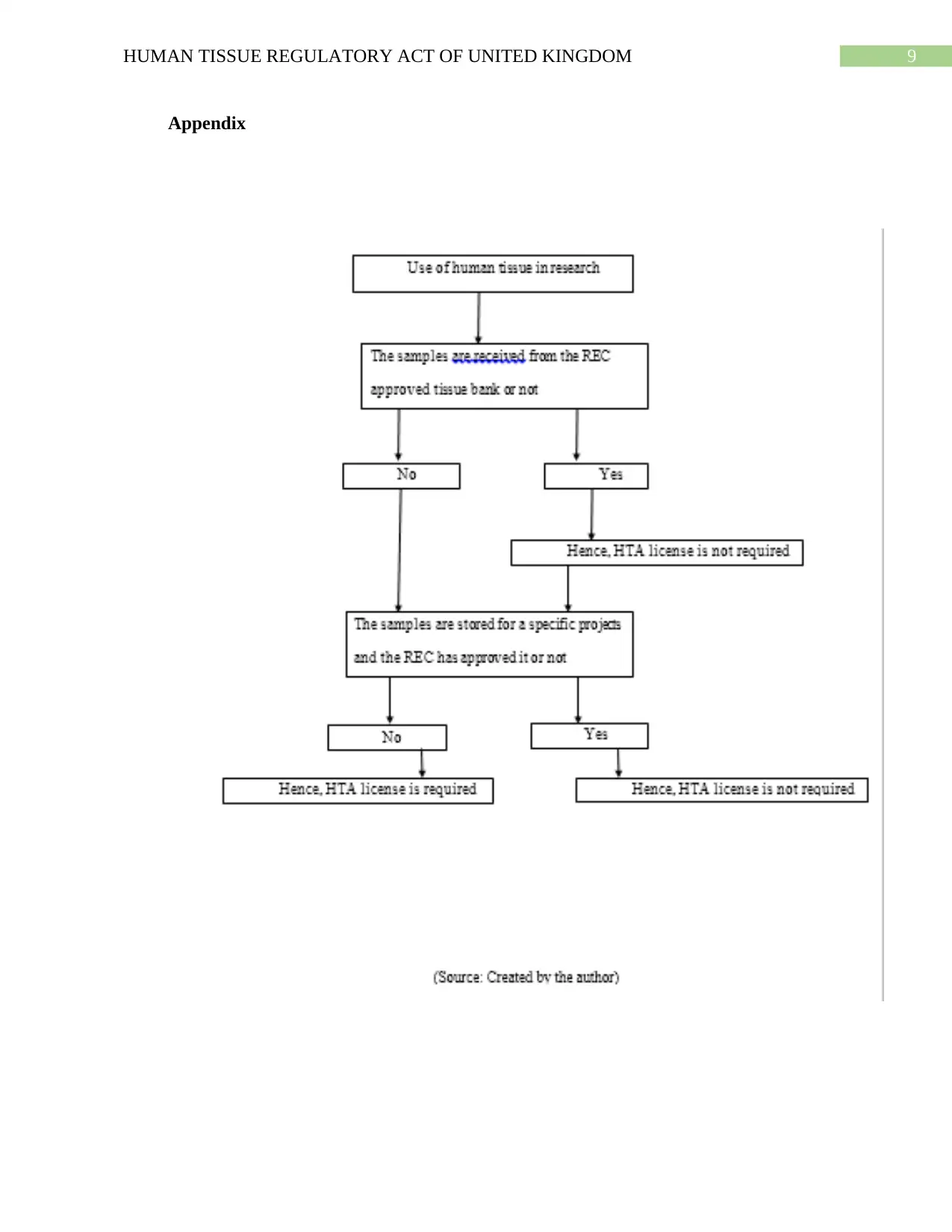
ethics (Health Research Authority, 2017). The following flow chart describes the process of
consent and the consequences of not receiving it from the donor. This flowchart also describes
the process in which the consent is required or not required. Detailed flowchart has been added
in the appendix section.
strength is the research sector is able to conduct their research on cell lines, which are with
complete details and consent letter. The weaknesses are also associated with it and people are
complaining that surgeons are extracting the organs of their loved ones who are on deathbeds
and are providing fake consent letter in return. The Organ transplant act 2012 need to be more
strong and harsh on such medical institutions where such practices are followed.
Procedure
The first procedure of using human tissue for research is taking consent of the patient
before using his bone marrow or tissue and it falls under the code of ethics by the Human Tissue
act 2004. However, it is not important to collect the consent letter of the donor always, if the
tissue is existing holding or is kept with the researcher before September 2006. In this case, the
researcher do not need any consent to continue with his research (The Human Tissue Q and S
Regulations, 2017). The reason is the law was implemented after the researcher collected the
tissue and therefore, consent is not required. Furthermore, if the tissue is imported or the
researcher is not been able to identify the donor of the tissue and the recognized Research Ethics
Committee (REC) approved the research, then the consent is not necessary as per the code of
ethics (Health Research Authority, 2017). The following flow chart describes the process of
consent and the consequences of not receiving it from the donor. This flowchart also describes
the process in which the consent is required or not required. Detailed flowchart has been added
in the appendix section.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
4HUMAN TISSUE REGULATORY ACT OF UNITED KINGDOM
From the above flow diagram, it is quite evident that the role of Recognized Ethical
Committee is commendable in the entire procedure of the ethical rules and regulations. Further,
under the rules and regulations of the Human Tissue Act, approval on ethical ground can be
given by the REC. these RECs need to be approved by the United Kingdom Ethics Committee
Authority (UKECA) (Brisson et al. 2012). Under the clinical trial regulation, any REC
recognized by UKECA can approve the use of human tissue throughout the England, Northern
Ireland, and Wales. All these Recs work under the roof of the government driven Governance
Arrangements for Research and Ethics Committee (GAREC). Hence, screening of all these
RECs are made possible in the United Kingdom.
From the above flow diagram, it is quite evident that the role of Recognized Ethical
Committee is commendable in the entire procedure of the ethical rules and regulations. Further,
under the rules and regulations of the Human Tissue Act, approval on ethical ground can be
given by the REC. these RECs need to be approved by the United Kingdom Ethics Committee
Authority (UKECA) (Brisson et al. 2012). Under the clinical trial regulation, any REC
recognized by UKECA can approve the use of human tissue throughout the England, Northern
Ireland, and Wales. All these Recs work under the roof of the government driven Governance
Arrangements for Research and Ethics Committee (GAREC). Hence, screening of all these
RECs are made possible in the United Kingdom.

5HUMAN TISSUE REGULATORY ACT OF UNITED KINGDOM
However, the prime concern is about those tissues that are being imported from different
locations and RECs approve those tissues without any consent letter. The risk factor associated
with it is the unidentified resource from which the tissue has been taken. This process can also be
debatable, as the researcher does not know the collecting procedure of the tissue. Hence, ethical
regulations can be violated using this procedure, because it is important to know the source of
the tissue used in the research as per the HT act code of research (Medical Research Council
2017). Hence, the HT act talks about these issues as well in its first code, which is consent and
ethics. According to the provision, the tissue bank that provides the researcher with the imported
tissue, need to collect the consent letter from the donor and it is the bank’s responsibility to
collect it. . The License gives right to store the tissue for research. Any obligation for ethical
support rest on the policy of the association storing the tissue (HTA code of Practice and
Standards, 2017).
The positive and negative outcomes of the ethical procedure to use human tissue in
research are-
The first positive outcome of the procedure to use human tissue in research is the consent
letter. This letter allows the researcher to know about the donor and his or her health
complication. As the tissue is the part of the donor’s body, complication of the donor can affect
the research as well. Secondly, presence of recognized ethics committee to approve the use of
human tissue in research. Third strength is the provision of storage that let the researcher store
the cell line for future uses. However, there are negative outcome as well. A single application
can be made for ethical review of several human tissue line. However, the chances of corruption
are less, but researchers can generate fake reviews in the case. Second negative outcome is
drastic as well, in which if a hospital bears a license for research, the tissue collected by it will
However, the prime concern is about those tissues that are being imported from different
locations and RECs approve those tissues without any consent letter. The risk factor associated
with it is the unidentified resource from which the tissue has been taken. This process can also be
debatable, as the researcher does not know the collecting procedure of the tissue. Hence, ethical
regulations can be violated using this procedure, because it is important to know the source of
the tissue used in the research as per the HT act code of research (Medical Research Council
2017). Hence, the HT act talks about these issues as well in its first code, which is consent and
ethics. According to the provision, the tissue bank that provides the researcher with the imported
tissue, need to collect the consent letter from the donor and it is the bank’s responsibility to
collect it. . The License gives right to store the tissue for research. Any obligation for ethical
support rest on the policy of the association storing the tissue (HTA code of Practice and
Standards, 2017).
The positive and negative outcomes of the ethical procedure to use human tissue in
research are-
The first positive outcome of the procedure to use human tissue in research is the consent
letter. This letter allows the researcher to know about the donor and his or her health
complication. As the tissue is the part of the donor’s body, complication of the donor can affect
the research as well. Secondly, presence of recognized ethics committee to approve the use of
human tissue in research. Third strength is the provision of storage that let the researcher store
the cell line for future uses. However, there are negative outcome as well. A single application
can be made for ethical review of several human tissue line. However, the chances of corruption
are less, but researchers can generate fake reviews in the case. Second negative outcome is
drastic as well, in which if a hospital bears a license for research, the tissue collected by it will

6HUMAN TISSUE REGULATORY ACT OF UNITED KINGDOM
not require consent letter. this unethical rule affects the research as the donor information is not
always correct.
Storage of the Human Tissue is also covered in the HT act 2006 and according to the
section 16(7); storage does not include the incidental to transportation. It is defines as incidental
to transportation if the tissue is stored for an hour or day. In this situation license is not required.
However, if the researchers store the tissue for a scheduled purpose, requires a license that has to
be given by the HTA or the RECs (Human Tissue Authority 2017). Hence, from the above
review of the sections of the Human Tissue Act 2006, it is quite evident that the use of human
tissue in UK is regulated strongly to allow the ethical use of tissues in the research for the
beneficiary of the humankind.
not require consent letter. this unethical rule affects the research as the donor information is not
always correct.
Storage of the Human Tissue is also covered in the HT act 2006 and according to the
section 16(7); storage does not include the incidental to transportation. It is defines as incidental
to transportation if the tissue is stored for an hour or day. In this situation license is not required.
However, if the researchers store the tissue for a scheduled purpose, requires a license that has to
be given by the HTA or the RECs (Human Tissue Authority 2017). Hence, from the above
review of the sections of the Human Tissue Act 2006, it is quite evident that the use of human
tissue in UK is regulated strongly to allow the ethical use of tissues in the research for the
beneficiary of the humankind.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

7HUMAN TISSUE REGULATORY ACT OF UNITED KINGDOM
References
Adey, A., Burton, J.N., Kitzman, J.O., Hiatt, J.B., Lewis, A.P., Martin, B.K., Qiu, R., Lee, C. and
Shendure, J., 2013. The haplotype-resolved genome and epigenome of the aneuploid HeLa
cancer cell line. Nature, 500(7461), pp.207-211.
Best, J.W. and Kahn, J.V., 2016. Research in education. Pearson Education India.
https://scholar.google.co.in/scholar?
hl=en&as_sdt=0%2C5&as_ylo=2012&as_yhi=2017&q=Best%2C+J.W.+and+Kahn%2C+J.V.
%2C+2016.+Research+in+education.+Pearson+Education+India.&btnG
Health Research Authority (2017). Use of human tissue in research. [online] Health Research
Authority. Available at: https://www.hra.nhs.uk/planning-and-improving-research/policies-
standards-legislation/use-tissue-research/ [Accessed 10 Nov. 2017].
HTA code of Practice and Standards (2017). HTA Codes of Practice and Standards | Human
Tissue Authority. [online] Hta.gov.uk. Available at: https://www.hta.gov.uk/hta-codes-practice-
and-standards-0 [Accessed 23 Oct. 2017].
Human Tissue Act (2017). Human Tissue Act 2004. [online] Legislation.gov.uk. Available at:
http://www.legislation.gov.uk/ukpga/2004/30/contents [Accessed 23 Oct. 2017].
Human Tissue Authority (2017). Find out what the HTA can do for you | Human Tissue
Authority. [online] Hta.gov.uk. Available at: https://www.hta.gov.uk/ [Accessed 23 Oct. 2017].
Lonsdale, J., Thomas, J., Salvatore, M., Phillips, R., Lo, E., Shad, S., Hasz, R., Walters, G.,
Garcia, F., Young, N. and Foster, B., 2013. The genotype-tissue expression (GTEx)
project. Nature genetics, 45(6), pp.580-585.
References
Adey, A., Burton, J.N., Kitzman, J.O., Hiatt, J.B., Lewis, A.P., Martin, B.K., Qiu, R., Lee, C. and
Shendure, J., 2013. The haplotype-resolved genome and epigenome of the aneuploid HeLa
cancer cell line. Nature, 500(7461), pp.207-211.
Best, J.W. and Kahn, J.V., 2016. Research in education. Pearson Education India.
https://scholar.google.co.in/scholar?
hl=en&as_sdt=0%2C5&as_ylo=2012&as_yhi=2017&q=Best%2C+J.W.+and+Kahn%2C+J.V.
%2C+2016.+Research+in+education.+Pearson+Education+India.&btnG
Health Research Authority (2017). Use of human tissue in research. [online] Health Research
Authority. Available at: https://www.hra.nhs.uk/planning-and-improving-research/policies-
standards-legislation/use-tissue-research/ [Accessed 10 Nov. 2017].
HTA code of Practice and Standards (2017). HTA Codes of Practice and Standards | Human
Tissue Authority. [online] Hta.gov.uk. Available at: https://www.hta.gov.uk/hta-codes-practice-
and-standards-0 [Accessed 23 Oct. 2017].
Human Tissue Act (2017). Human Tissue Act 2004. [online] Legislation.gov.uk. Available at:
http://www.legislation.gov.uk/ukpga/2004/30/contents [Accessed 23 Oct. 2017].
Human Tissue Authority (2017). Find out what the HTA can do for you | Human Tissue
Authority. [online] Hta.gov.uk. Available at: https://www.hta.gov.uk/ [Accessed 23 Oct. 2017].
Lonsdale, J., Thomas, J., Salvatore, M., Phillips, R., Lo, E., Shad, S., Hasz, R., Walters, G.,
Garcia, F., Young, N. and Foster, B., 2013. The genotype-tissue expression (GTEx)
project. Nature genetics, 45(6), pp.580-585.

8HUMAN TISSUE REGULATORY ACT OF UNITED KINGDOM
Medical Research Council (2017). Human tissue - Research - Medical Research Council.
[online] Mrc.ac.uk. Available at: https://www.mrc.ac.uk/research/facilities-and-resources-for-
researchers/regulatory-support-centre/human-tissue/ [Accessed 23 Oct. 2017].
The Human Tissue Q and S Regulations (2017). The Human Tissue (Quality and Safety for
Human Application) Regulations 2007. [online] Legislation.gov.uk. Available at:
http://www.legislation.gov.uk/uksi/2007/1523/contents/made [Accessed 23 Oct. 2017].
Whitburn, J., Marsden, G. and Sooriakumaran, P., 2017. The Human Tissue Act: a guide for
clinical researchers. Journal of Clinical Urology, p.2051415817719838.
Medical Research Council (2017). Human tissue - Research - Medical Research Council.
[online] Mrc.ac.uk. Available at: https://www.mrc.ac.uk/research/facilities-and-resources-for-
researchers/regulatory-support-centre/human-tissue/ [Accessed 23 Oct. 2017].
The Human Tissue Q and S Regulations (2017). The Human Tissue (Quality and Safety for
Human Application) Regulations 2007. [online] Legislation.gov.uk. Available at:
http://www.legislation.gov.uk/uksi/2007/1523/contents/made [Accessed 23 Oct. 2017].
Whitburn, J., Marsden, G. and Sooriakumaran, P., 2017. The Human Tissue Act: a guide for
clinical researchers. Journal of Clinical Urology, p.2051415817719838.
9HUMAN TISSUE REGULATORY ACT OF UNITED KINGDOM
Appendix
Appendix
1 out of 10
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
