Comprehensive Report on Hygiene and Toxicology: HCN Exposure
VerifiedAdded on 2019/11/12
|12
|3262
|273
Report
AI Summary
This report provides a comprehensive overview of hydrogen cyanide (HCN) toxicology, beginning with its historical context and its mechanism of action as an inhibitor of cellular respiration. It details various sources of exposure, including inhalation, oral ingestion, and dermal absorption, highlighting the associated health effects such as vertigo, headache, and severe poisoning leading to cardiac and neurological abnormalities. The report emphasizes the importance of occupational hygiene practices and adherence to exposure standards set by organizations like OSHA and Safe Work Australia, outlining control measures like elimination, substitution, and the use of personal protective equipment. Furthermore, it delves into the toxicokinetics and toxicodynamics of HCN, explaining its absorption, distribution, metabolism via rhodanese, and elimination, as well as its interaction with cytochrome oxidase and resulting histotoxic anoxia. The document concludes by underscoring the necessity of understanding and mitigating the risks associated with HCN exposure.

Running head: HYGIENE AND TOXICOLOGY
Hygiene and toxicology
Name of the Student
Name of the University
Author Note
Hygiene and toxicology
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1HYGIENE AND TOXICOLOGY
Executive summary
Toxicology is a branch of science that analyses relationship between a chemical and its adverse effects.
Hydrogen cyanide is a colourless gas that is fast acting and potentially deadly. It is extremely dangerous
to human beings irrespective of the exposure route. It binds to cytochrome oxidase and disrupts the
electron transport system. Thus, cellular respiration gets inhibited. Oxygen unavailability in the tissues
leads to asphyxia, incapacitation and death. Most common symptoms include vertigo, headache,
tachypnea, vomiting, and nausea, lack of motor coordination, convulsions and low pulse rate. Respiratory
rate gets increased followed by collapse and arrest. 10ppm has been set as the exposure limit to hydrogen
cyanide at the workplace by OSHA. This report will illustrate the toxicity of HCN by discussing the
different routes of exposure and the common symptoms. The report will also elaborate the standard codes
of practice that one needs to follow to avoid cyanide exposure. It will further shed light on the
toxicokinetics and toxicodynamics of HCN.
Executive summary
Toxicology is a branch of science that analyses relationship between a chemical and its adverse effects.
Hydrogen cyanide is a colourless gas that is fast acting and potentially deadly. It is extremely dangerous
to human beings irrespective of the exposure route. It binds to cytochrome oxidase and disrupts the
electron transport system. Thus, cellular respiration gets inhibited. Oxygen unavailability in the tissues
leads to asphyxia, incapacitation and death. Most common symptoms include vertigo, headache,
tachypnea, vomiting, and nausea, lack of motor coordination, convulsions and low pulse rate. Respiratory
rate gets increased followed by collapse and arrest. 10ppm has been set as the exposure limit to hydrogen
cyanide at the workplace by OSHA. This report will illustrate the toxicity of HCN by discussing the
different routes of exposure and the common symptoms. The report will also elaborate the standard codes
of practice that one needs to follow to avoid cyanide exposure. It will further shed light on the
toxicokinetics and toxicodynamics of HCN.

2HYGIENE AND TOXICOLOGY
Table of Contents
1. Introduction............................................................................................................................................3
2. Discussion..............................................................................................................................................3
a. Sources of exposure...........................................................................................................................3
b. Health effects from exposure.............................................................................................................4
c. Standards and codes of practice.........................................................................................................5
d. Exposure standards............................................................................................................................6
e. Toxicokinetics....................................................................................................................................7
f. Toxicodynamics.................................................................................................................................8
3. Conclusion.............................................................................................................................................9
References....................................................................................................................................................10
Table of Contents
1. Introduction............................................................................................................................................3
2. Discussion..............................................................................................................................................3
a. Sources of exposure...........................................................................................................................3
b. Health effects from exposure.............................................................................................................4
c. Standards and codes of practice.........................................................................................................5
d. Exposure standards............................................................................................................................6
e. Toxicokinetics....................................................................................................................................7
f. Toxicodynamics.................................................................................................................................8
3. Conclusion.............................................................................................................................................9
References....................................................................................................................................................10
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3HYGIENE AND TOXICOLOGY
1. Introduction
Toxicology refers to the science of poisons. The history of toxicology dates back to the time of cave
dwellers. They recognized poisonous animals and plants extracts for warfare and hunting. This branch of
science overlaps with chemistry, biology, medicine and nursing. It analyses the relationship between a
chemical dose and its adverse effects on a particular organism. Any substance that exhibits immediate
adverse effects is termed as a toxin. These toxins can be systemic (affecting the entire body) or organ
specific. These toxins can be chemical agents (cyanide), physical agents (radiations) and biological agents
(snake venom). Hydrogen cyanide is a potentially deadly and fast acting chemical that prevents oxygen
utilization in the body cells. Hydrogen cyanide exists in the form of a colourless gas. Its most toxic effect
is the inhibition of enzymes, which contain metals. This report aims to illustrate the different sources of
cyanide exposure, the adverse health effects, toxico-kinetics and toxico-dynamics of HCN poisoning.
2. Discussion
a. Sources of exposure
Hydrogen cyanide mainly affects cellular metabolism. It inhibits aerobic metabolism by acting on
cytochrome oxidase. The enzyme is a terminal component of the respiratory chain. Hydrogen cyanide is
readily absorbed by the human body through the lungs, mucous membrane and skin. Exposure of any
body part to HCN results in acute intoxication. Occupational exposures occur due to inhalation, skin
absorption or oral routes.
1) Inhalation- Following inhalation, HCN gets rapidly and readily absorbed by the lungs. The
symptoms of cyanide poisoning begin a few seconds after the exposure and can cause death in the
affected person within a few minutes. Dust particles that contain cyanide components if inhaled
produce toxicity in the body. HCN acts as a lung irritant and creates localized damage in the
lungs. This damage manifests in the form of dryness, pulmonary congestion and burning
1. Introduction
Toxicology refers to the science of poisons. The history of toxicology dates back to the time of cave
dwellers. They recognized poisonous animals and plants extracts for warfare and hunting. This branch of
science overlaps with chemistry, biology, medicine and nursing. It analyses the relationship between a
chemical dose and its adverse effects on a particular organism. Any substance that exhibits immediate
adverse effects is termed as a toxin. These toxins can be systemic (affecting the entire body) or organ
specific. These toxins can be chemical agents (cyanide), physical agents (radiations) and biological agents
(snake venom). Hydrogen cyanide is a potentially deadly and fast acting chemical that prevents oxygen
utilization in the body cells. Hydrogen cyanide exists in the form of a colourless gas. Its most toxic effect
is the inhibition of enzymes, which contain metals. This report aims to illustrate the different sources of
cyanide exposure, the adverse health effects, toxico-kinetics and toxico-dynamics of HCN poisoning.
2. Discussion
a. Sources of exposure
Hydrogen cyanide mainly affects cellular metabolism. It inhibits aerobic metabolism by acting on
cytochrome oxidase. The enzyme is a terminal component of the respiratory chain. Hydrogen cyanide is
readily absorbed by the human body through the lungs, mucous membrane and skin. Exposure of any
body part to HCN results in acute intoxication. Occupational exposures occur due to inhalation, skin
absorption or oral routes.
1) Inhalation- Following inhalation, HCN gets rapidly and readily absorbed by the lungs. The
symptoms of cyanide poisoning begin a few seconds after the exposure and can cause death in the
affected person within a few minutes. Dust particles that contain cyanide components if inhaled
produce toxicity in the body. HCN acts as a lung irritant and creates localized damage in the
lungs. This damage manifests in the form of dryness, pulmonary congestion and burning
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4HYGIENE AND TOXICOLOGY
sensation in the throat. 0.3mg/l inhalation of HCN is considered lethal. After 10 minutes of
exposure to the deadly gas, a dose of 0.2mg/l (181 ppm) is considered as a lethal dose (1).
Children generally receive larger doses of the toxic gas when compared to adults who are
exposed to the same levels of HCN due to the larger lung surface area and body weight ratio.
2) Oral routes- Ingestion of solutions that contain cyanide salts or hydrogen cyanide are fatal. These
solutions get rapidly absorbed form the alimentary canal when compared to cyanide salts. Higher
salt doses produce severe intoxication. Lower doses delay death by an hour or more owing to
their exhibition of symptoms at a slower rate. These solutions are strong and corrosive in nature.
They lead to inflammation and ulcer formation in the stomach.
3) Dermal routes- Hydrogen cyanide may be absorbed across uninjured skin primarily and lead to
systemic toxicity within the human body. With an increase in pH of the solution containing
cyanide, the rate of absorption across the skin increases. This occurs due to the presence of
unionized HCN at low pH levels. It can lead to breathing abnormalities like Cheyne Stokes
respiration, plasma extravasations and peripheral vasoconstriction (2). The person can also enter a
state of coma if parts of skin get immersed in cisterns that contain potassium or copper cyanide
solutions.
4) Ocular- Cyanides can also get absorbed through the conjunctiva present in the eyes, in addition to
dermal routes.
b. Health effects from exposure
Some of the most common health effects are:
Severe hydrogen cyanide poisoning can lead to leads to abnormalities in the heartbeat. The
heartbeat may get lowered, blood pressure reduces and the person can even die.
A victim exposed to systemic cyanide poisoning often reports of breathlessness and tightening of
the chest muscles. Pulmonary findings show an increase in respiration depth and rapid breathing.
As the effect of poison increases, the respirations become slow and the person gasps for breath.
sensation in the throat. 0.3mg/l inhalation of HCN is considered lethal. After 10 minutes of
exposure to the deadly gas, a dose of 0.2mg/l (181 ppm) is considered as a lethal dose (1).
Children generally receive larger doses of the toxic gas when compared to adults who are
exposed to the same levels of HCN due to the larger lung surface area and body weight ratio.
2) Oral routes- Ingestion of solutions that contain cyanide salts or hydrogen cyanide are fatal. These
solutions get rapidly absorbed form the alimentary canal when compared to cyanide salts. Higher
salt doses produce severe intoxication. Lower doses delay death by an hour or more owing to
their exhibition of symptoms at a slower rate. These solutions are strong and corrosive in nature.
They lead to inflammation and ulcer formation in the stomach.
3) Dermal routes- Hydrogen cyanide may be absorbed across uninjured skin primarily and lead to
systemic toxicity within the human body. With an increase in pH of the solution containing
cyanide, the rate of absorption across the skin increases. This occurs due to the presence of
unionized HCN at low pH levels. It can lead to breathing abnormalities like Cheyne Stokes
respiration, plasma extravasations and peripheral vasoconstriction (2). The person can also enter a
state of coma if parts of skin get immersed in cisterns that contain potassium or copper cyanide
solutions.
4) Ocular- Cyanides can also get absorbed through the conjunctiva present in the eyes, in addition to
dermal routes.
b. Health effects from exposure
Some of the most common health effects are:
Severe hydrogen cyanide poisoning can lead to leads to abnormalities in the heartbeat. The
heartbeat may get lowered, blood pressure reduces and the person can even die.
A victim exposed to systemic cyanide poisoning often reports of breathlessness and tightening of
the chest muscles. Pulmonary findings show an increase in respiration depth and rapid breathing.
As the effect of poison increases, the respirations become slow and the person gasps for breath.

5HYGIENE AND TOXICOLOGY
The person may develop a blue color in the skin and on the finger nails. Fluids may get
accumulated in the lungs.
The person may feel dizziness, nausea, anxiety, vomiting, weakness and headache. With progress
in poisoning, a person may also develop hallucinations, convulsions, lockjaw, and tetanus spasm
and can lose consciousness or enter a state of coma.
Exposure to liquid hydrogen cyanide leads to skin irritation and skin burns (6).
Severe poisoning leads to metabolic acidosis and increases the level of lactic acids in the blood.
Irritation and swelling in eyes are observed.
Deficiency of oxygen causes brain damage and neurologic sequelae like memory deficit,
personality changes and voluntary muscle disturbances.
Workers who are suffering from chronic HCN exposure report headache, fatigue, eye irritation,
discomfort in chest, loss of appetite, palpitation and nose-bleeds.
c. Standards and codes of practice
Maintenance of occupational hygiene practice is essential to prevent the incidence of several diseases
among workers. Exposure standards represent the concentrations of airborne chemicals in the breathing
zone of workers that can cause undue discomfort or adverse health effects. The principal aim of these
standards is to formulate specific targets that will help to reduce the risk of these adverse health
conditions. The exposure standards are given effect under all jurisdictions and are therefore legally
enforced. These regulations ensure that a business or workplace must not exceed the minimum standard
limits of exposure (5). The ALARP principle is followed while maintaining hygiene standards, which
state that though occupational risks cannot be completely avoided, they should be reduced to tolerable
levels. It helps to assess the symptoms, potential health effects, toxicity levels and decision formulation
regarding the implementation of control measures (3). The stages involved in these standards are
described below:
The person may develop a blue color in the skin and on the finger nails. Fluids may get
accumulated in the lungs.
The person may feel dizziness, nausea, anxiety, vomiting, weakness and headache. With progress
in poisoning, a person may also develop hallucinations, convulsions, lockjaw, and tetanus spasm
and can lose consciousness or enter a state of coma.
Exposure to liquid hydrogen cyanide leads to skin irritation and skin burns (6).
Severe poisoning leads to metabolic acidosis and increases the level of lactic acids in the blood.
Irritation and swelling in eyes are observed.
Deficiency of oxygen causes brain damage and neurologic sequelae like memory deficit,
personality changes and voluntary muscle disturbances.
Workers who are suffering from chronic HCN exposure report headache, fatigue, eye irritation,
discomfort in chest, loss of appetite, palpitation and nose-bleeds.
c. Standards and codes of practice
Maintenance of occupational hygiene practice is essential to prevent the incidence of several diseases
among workers. Exposure standards represent the concentrations of airborne chemicals in the breathing
zone of workers that can cause undue discomfort or adverse health effects. The principal aim of these
standards is to formulate specific targets that will help to reduce the risk of these adverse health
conditions. The exposure standards are given effect under all jurisdictions and are therefore legally
enforced. These regulations ensure that a business or workplace must not exceed the minimum standard
limits of exposure (5). The ALARP principle is followed while maintaining hygiene standards, which
state that though occupational risks cannot be completely avoided, they should be reduced to tolerable
levels. It helps to assess the symptoms, potential health effects, toxicity levels and decision formulation
regarding the implementation of control measures (3). The stages involved in these standards are
described below:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
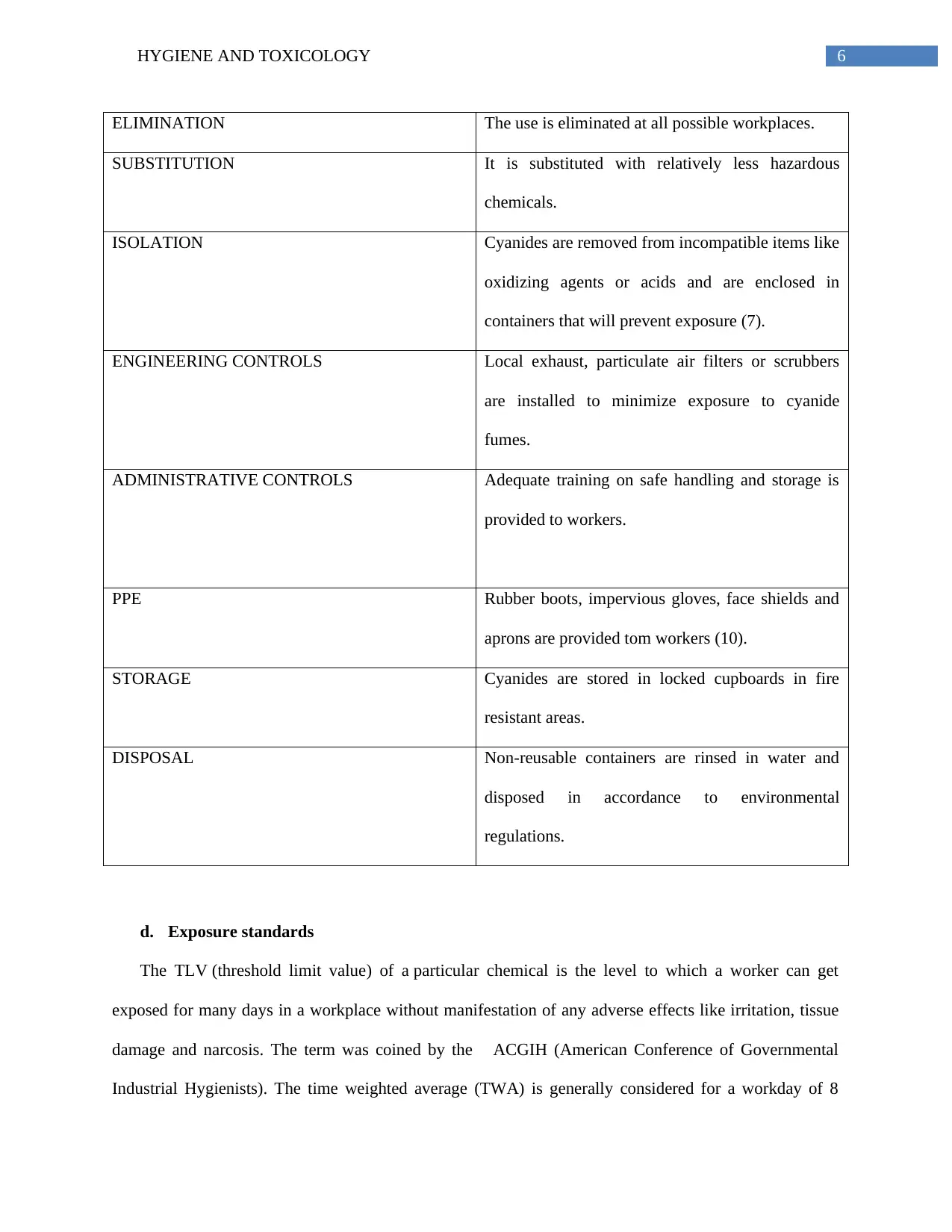
6HYGIENE AND TOXICOLOGY
ELIMINATION The use is eliminated at all possible workplaces.
SUBSTITUTION It is substituted with relatively less hazardous
chemicals.
ISOLATION Cyanides are removed from incompatible items like
oxidizing agents or acids and are enclosed in
containers that will prevent exposure (7).
ENGINEERING CONTROLS Local exhaust, particulate air filters or scrubbers
are installed to minimize exposure to cyanide
fumes.
ADMINISTRATIVE CONTROLS Adequate training on safe handling and storage is
provided to workers.
PPE Rubber boots, impervious gloves, face shields and
aprons are provided tom workers (10).
STORAGE Cyanides are stored in locked cupboards in fire
resistant areas.
DISPOSAL Non-reusable containers are rinsed in water and
disposed in accordance to environmental
regulations.
d. Exposure standards
The TLV (threshold limit value) of a particular chemical is the level to which a worker can get
exposed for many days in a workplace without manifestation of any adverse effects like irritation, tissue
damage and narcosis. The term was coined by the ACGIH (American Conference of Governmental
Industrial Hygienists). The time weighted average (TWA) is generally considered for a workday of 8
ELIMINATION The use is eliminated at all possible workplaces.
SUBSTITUTION It is substituted with relatively less hazardous
chemicals.
ISOLATION Cyanides are removed from incompatible items like
oxidizing agents or acids and are enclosed in
containers that will prevent exposure (7).
ENGINEERING CONTROLS Local exhaust, particulate air filters or scrubbers
are installed to minimize exposure to cyanide
fumes.
ADMINISTRATIVE CONTROLS Adequate training on safe handling and storage is
provided to workers.
PPE Rubber boots, impervious gloves, face shields and
aprons are provided tom workers (10).
STORAGE Cyanides are stored in locked cupboards in fire
resistant areas.
DISPOSAL Non-reusable containers are rinsed in water and
disposed in accordance to environmental
regulations.
d. Exposure standards
The TLV (threshold limit value) of a particular chemical is the level to which a worker can get
exposed for many days in a workplace without manifestation of any adverse effects like irritation, tissue
damage and narcosis. The term was coined by the ACGIH (American Conference of Governmental
Industrial Hygienists). The time weighted average (TWA) is generally considered for a workday of 8
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7HYGIENE AND TOXICOLOGY
hours, for 5 days per week. The short term exposure limits (STEL) are bound to remain within the TWA
levels. The levels can exceed TWA by 3 times only for a maximum time period of 30 minutes in a
workday. Rates of exposure 5 times beyond the permissible limits are not allowed. Section 59 (10) of the
Factories Act cites some permissible exposure levels for toxic substances. PEL refers to the maximum
TWA concentration of a hazardous substance to which a person can get exposed (15). Long term PEL
considers 8 hour working day for 40 hours per week as the permissible limit. On the other hand, for short
term PEL, 15 minutes during a workday are regarded as the exposure level. Short term PEL for hydrogen
cyanide are 4.7 ppm and 5 mg/m3. Accoridng to the Safe Work Australia standards, HCN exposure limits
are 10 peak for TWA/ppm and 11 peak for TWA/mg/m3.
e. Toxicokinetics
Following inhalation, dermal exposure or entry through oral routes, hydrogen cyanide gets rapidly
absorbed by the cells. Cyanide exists in an equilibrium state as undissociated HCN and as an anion.
Hydrogen cyanide exists in ionised form at the physiological pH of 7.4 owing to its pKa value of 9.21.
The distribution of cyanide ions is limited across biological membranes. On the other hand, non-ionised
hydrogen cyanide is readily able to cross the membranes. On crossing the membrane, it gets distributed
through the body cells rapidly. The highest concentration of cyanide is found in the liver upon oral
administration. Maximum concentration of cyanide is found in the brain and the heart irrespective of any
routes of exposure (12). These two organs are most affected by the toxic levels. Whole blood levels with
minimum lethality have been found to be near the range of 250-300 μg/dl. The major pathway for
hydrogen cyanide metabolism is the by the process of detoxification that occurs on the liver in the
presence of 2 mitochondrial sulfur transferase enzyme: thiosulfate cyanide sulfurtransferase (rhodanese)
and β-mercaptopyruvate cyanide sulfurtransferase (4). These enzymes catalyse the transfer of sulfane
sulfur present in thiosulphate to cyanide ions that forms thiocyanate. Rhodanese is the primary pathway
that is responsible for 80% detoxification of cyanide. The concentration of this enzyme is highest in the
liver. Sulfur donor availability acts as the limiting factor in detoxification by this pathway during events
hours, for 5 days per week. The short term exposure limits (STEL) are bound to remain within the TWA
levels. The levels can exceed TWA by 3 times only for a maximum time period of 30 minutes in a
workday. Rates of exposure 5 times beyond the permissible limits are not allowed. Section 59 (10) of the
Factories Act cites some permissible exposure levels for toxic substances. PEL refers to the maximum
TWA concentration of a hazardous substance to which a person can get exposed (15). Long term PEL
considers 8 hour working day for 40 hours per week as the permissible limit. On the other hand, for short
term PEL, 15 minutes during a workday are regarded as the exposure level. Short term PEL for hydrogen
cyanide are 4.7 ppm and 5 mg/m3. Accoridng to the Safe Work Australia standards, HCN exposure limits
are 10 peak for TWA/ppm and 11 peak for TWA/mg/m3.
e. Toxicokinetics
Following inhalation, dermal exposure or entry through oral routes, hydrogen cyanide gets rapidly
absorbed by the cells. Cyanide exists in an equilibrium state as undissociated HCN and as an anion.
Hydrogen cyanide exists in ionised form at the physiological pH of 7.4 owing to its pKa value of 9.21.
The distribution of cyanide ions is limited across biological membranes. On the other hand, non-ionised
hydrogen cyanide is readily able to cross the membranes. On crossing the membrane, it gets distributed
through the body cells rapidly. The highest concentration of cyanide is found in the liver upon oral
administration. Maximum concentration of cyanide is found in the brain and the heart irrespective of any
routes of exposure (12). These two organs are most affected by the toxic levels. Whole blood levels with
minimum lethality have been found to be near the range of 250-300 μg/dl. The major pathway for
hydrogen cyanide metabolism is the by the process of detoxification that occurs on the liver in the
presence of 2 mitochondrial sulfur transferase enzyme: thiosulfate cyanide sulfurtransferase (rhodanese)
and β-mercaptopyruvate cyanide sulfurtransferase (4). These enzymes catalyse the transfer of sulfane
sulfur present in thiosulphate to cyanide ions that forms thiocyanate. Rhodanese is the primary pathway
that is responsible for 80% detoxification of cyanide. The concentration of this enzyme is highest in the
liver. Sulfur donor availability acts as the limiting factor in detoxification by this pathway during events

8HYGIENE AND TOXICOLOGY
of acute cyanide intoxication. Cyanide metabolism slows down when the reserves of sulfur donor ions get
depleted. Thus, it can be stated that sodium thiosulphate accelerate the inactivation of cyanideby acting as
an antidote (16).
Exhalation from the lungs (1-2%) ids one route of cyanide elimination forms the body. There are
several minor pathways that help in its elimination (<15%). These pathways involve conversion of
cyanide to 2-aminothiazoline-4-carboxylicn acid, formation of cyanocobalamine by combining cyanide
with hydroxycobalamine and incorporating into 1-C metabolic pool (14). Cyanide metabolites are also
eliminated in the form of thiocyanate by urination. Urine thiocyanate levels are used as markers for
cyanide poisoning.
f. Toxicodynamics
HCN shows high affinity for sulfur compounds like sulfanes that contain 2 covalently bonded sulfur
atoms that carry unequal charge. It also shows affinity for cobalt and ferric ions containing metallic
compounds. Cyanide forms a combination with ferric ions in cytochrome oxidase, located in the
mitochondria and prevents electron transport system in the cytoplasm. This stops oxidative
phosphorylation and ATP synthesis. Due to prevention of oxidative metabolism mechanism, an increased
demand is created on anerobic glycolysis, which leads to lactic acid accumulation in the muscles and
produces severe disbalance in the composition of acids and base (11). HCN leads to activation of voltage
sensitive and receptor operated calcium channels, inhibition of antioxidants enzymes like catalase and
superoxide dismutase and reactive oxygen species generation. It also leads to failure in utilization of
oxygen and causes histotoxic anoxia. There occurs a shift to anaerobic respiration that elevates plasma
lactate concentrations. An initial increase in cardiac output is observed that is soon followed by a
decrease. This leads to a fall in blood pressure and causes vasodilation. It also creates visual disturbances
by impairing the capacity to focus on an object and mydriasis (dilation of the pupil). Stimulation of the
chemoreceptors that are situated near aortic bifurcation leads to respiratory problems and the person gaps
for breath (13). This is followed by hyperventilation. Cyanide poisoning leads to an increase in
of acute cyanide intoxication. Cyanide metabolism slows down when the reserves of sulfur donor ions get
depleted. Thus, it can be stated that sodium thiosulphate accelerate the inactivation of cyanideby acting as
an antidote (16).
Exhalation from the lungs (1-2%) ids one route of cyanide elimination forms the body. There are
several minor pathways that help in its elimination (<15%). These pathways involve conversion of
cyanide to 2-aminothiazoline-4-carboxylicn acid, formation of cyanocobalamine by combining cyanide
with hydroxycobalamine and incorporating into 1-C metabolic pool (14). Cyanide metabolites are also
eliminated in the form of thiocyanate by urination. Urine thiocyanate levels are used as markers for
cyanide poisoning.
f. Toxicodynamics
HCN shows high affinity for sulfur compounds like sulfanes that contain 2 covalently bonded sulfur
atoms that carry unequal charge. It also shows affinity for cobalt and ferric ions containing metallic
compounds. Cyanide forms a combination with ferric ions in cytochrome oxidase, located in the
mitochondria and prevents electron transport system in the cytoplasm. This stops oxidative
phosphorylation and ATP synthesis. Due to prevention of oxidative metabolism mechanism, an increased
demand is created on anerobic glycolysis, which leads to lactic acid accumulation in the muscles and
produces severe disbalance in the composition of acids and base (11). HCN leads to activation of voltage
sensitive and receptor operated calcium channels, inhibition of antioxidants enzymes like catalase and
superoxide dismutase and reactive oxygen species generation. It also leads to failure in utilization of
oxygen and causes histotoxic anoxia. There occurs a shift to anaerobic respiration that elevates plasma
lactate concentrations. An initial increase in cardiac output is observed that is soon followed by a
decrease. This leads to a fall in blood pressure and causes vasodilation. It also creates visual disturbances
by impairing the capacity to focus on an object and mydriasis (dilation of the pupil). Stimulation of the
chemoreceptors that are situated near aortic bifurcation leads to respiratory problems and the person gaps
for breath (13). This is followed by hyperventilation. Cyanide poisoning leads to an increase in
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9HYGIENE AND TOXICOLOGY
enkephalin release, that manifests in the form of reduced awareness, convulsions and loss of
consciousness. Cyanide also affects the cardiac cells and produces negative inotropy and arrthymia. MRI
and PET scans reveal lesions in the cerebellum, globus pallidus and substantia nigra following acute
cyanide poisoning. It also reduces metabolism of glucose in the cerebrum. Chronic exposure has been
associated with tobacco amblyopia, neuropathy and Leber’s hereditary optic atrophy.
3. Conclusion
Thus, it can be concluded that toxicology is the branch of science that studies the harmful effects of
chemical or biological compounds on the living body. Hydrogen cyanide is a deadly poison that acts
rapidly on the cells and inhibits the enzyme cytochrome oxidase. The possible routes of exposure include
inhalation of smoke, workplaces like metal polishing industries and exposure to insecticides and liquid
cyanide solutions. Several laws have been formulated by the governments to regulate the permissible
exposure limits in order to prevent impairment of cellular respiration. Thus, it can be concluded that the
probable sources of exposure should be identified and legislations should be imposed to prevent cyanide
contamination among people.
enkephalin release, that manifests in the form of reduced awareness, convulsions and loss of
consciousness. Cyanide also affects the cardiac cells and produces negative inotropy and arrthymia. MRI
and PET scans reveal lesions in the cerebellum, globus pallidus and substantia nigra following acute
cyanide poisoning. It also reduces metabolism of glucose in the cerebrum. Chronic exposure has been
associated with tobacco amblyopia, neuropathy and Leber’s hereditary optic atrophy.
3. Conclusion
Thus, it can be concluded that toxicology is the branch of science that studies the harmful effects of
chemical or biological compounds on the living body. Hydrogen cyanide is a deadly poison that acts
rapidly on the cells and inhibits the enzyme cytochrome oxidase. The possible routes of exposure include
inhalation of smoke, workplaces like metal polishing industries and exposure to insecticides and liquid
cyanide solutions. Several laws have been formulated by the governments to regulate the permissible
exposure limits in order to prevent impairment of cellular respiration. Thus, it can be concluded that the
probable sources of exposure should be identified and legislations should be imposed to prevent cyanide
contamination among people.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10HYGIENE AND TOXICOLOGY
References
1) Anseeuw K, Delvau N, Burillo-Putze G, De Iaco F, Geldner G, Holmström P, Lambert Y, Sabbe
M. Cyanide poisoning by fire smoke inhalation: a European expert consensus. European Journal
of Emergency Medicine. 2013 Feb 1;20(1):2-9. Retrieved from: http://journals.lww.com/euro-
emergencymed/Fulltext/2013/02000/Cyanide_poisoning_by_fire_smoke_inhalation___a.2.aspx
2) Ballantyne BR. The forensic diagnosis of acute cyanide poisoning. Forensic toxicology (B.
Ballantyne, ed.). 2013 Oct 22:99-113. Retrieved from: https://books.google.co.in/books?
hl=en&lr=&id=3gQlBQAAQBAJ&oi=fnd&pg=PA99&dq=The+forensic+diagnosis+of+acute+c
yanide+poisoning.
+&ots=ewYfM6KWRJ&sig=_3XbH9_F6hrBVHq8VW0kyDXK3Xs#v=onepage&q=The
%20forensic%20diagnosis%20of%20acute%20cyanide%20poisoning.&f=false
3) Ballatori N, Cherian MG, Dawson DC, Delnomdedieu M, Druet P, Fisher BR, Goering P, Goyer
RA, Himeno S, Imura N, Jeffrey EH. Toxicology of metals: biochemical aspects. Springer
Science & Business Media; 2012 Dec 6. Retrieved from: https://books.google.co.in/books?
hl=en&lr=&id=LfP0CAAAQBAJ&oi=fnd&pg=PA1&dq=3)%09Ballatori+N,+Cherian+MG,
+Dawson+DC,+Delnomdedieu+M,+Druet+P,+Fisher+BR,+Goering+P,+Goyer+RA,+Himeno+S,
+Imura+N,+Jeffrey+EH.+Toxicology+of+metals:+biochemical+aspects.+Springer+Science+
%26+Business+Media
%3B+2012+Dec+6.&ots=GPdBSb5yhV&sig=SurKSMi0zYIpJfXTCrxBmkB3gGY#v=onepage
&q&f=false
4) Bhandari RK, Oda RP, Petrikovics I, Thompson DE, Brenner M, Mahon SB, Bebarta VS,
Rockwood GA, Logue BA. Cyanide toxicokinetics: the behavior of cyanide, thiocyanate and 2-
amino-2-thiazoline-4-carboxylic acid in multiple animal models. Journal of analytical toxicology.
2014 May 1;38(4):218-25. Retrieved from:
https://academic.oup.com/jat/article/38/4/218/834049/Cyanide-Toxicokinetics-The-Behavior-of-
Cyanide
5) Brent J. Critical care toxicology. Burkhart K, Dargan P, Hatten B, Megarbane B, Palmer R,
editors. Springer International Publishing; 2015. Retrieved from:
https://www.researchgate.net/profile/Laura_Bechtel/publication/51429130_Critcal_Care_Toxicol
ogy/links/545243f50cf2bf864cbb3a94.pdf
6) Curry SC, Spyres MB. Cyanide: hydrogen cyanide, inorganic cyanide salts, and nitriles. Critical
Care Toxicology. 2016:1-21. Retrieved from:
https://link.springer.com/referenceworkentry/10.1007/978-3-319-20790-2_101-1#page-1
7) Grabowska T, Skowronek R, Nowicka J, Sybirska H. Prevalence of hydrogen cyanide and
carboxyhaemoglobin in victims of smoke inhalation during enclosed-space fires: a combined
toxicological risk. Clinical toxicology. 2012 Sep 1;50(8):759-63. Retrieved from:
http://www.tandfonline.com/doi/abs/10.3109/15563650.2012.714470
8) Magnusson R, Nyholm S, Åstot C. Analysis of hydrogen cyanide in air in a case of attempted
cyanide poisoning. Forensic science international. 2012 Oct 10;222(1):e7-12. Retrieved from:
http://www.sciencedirect.com/science/article/pii/S0379073812002575
References
1) Anseeuw K, Delvau N, Burillo-Putze G, De Iaco F, Geldner G, Holmström P, Lambert Y, Sabbe
M. Cyanide poisoning by fire smoke inhalation: a European expert consensus. European Journal
of Emergency Medicine. 2013 Feb 1;20(1):2-9. Retrieved from: http://journals.lww.com/euro-
emergencymed/Fulltext/2013/02000/Cyanide_poisoning_by_fire_smoke_inhalation___a.2.aspx
2) Ballantyne BR. The forensic diagnosis of acute cyanide poisoning. Forensic toxicology (B.
Ballantyne, ed.). 2013 Oct 22:99-113. Retrieved from: https://books.google.co.in/books?
hl=en&lr=&id=3gQlBQAAQBAJ&oi=fnd&pg=PA99&dq=The+forensic+diagnosis+of+acute+c
yanide+poisoning.
+&ots=ewYfM6KWRJ&sig=_3XbH9_F6hrBVHq8VW0kyDXK3Xs#v=onepage&q=The
%20forensic%20diagnosis%20of%20acute%20cyanide%20poisoning.&f=false
3) Ballatori N, Cherian MG, Dawson DC, Delnomdedieu M, Druet P, Fisher BR, Goering P, Goyer
RA, Himeno S, Imura N, Jeffrey EH. Toxicology of metals: biochemical aspects. Springer
Science & Business Media; 2012 Dec 6. Retrieved from: https://books.google.co.in/books?
hl=en&lr=&id=LfP0CAAAQBAJ&oi=fnd&pg=PA1&dq=3)%09Ballatori+N,+Cherian+MG,
+Dawson+DC,+Delnomdedieu+M,+Druet+P,+Fisher+BR,+Goering+P,+Goyer+RA,+Himeno+S,
+Imura+N,+Jeffrey+EH.+Toxicology+of+metals:+biochemical+aspects.+Springer+Science+
%26+Business+Media
%3B+2012+Dec+6.&ots=GPdBSb5yhV&sig=SurKSMi0zYIpJfXTCrxBmkB3gGY#v=onepage
&q&f=false
4) Bhandari RK, Oda RP, Petrikovics I, Thompson DE, Brenner M, Mahon SB, Bebarta VS,
Rockwood GA, Logue BA. Cyanide toxicokinetics: the behavior of cyanide, thiocyanate and 2-
amino-2-thiazoline-4-carboxylic acid in multiple animal models. Journal of analytical toxicology.
2014 May 1;38(4):218-25. Retrieved from:
https://academic.oup.com/jat/article/38/4/218/834049/Cyanide-Toxicokinetics-The-Behavior-of-
Cyanide
5) Brent J. Critical care toxicology. Burkhart K, Dargan P, Hatten B, Megarbane B, Palmer R,
editors. Springer International Publishing; 2015. Retrieved from:
https://www.researchgate.net/profile/Laura_Bechtel/publication/51429130_Critcal_Care_Toxicol
ogy/links/545243f50cf2bf864cbb3a94.pdf
6) Curry SC, Spyres MB. Cyanide: hydrogen cyanide, inorganic cyanide salts, and nitriles. Critical
Care Toxicology. 2016:1-21. Retrieved from:
https://link.springer.com/referenceworkentry/10.1007/978-3-319-20790-2_101-1#page-1
7) Grabowska T, Skowronek R, Nowicka J, Sybirska H. Prevalence of hydrogen cyanide and
carboxyhaemoglobin in victims of smoke inhalation during enclosed-space fires: a combined
toxicological risk. Clinical toxicology. 2012 Sep 1;50(8):759-63. Retrieved from:
http://www.tandfonline.com/doi/abs/10.3109/15563650.2012.714470
8) Magnusson R, Nyholm S, Åstot C. Analysis of hydrogen cyanide in air in a case of attempted
cyanide poisoning. Forensic science international. 2012 Oct 10;222(1):e7-12. Retrieved from:
http://www.sciencedirect.com/science/article/pii/S0379073812002575

11HYGIENE AND TOXICOLOGY
9) Petrikovics I, Budai M, Kovacs K, Thompson DE. Past, present and future of cyanide antagonism
research: from the early remedies to the current therapies. World journal of methodology. 2015
Jun 26;5(2):88. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4482825/
10) Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC. Management of cyanide poisoning.
Emergency Medicine Australasia. 2012 Jun 1;24(3):225-38. Retrieved from:
http://onlinelibrary.wiley.com/doi/10.1111/j.1742-6723.2012.01538.x/full
11) Srinivasan S, Avadhani NG. Cytochrome c oxidase dysfunction in oxidative stress. Free Radical
Biology and Medicine. 2012 Sep 15;53(6):1252-63. Retrieved from:
http://www.sciencedirect.com/science/article/pii/S0891584912004169
12) Stamyr K, Mörk AK, Johanson G. Physiologically based pharmacokinetic modeling of hydrogen
cyanide levels in human breath. Archives of toxicology. 2015 Aug 1;89(8):1287-96. Retrieved
from: https://link.springer.com/article/10.1007/s00204-014-1310-y
13) Stamyr K, Thelander G, Ernstgård L, Ahlner J, Johanson G. Swedish forensic data 1992–2009
suggest hydrogen cyanide as an important cause of death in fire victims. Inhalation toxicology.
2012 Feb 1;24(3):194-9. Retrieved from:
http://www.tandfonline.com/doi/abs/10.3109/08958378.2012.660285
14) Thompson JP, Marrs TC. Hydroxocobalamin in cyanide poisoning. Clinical Toxicology. 2012
Dec 1;50(10):875-85. Retrieved from:
http://www.tandfonline.com/doi/abs/10.3109/15563650.2012.742197
15) Vale A. Cyanide. Medicine. 2012 Mar 31;40(3):121. Retrieved from:
http://www.sciencedirect.com/science/article/pii/S1357303911003410
16) W Borron, S. and J Baud, F., 2012. Antidotes for acute cyanide poisoning. Current
pharmaceutical biotechnology, 13(10), pp.1940-1948. Retrieved from:
http://www.ingentaconnect.com/content/ben/cpb/2012/00000013/00000010/art00011
9) Petrikovics I, Budai M, Kovacs K, Thompson DE. Past, present and future of cyanide antagonism
research: from the early remedies to the current therapies. World journal of methodology. 2015
Jun 26;5(2):88. Retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4482825/
10) Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC. Management of cyanide poisoning.
Emergency Medicine Australasia. 2012 Jun 1;24(3):225-38. Retrieved from:
http://onlinelibrary.wiley.com/doi/10.1111/j.1742-6723.2012.01538.x/full
11) Srinivasan S, Avadhani NG. Cytochrome c oxidase dysfunction in oxidative stress. Free Radical
Biology and Medicine. 2012 Sep 15;53(6):1252-63. Retrieved from:
http://www.sciencedirect.com/science/article/pii/S0891584912004169
12) Stamyr K, Mörk AK, Johanson G. Physiologically based pharmacokinetic modeling of hydrogen
cyanide levels in human breath. Archives of toxicology. 2015 Aug 1;89(8):1287-96. Retrieved
from: https://link.springer.com/article/10.1007/s00204-014-1310-y
13) Stamyr K, Thelander G, Ernstgård L, Ahlner J, Johanson G. Swedish forensic data 1992–2009
suggest hydrogen cyanide as an important cause of death in fire victims. Inhalation toxicology.
2012 Feb 1;24(3):194-9. Retrieved from:
http://www.tandfonline.com/doi/abs/10.3109/08958378.2012.660285
14) Thompson JP, Marrs TC. Hydroxocobalamin in cyanide poisoning. Clinical Toxicology. 2012
Dec 1;50(10):875-85. Retrieved from:
http://www.tandfonline.com/doi/abs/10.3109/15563650.2012.742197
15) Vale A. Cyanide. Medicine. 2012 Mar 31;40(3):121. Retrieved from:
http://www.sciencedirect.com/science/article/pii/S1357303911003410
16) W Borron, S. and J Baud, F., 2012. Antidotes for acute cyanide poisoning. Current
pharmaceutical biotechnology, 13(10), pp.1940-1948. Retrieved from:
http://www.ingentaconnect.com/content/ben/cpb/2012/00000013/00000010/art00011
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 12
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.