Efficacy of Hypertonic Saline in Pediatric Cerebral Edema Treatment
VerifiedAdded on 2023/05/23
|9
|5169
|120
Report
AI Summary
This retrospective study, conducted in the Pediatric Intensive Care Unit at Çukurova University Faculty of Medicine, evaluates the efficacy and side effects of hypertonic saline (HS) compared to mannitol in treating cerebral edema in children. The study included 67 patients divided into three groups: those treated with mannitol, HS, and both. The results indicate that hypertonic saline, either alone or in combination with mannitol, was associated with a significantly shorter duration of comatose state and lower mortality rates compared to mannitol alone. The study closely monitored patients for various parameters, including serum sodium levels, osmolarity, and complications like hyperchloremic metabolic acidosis and hypotension. The findings suggest that hypertonic saline may be a more effective treatment option for cerebral edema than mannitol, although intracranial pressure monitoring was not applied. The study emphasizes the importance of maintaining intravascular volume and carefully monitoring electrolyte imbalances during treatment. Multivariate analysis found no significant impact of age, gender, or the cause of cerebral edema on outcomes.

Original Articles
INDIAN PEDIATRICS 771 VOLUME 43__SEPTEMBER 17, 2006
Hypertonic Saline Treatment in Children with Cerebral Edema
Yildizdas D, Altunbasak S*, Celik U+ and Herguner O*
Pediatric Intensive Care Unit, *Department of Pediatric Neurology and +Department of Pediatric Infectious
Diseases, Çukurova University Faculty of Medicine, Adana, Turkey.
Correspondence to: Dr. Dinçer Yildizdas, Çukurova University Faculty of Medicine, Pediatric Intensive Care
Unit, 01330/Adana/Turkey.
E-mail: rdy90@hotmail.com dyildizdas@cu.edu.tr
Manuscript received: May 18, 2005; Initial review completed: August 3, 2005;
Revision accepted: March 20, 2006.
Objective: To compare the efficacy and side effects of hypertronic saline and mannitol use in
cerebral edema. Design: Retrospective study. Setting: Pediatric intensive care unit. Subjects: 67
patients with cerebral edema. Methods: Patients with cerebral edema treated with either mannitol
or hypertronic saline (HS) (Group II: n = 25), and both mannitol and HS (Group III: n = 20) were
evaluated retrospectively. Cerebral edema and increased intracranial pressure were based on the
clinical and/or radiological (CT, MR) findings. When treating with both mannitol and HS (Group
IIIA), if patients serum osmality was greater than 325 mosmol/L, mannitol was stopped and
patients were treated with only HS (Group IIIB). All patients were closely monitored for fever,
pulse, blood pressure, central venous pressure (CVP), oxygen saturation, volume of fluid intake
and urine output. Mannitol was given at a dose of 0.25-0.5 g/kg while the hypertonic saline was
given as 3% saline to maintain the serum-Na within the range of 155-165 mEq/L. Results: There
was no statistically significant difference in terms of Glasgow coma scale, age, gender, and
etiologic distribution between the groups. And also distribution of the other treatments given for
cerebral edema is not significiant. Mannitol was given for a total dose of 9.3 ± 5.0 (2-16) doses in
Group I, and 6.5 ± 2.8 (2-10) doses in Group III. Hypertonic saline was infused for 4-25 times in
Group II. Although there was no statistically significant difference in the highest serum Na and
osmolarity levels of the groups, duration of comatose state and mortality rate were significantly
lower in Group II and Group III A/B. Patients who received only HS were subdivided according to
their serum Na concentrations into 2 groups as those between 150-160 mEq/L and those between
160-170 mEq/L. The duration of comatose state and mortality was not different in patients with
serum-Na of 150-160 mEq/L and in patients with 160-170 mEq/L in the hypertonic saline receiving
patients. Four patients in the group II developed hyperchloremic metabolic acidosis and 2 patients
in the group I had hypotension. As two patients in group II had diabetes insipidus and one patient
had renal failure in group I, the treatment was terminated. The causes of death were septic shock,
ventilator associated pneumonia with acute respiratory distress syndrome, progressive cerebral
edema and cerebral edema with pulmonary edema. Multivariate analysis showed that age, gender,
cause of cerebral edema, electrolyte imbalance, hyperglycemia and hyper-ventilation had no
significant impact on outcome. Conclusions: Hypertonic saline seems to be more effective than
mannitol in the cerebral edema.
Key words: Cerebral edema, Hypertonic saline, Mannitol.
INDIAN PEDIATRICS 771 VOLUME 43__SEPTEMBER 17, 2006
Hypertonic Saline Treatment in Children with Cerebral Edema
Yildizdas D, Altunbasak S*, Celik U+ and Herguner O*
Pediatric Intensive Care Unit, *Department of Pediatric Neurology and +Department of Pediatric Infectious
Diseases, Çukurova University Faculty of Medicine, Adana, Turkey.
Correspondence to: Dr. Dinçer Yildizdas, Çukurova University Faculty of Medicine, Pediatric Intensive Care
Unit, 01330/Adana/Turkey.
E-mail: rdy90@hotmail.com dyildizdas@cu.edu.tr
Manuscript received: May 18, 2005; Initial review completed: August 3, 2005;
Revision accepted: March 20, 2006.
Objective: To compare the efficacy and side effects of hypertronic saline and mannitol use in
cerebral edema. Design: Retrospective study. Setting: Pediatric intensive care unit. Subjects: 67
patients with cerebral edema. Methods: Patients with cerebral edema treated with either mannitol
or hypertronic saline (HS) (Group II: n = 25), and both mannitol and HS (Group III: n = 20) were
evaluated retrospectively. Cerebral edema and increased intracranial pressure were based on the
clinical and/or radiological (CT, MR) findings. When treating with both mannitol and HS (Group
IIIA), if patients serum osmality was greater than 325 mosmol/L, mannitol was stopped and
patients were treated with only HS (Group IIIB). All patients were closely monitored for fever,
pulse, blood pressure, central venous pressure (CVP), oxygen saturation, volume of fluid intake
and urine output. Mannitol was given at a dose of 0.25-0.5 g/kg while the hypertonic saline was
given as 3% saline to maintain the serum-Na within the range of 155-165 mEq/L. Results: There
was no statistically significant difference in terms of Glasgow coma scale, age, gender, and
etiologic distribution between the groups. And also distribution of the other treatments given for
cerebral edema is not significiant. Mannitol was given for a total dose of 9.3 ± 5.0 (2-16) doses in
Group I, and 6.5 ± 2.8 (2-10) doses in Group III. Hypertonic saline was infused for 4-25 times in
Group II. Although there was no statistically significant difference in the highest serum Na and
osmolarity levels of the groups, duration of comatose state and mortality rate were significantly
lower in Group II and Group III A/B. Patients who received only HS were subdivided according to
their serum Na concentrations into 2 groups as those between 150-160 mEq/L and those between
160-170 mEq/L. The duration of comatose state and mortality was not different in patients with
serum-Na of 150-160 mEq/L and in patients with 160-170 mEq/L in the hypertonic saline receiving
patients. Four patients in the group II developed hyperchloremic metabolic acidosis and 2 patients
in the group I had hypotension. As two patients in group II had diabetes insipidus and one patient
had renal failure in group I, the treatment was terminated. The causes of death were septic shock,
ventilator associated pneumonia with acute respiratory distress syndrome, progressive cerebral
edema and cerebral edema with pulmonary edema. Multivariate analysis showed that age, gender,
cause of cerebral edema, electrolyte imbalance, hyperglycemia and hyper-ventilation had no
significant impact on outcome. Conclusions: Hypertonic saline seems to be more effective than
mannitol in the cerebral edema.
Key words: Cerebral edema, Hypertonic saline, Mannitol.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

INDIAN PEDIATRICS 772 VOLUME 43__SEPTEMBER 17, 2006
YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
Hyperosmolar treatment is one of the
important methods for treating cerebral
edema, and has been employed since early
1960(1). Urea, glycerol and mannitol were
used to treatment of this condition in the early
years, but then urea and glycerol were soon
abandoned because of low efficacy. Mannitol
is still used extensively. Side effects like
rebound effect, serum electrolyte imbalance
and hypovolemia have led to the continued
search for other osmotically active agents.
One of them is hypertonic saline.
Studies using isotopic techniques and ion
selective microelectrodes have shown that the
blood-brain barrier (BBB) is impermeable to
sodium (Na) and chloride (Cl) ions with the
reflection coefficient for Na and Cl deter-
mined to be 1.0 and 0.9 for mannitol(2-4). It
has been shown that both in animals with and
without intracranial pathology, hypertonic
saline (HS) reduces brain water content(5,6)
and in traumatized animal models it has more
favorable results than mannitol(7,8).
We have been using 3% hypertonic saline
in patients with cerebral edema since 2002. In
this retrospective study, we aimed to show the
effects of hypertonic saline in children with
cerebral edema and to compare this treatment
modality with mannitol in terms of efficacy
and side effects.
Subjects and Methods
Patients with cerebral edema treated with
either mannitol or HS, and both mannitol and
HS in the Pediatric Intensive Care Unit,
Çukurova University, School of Medicine
between June 2002 and May 2004 were
evaluated retrospectively. The patients were
divided into 3 groups according to the
hyperosmolar agent applied:
Group I: Group receiving only mannitol
Group II: Group receiving only HS
Group III: Group receiving both mannitol and
HS. When treating with both mannitol and HS
(Group IIIA), if patients serum osmality was
greater than 325 mosmol/L, mannitol was
stopped and patients were treated with only
HS (Group IIIB)
Cerebral edema and increased intracranial
pressure were based on the clinical and/or
radiological (CT, MR) findings(9,10). Clini-
cal findings included low consciousness level
(GCS <8) plus one or more of the followings:
Unequal, dilated or unreactive pupils, loss of
brain stem reflexes (light and oculocephalic),
cranial nerve palsies (III, VI) and Cushing's
triad. Radiological findings included one or
more of the followings: Effacement of the
basal cisterns, thin, slit-like or completely
obliterated ventricles, obliterated cortical
sulci, shift in the midline, and temporal lobe
or cerebellar tonsils herniation(9,10). All
patients were closely monitored for fever,
pulse, blood pressure, central venous pressure
(CVP), oxygen saturation, volume of fluid in-
take and urine output. None of the patients had
pulmonary problem. By CVP monitoring,
blood pressure, renal function tests, and fluid
intake and urine output, every effort was made
to keep the intravascular volume within the
normal limits; hypervolemia or hypovolemia
were avoided. Fluid replacement and
maintenence fluid treatment were given to
maintain CVP of 5-10 cm H2O. In addition to
the hyperosmolar treatment the patients were
ventilated to keep the PCO 2 at 30-35 mmHg
for a period 48-72 hours. Intracranial pressure
monitoring could not be applied to any
patient. All patients were given midazolam
(in a dose of 0.1 mg/kg/h, increased as
needed) for sedation and fentanyl (in a dose of
1 μg/kg/h, increased as needed) for analgesia.
Patients were treated with the neuromuscular
blocking agent vecuronium (0.1 mg/kg), if
there was patient- ventilator asynchrony even
YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
Hyperosmolar treatment is one of the
important methods for treating cerebral
edema, and has been employed since early
1960(1). Urea, glycerol and mannitol were
used to treatment of this condition in the early
years, but then urea and glycerol were soon
abandoned because of low efficacy. Mannitol
is still used extensively. Side effects like
rebound effect, serum electrolyte imbalance
and hypovolemia have led to the continued
search for other osmotically active agents.
One of them is hypertonic saline.
Studies using isotopic techniques and ion
selective microelectrodes have shown that the
blood-brain barrier (BBB) is impermeable to
sodium (Na) and chloride (Cl) ions with the
reflection coefficient for Na and Cl deter-
mined to be 1.0 and 0.9 for mannitol(2-4). It
has been shown that both in animals with and
without intracranial pathology, hypertonic
saline (HS) reduces brain water content(5,6)
and in traumatized animal models it has more
favorable results than mannitol(7,8).
We have been using 3% hypertonic saline
in patients with cerebral edema since 2002. In
this retrospective study, we aimed to show the
effects of hypertonic saline in children with
cerebral edema and to compare this treatment
modality with mannitol in terms of efficacy
and side effects.
Subjects and Methods
Patients with cerebral edema treated with
either mannitol or HS, and both mannitol and
HS in the Pediatric Intensive Care Unit,
Çukurova University, School of Medicine
between June 2002 and May 2004 were
evaluated retrospectively. The patients were
divided into 3 groups according to the
hyperosmolar agent applied:
Group I: Group receiving only mannitol
Group II: Group receiving only HS
Group III: Group receiving both mannitol and
HS. When treating with both mannitol and HS
(Group IIIA), if patients serum osmality was
greater than 325 mosmol/L, mannitol was
stopped and patients were treated with only
HS (Group IIIB)
Cerebral edema and increased intracranial
pressure were based on the clinical and/or
radiological (CT, MR) findings(9,10). Clini-
cal findings included low consciousness level
(GCS <8) plus one or more of the followings:
Unequal, dilated or unreactive pupils, loss of
brain stem reflexes (light and oculocephalic),
cranial nerve palsies (III, VI) and Cushing's
triad. Radiological findings included one or
more of the followings: Effacement of the
basal cisterns, thin, slit-like or completely
obliterated ventricles, obliterated cortical
sulci, shift in the midline, and temporal lobe
or cerebellar tonsils herniation(9,10). All
patients were closely monitored for fever,
pulse, blood pressure, central venous pressure
(CVP), oxygen saturation, volume of fluid in-
take and urine output. None of the patients had
pulmonary problem. By CVP monitoring,
blood pressure, renal function tests, and fluid
intake and urine output, every effort was made
to keep the intravascular volume within the
normal limits; hypervolemia or hypovolemia
were avoided. Fluid replacement and
maintenence fluid treatment were given to
maintain CVP of 5-10 cm H2O. In addition to
the hyperosmolar treatment the patients were
ventilated to keep the PCO 2 at 30-35 mmHg
for a period 48-72 hours. Intracranial pressure
monitoring could not be applied to any
patient. All patients were given midazolam
(in a dose of 0.1 mg/kg/h, increased as
needed) for sedation and fentanyl (in a dose of
1 μg/kg/h, increased as needed) for analgesia.
Patients were treated with the neuromuscular
blocking agent vecuronium (0.1 mg/kg), if
there was patient- ventilator asynchrony even
YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
INDIAN PEDIATRICS 773 VOLUME 43__SEPTEMBER 17, 2006
on midazolam and fentanyl infusion. In Group
I, patients were treated with 0.5 g/kg mannitol
for the first two doses and if needed the main-
tenance doses were 0.25 g/kg/dose. Hyper-
tonic saline was given to provide a serum-Na
level of 155-165 mEq/L. Extra boluses were
given depending on the serum Na level.
Hypertonic saline was applied both as an
infusion and in bolus form. The infusion rate
was 0.5-2 mL/kg/h and each bolus was
applied as 1 mL/kg for 15 minutes. Na level
and osmality sample were obtained after each
bolus and/or one for every 3 hours. At GCS
≥8, cerebral edema treatment was terminated.
The serum Na concentration was reduced to
the normal range gradually over 2-3 days. If
any clinical improvement has not been re-
ceived in spite of hypertonic saline, mannitol
and hyperventilation treatment, we used pen-
tothal infusion (Na-thiopental 5-10 mg/kg
loading followed by 3-5 mg/kg/h mainte-
nance).
Complications (Hyperchloremic meta-
bolic acidosis, renal failure, subarachnoid
hemorrhage, central pontine myelinosis,
coagulopathy disorder, pulmonary edema,
hypokalemia and hemolysis) were recorded.
The protocol was reviewed and approved
by the local ethics committee of the Faculty of
Medicine. Statistical analysis was done by
SPSS-10.0 (SPSS, Chicago, IL, USA). Chi-
square and t tests were used for independent
samples and when necessary Mann-Whitney
U tests were performed. Survivors and non-
survivors were compared using multivariate
analysis to identify variables having a
significant association with mortality.
Results
There were 22 children in Group I. Of
these, 9 (41%) were male and 13 (59%) were
female and their mean age was 67.9 ± 46.4
(range 1-120) months. Group II consisted of
25 children, 12 (48%) male, 13 (52%) female,
whose mean age was 68.4 ± 50.3 (range 2-
144) months. Group III had 20 children (10
male and 10 female) with a mean age of 70.5 ±
52.2 (range 5-180) months. There were no
statistically significant difference between
groups in terms of age (P = 0.5) and gender
(P = 0.4). The Glasgow Coma Scale of the
patients in groups I, II and III at presentation
were 4.4 ± 1.3 (3-7), 4.5 ± 1.1 (3-7), and 4.3 ±
1.3 (3-7), respectively (P = 0.85). All patients
received midazolam and fentanyl and 7
patients in Group I, 7 in Group II and 9 in
Group III were treated with vecuronium.
The etiologic causes are shown in Table I.
We could not identify any significant
difference among the groups in etiological
causes (P = 0.8) (Table I). Cranial tomography
was performed in all patients, and also MRI
was performed in 22 patients. Neither
hypothermia nor decompressive craniectomy
had to be done in any of patients.
Mannitol was given for a total dose of
TABLE I–Causes of Cerebral Edema in various Groups
Etiology Group I (n = 22) Group II(n = 25) Group III(n = 20)
Meningoencephalitis 9 (40%) 10 (40%) 9 (45%)
Hypoxic ischemic encephalopathy 4 (18%) 6 (24%) 4 (20%)
Intracranial hemorrhage 4 (18%) 5 (20%) 3 (15%)
Meningitis 2 (9%) 2 (8%) 2 (10%)
Metabolic encephalopathy 3 (15%) 2 (8%) 2 (10%)
INDIAN PEDIATRICS 773 VOLUME 43__SEPTEMBER 17, 2006
on midazolam and fentanyl infusion. In Group
I, patients were treated with 0.5 g/kg mannitol
for the first two doses and if needed the main-
tenance doses were 0.25 g/kg/dose. Hyper-
tonic saline was given to provide a serum-Na
level of 155-165 mEq/L. Extra boluses were
given depending on the serum Na level.
Hypertonic saline was applied both as an
infusion and in bolus form. The infusion rate
was 0.5-2 mL/kg/h and each bolus was
applied as 1 mL/kg for 15 minutes. Na level
and osmality sample were obtained after each
bolus and/or one for every 3 hours. At GCS
≥8, cerebral edema treatment was terminated.
The serum Na concentration was reduced to
the normal range gradually over 2-3 days. If
any clinical improvement has not been re-
ceived in spite of hypertonic saline, mannitol
and hyperventilation treatment, we used pen-
tothal infusion (Na-thiopental 5-10 mg/kg
loading followed by 3-5 mg/kg/h mainte-
nance).
Complications (Hyperchloremic meta-
bolic acidosis, renal failure, subarachnoid
hemorrhage, central pontine myelinosis,
coagulopathy disorder, pulmonary edema,
hypokalemia and hemolysis) were recorded.
The protocol was reviewed and approved
by the local ethics committee of the Faculty of
Medicine. Statistical analysis was done by
SPSS-10.0 (SPSS, Chicago, IL, USA). Chi-
square and t tests were used for independent
samples and when necessary Mann-Whitney
U tests were performed. Survivors and non-
survivors were compared using multivariate
analysis to identify variables having a
significant association with mortality.
Results
There were 22 children in Group I. Of
these, 9 (41%) were male and 13 (59%) were
female and their mean age was 67.9 ± 46.4
(range 1-120) months. Group II consisted of
25 children, 12 (48%) male, 13 (52%) female,
whose mean age was 68.4 ± 50.3 (range 2-
144) months. Group III had 20 children (10
male and 10 female) with a mean age of 70.5 ±
52.2 (range 5-180) months. There were no
statistically significant difference between
groups in terms of age (P = 0.5) and gender
(P = 0.4). The Glasgow Coma Scale of the
patients in groups I, II and III at presentation
were 4.4 ± 1.3 (3-7), 4.5 ± 1.1 (3-7), and 4.3 ±
1.3 (3-7), respectively (P = 0.85). All patients
received midazolam and fentanyl and 7
patients in Group I, 7 in Group II and 9 in
Group III were treated with vecuronium.
The etiologic causes are shown in Table I.
We could not identify any significant
difference among the groups in etiological
causes (P = 0.8) (Table I). Cranial tomography
was performed in all patients, and also MRI
was performed in 22 patients. Neither
hypothermia nor decompressive craniectomy
had to be done in any of patients.
Mannitol was given for a total dose of
TABLE I–Causes of Cerebral Edema in various Groups
Etiology Group I (n = 22) Group II(n = 25) Group III(n = 20)
Meningoencephalitis 9 (40%) 10 (40%) 9 (45%)
Hypoxic ischemic encephalopathy 4 (18%) 6 (24%) 4 (20%)
Intracranial hemorrhage 4 (18%) 5 (20%) 3 (15%)
Meningitis 2 (9%) 2 (8%) 2 (10%)
Metabolic encephalopathy 3 (15%) 2 (8%) 2 (10%)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

INDIAN PEDIATRICS 774 VOLUME 43__SEPTEMBER 17, 2006
YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
9.3 ± 5.0 (2-16) doses in Group I and
6.5 ± 2.8(2-10) doses in Group III. Hypertonic
saline was infused for 4-25 times in Group II.
The highest serum Na and osmolarity levels
during mannitol, hypertonic saline, and HS +
mannitol (Group III subgroup A and B)
infusions are shown in Table II. Although
there was no statistically significant difference
in the highest serum Na and osmolarity levels
of the groups (P = 0.8, P = 0.5, respectively);
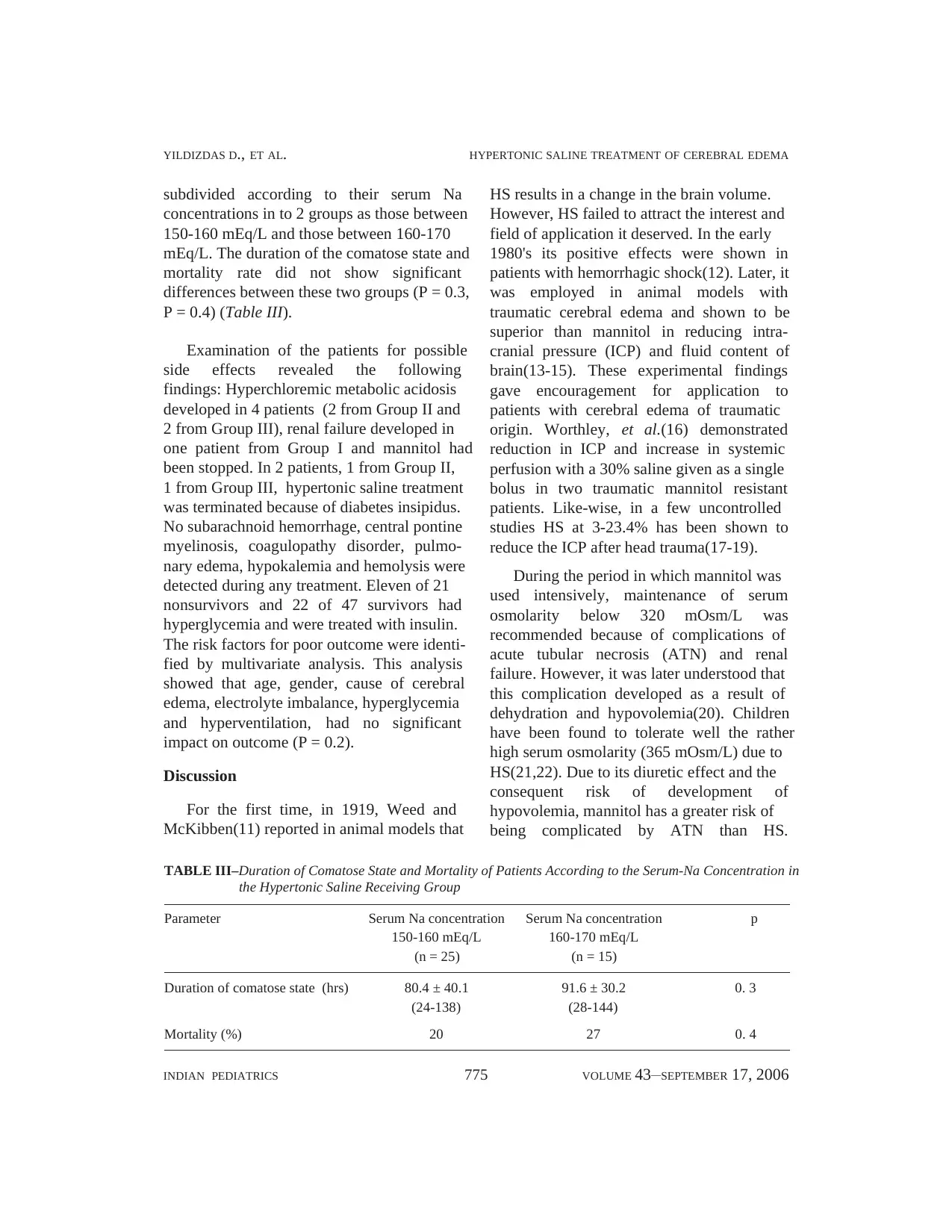
duration of comatose state (Table II, Fig 1)
and mortality rate (Table II) were significantly
lower in Group II and Group III A/B. Of 67
patients, 46 survived and 21 (31.3%) died.
The causes of death were septic shock
(Group I = 2, Group II = 2, Group III = 1),
ventilator associated pneumonia with acute
respiratory distress syndrome (Group I = 1,
Group II = 1), progressive cerebral edema
(Group I = 7, Group II = 2, Group III = 2) and
cerebral edema with pulmonary edema
(Group I = 1, Group III = 1).
Patients who received only HS were
TABLE II–Highest Serum Na Concentration, Osmolarity, Duration of Comatose State and Mortality Rate
Between Groups
Parameters Group I (n = 22) Group II (n=25) Group III (n =20) p
A* B*
Osmolarity (mOsm/L) 321.5 ± 10.8 343.8 ± 20.4 325.0 ± 11.5 337.6 ± 19.8) 0. 5
(305-40) (313-74) (305-37) (309-37)
Serum-Na(mEq/L) 157.5 ± 8.8 137.5 ± 10.4 157.3 ± 9.3
(144-176) (130-150) (148-175) 0. 8
Duration of comatose 123.0 ± 48.2 88.6 ± 42.5 87.5 ± 26.1 87.5 ± 26.1
state (18-196) (28-138) (24-144) (24-144) 0. 004
Mortality (%) 50 25 20 20 0. 003
Subgroup A shows patients who received hypertonic saline and mannitol together.
Subgroup B shows patients who received hypertonic saline after stopping mannitol.
Fig.1. Duration of comatose state of groups.
300
200
100
0N = 22 25 20
Mannitol HS Mannitol+HS
GROUP
Duration of comatose state (hrs)
YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
9.3 ± 5.0 (2-16) doses in Group I and
6.5 ± 2.8(2-10) doses in Group III. Hypertonic
saline was infused for 4-25 times in Group II.
The highest serum Na and osmolarity levels
during mannitol, hypertonic saline, and HS +
mannitol (Group III subgroup A and B)
infusions are shown in Table II. Although
there was no statistically significant difference
in the highest serum Na and osmolarity levels
of the groups (P = 0.8, P = 0.5, respectively);
duration of comatose state (Table II, Fig 1)
and mortality rate (Table II) were significantly
lower in Group II and Group III A/B. Of 67
patients, 46 survived and 21 (31.3%) died.
The causes of death were septic shock
(Group I = 2, Group II = 2, Group III = 1),
ventilator associated pneumonia with acute
respiratory distress syndrome (Group I = 1,
Group II = 1), progressive cerebral edema
(Group I = 7, Group II = 2, Group III = 2) and
cerebral edema with pulmonary edema
(Group I = 1, Group III = 1).
Patients who received only HS were
TABLE II–Highest Serum Na Concentration, Osmolarity, Duration of Comatose State and Mortality Rate
Between Groups
Parameters Group I (n = 22) Group II (n=25) Group III (n =20) p
A* B*
Osmolarity (mOsm/L) 321.5 ± 10.8 343.8 ± 20.4 325.0 ± 11.5 337.6 ± 19.8) 0. 5
(305-40) (313-74) (305-37) (309-37)
Serum-Na(mEq/L) 157.5 ± 8.8 137.5 ± 10.4 157.3 ± 9.3
(144-176) (130-150) (148-175) 0. 8
Duration of comatose 123.0 ± 48.2 88.6 ± 42.5 87.5 ± 26.1 87.5 ± 26.1
state (18-196) (28-138) (24-144) (24-144) 0. 004
Mortality (%) 50 25 20 20 0. 003
Subgroup A shows patients who received hypertonic saline and mannitol together.
Subgroup B shows patients who received hypertonic saline after stopping mannitol.
Fig.1. Duration of comatose state of groups.
300
200
100
0N = 22 25 20
Mannitol HS Mannitol+HS
GROUP
Duration of comatose state (hrs)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
INDIAN PEDIATRICS 775 VOLUME 43__SEPTEMBER 17, 2006
subdivided according to their serum Na
concentrations in to 2 groups as those between
150-160 mEq/L and those between 160-170
mEq/L. The duration of the comatose state and
mortality rate did not show significant
differences between these two groups (P = 0.3,
P = 0.4) (Table III).
Examination of the patients for possible
side effects revealed the following
findings: Hyperchloremic metabolic acidosis
developed in 4 patients (2 from Group II and
2 from Group III), renal failure developed in
one patient from Group I and mannitol had
been stopped. In 2 patients, 1 from Group II,
1 from Group III, hypertonic saline treatment
was terminated because of diabetes insipidus.
No subarachnoid hemorrhage, central pontine
myelinosis, coagulopathy disorder, pulmo-
nary edema, hypokalemia and hemolysis were
detected during any treatment. Eleven of 21
nonsurvivors and 22 of 47 survivors had
hyperglycemia and were treated with insulin.
The risk factors for poor outcome were identi-
fied by multivariate analysis. This analysis
showed that age, gender, cause of cerebral
edema, electrolyte imbalance, hyperglycemia
and hyperventilation, had no significant
impact on outcome (P = 0.2).
Discussion
For the first time, in 1919, Weed and
McKibben(11) reported in animal models that
HS results in a change in the brain volume.
However, HS failed to attract the interest and
field of application it deserved. In the early
1980's its positive effects were shown in
patients with hemorrhagic shock(12). Later, it
was employed in animal models with
traumatic cerebral edema and shown to be
superior than mannitol in reducing intra-
cranial pressure (ICP) and fluid content of
brain(13-15). These experimental findings
gave encouragement for application to
patients with cerebral edema of traumatic
origin. Worthley, et al.(16) demonstrated
reduction in ICP and increase in systemic
perfusion with a 30% saline given as a single
bolus in two traumatic mannitol resistant
patients. Like-wise, in a few uncontrolled
studies HS at 3-23.4% has been shown to
reduce the ICP after head trauma(17-19).
During the period in which mannitol was
used intensively, maintenance of serum
osmolarity below 320 mOsm/L was
recommended because of complications of
acute tubular necrosis (ATN) and renal
failure. However, it was later understood that
this complication developed as a result of
dehydration and hypovolemia(20). Children
have been found to tolerate well the rather
high serum osmolarity (365 mOsm/L) due to
HS(21,22). Due to its diuretic effect and the
consequent risk of development of
hypovolemia, mannitol has a greater risk of
being complicated by ATN than HS.
TABLE III–Duration of Comatose State and Mortality of Patients According to the Serum-Na Concentration in
the Hypertonic Saline Receiving Group
Parameter Serum Na concentration Serum Na concentration p
150-160 mEq/L 160-170 mEq/L
(n = 25) (n = 15)
Duration of comatose state (hrs) 80.4 ± 40.1 91.6 ± 30.2 0. 3
(24-138) (28-144)
Mortality (%) 20 27 0. 4
INDIAN PEDIATRICS 775 VOLUME 43__SEPTEMBER 17, 2006
subdivided according to their serum Na
concentrations in to 2 groups as those between
150-160 mEq/L and those between 160-170
mEq/L. The duration of the comatose state and
mortality rate did not show significant
differences between these two groups (P = 0.3,
P = 0.4) (Table III).
Examination of the patients for possible
side effects revealed the following
findings: Hyperchloremic metabolic acidosis
developed in 4 patients (2 from Group II and
2 from Group III), renal failure developed in
one patient from Group I and mannitol had
been stopped. In 2 patients, 1 from Group II,
1 from Group III, hypertonic saline treatment
was terminated because of diabetes insipidus.
No subarachnoid hemorrhage, central pontine
myelinosis, coagulopathy disorder, pulmo-
nary edema, hypokalemia and hemolysis were
detected during any treatment. Eleven of 21
nonsurvivors and 22 of 47 survivors had
hyperglycemia and were treated with insulin.
The risk factors for poor outcome were identi-
fied by multivariate analysis. This analysis
showed that age, gender, cause of cerebral
edema, electrolyte imbalance, hyperglycemia
and hyperventilation, had no significant
impact on outcome (P = 0.2).
Discussion
For the first time, in 1919, Weed and
McKibben(11) reported in animal models that
HS results in a change in the brain volume.
However, HS failed to attract the interest and
field of application it deserved. In the early
1980's its positive effects were shown in
patients with hemorrhagic shock(12). Later, it
was employed in animal models with
traumatic cerebral edema and shown to be
superior than mannitol in reducing intra-
cranial pressure (ICP) and fluid content of
brain(13-15). These experimental findings
gave encouragement for application to
patients with cerebral edema of traumatic
origin. Worthley, et al.(16) demonstrated
reduction in ICP and increase in systemic
perfusion with a 30% saline given as a single
bolus in two traumatic mannitol resistant
patients. Like-wise, in a few uncontrolled
studies HS at 3-23.4% has been shown to
reduce the ICP after head trauma(17-19).
During the period in which mannitol was
used intensively, maintenance of serum
osmolarity below 320 mOsm/L was
recommended because of complications of
acute tubular necrosis (ATN) and renal
failure. However, it was later understood that
this complication developed as a result of
dehydration and hypovolemia(20). Children
have been found to tolerate well the rather
high serum osmolarity (365 mOsm/L) due to
HS(21,22). Due to its diuretic effect and the
consequent risk of development of
hypovolemia, mannitol has a greater risk of
being complicated by ATN than HS.
TABLE III–Duration of Comatose State and Mortality of Patients According to the Serum-Na Concentration in
the Hypertonic Saline Receiving Group
Parameter Serum Na concentration Serum Na concentration p
150-160 mEq/L 160-170 mEq/L
(n = 25) (n = 15)
Duration of comatose state (hrs) 80.4 ± 40.1 91.6 ± 30.2 0. 3
(24-138) (28-144)
Mortality (%) 20 27 0. 4

INDIAN PEDIATRICS 776 VOLUME 43__SEPTEMBER 17, 2006
YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
Comparative studies with mannitol have also
been conducted and published. In a pros-
pective, randomized study, Vialet, et al.(23)
showed that HS at 7.5% concentration admi-
nistered as an isovolemic bolus (2 mL/kg) was
more effective than 20% mannitol in reducing
the ICP in trauma patients. Another pros-
pective study conducted by Horn, et al.
(24) using 7.5% saline administered as bolus
infusion to patients with elevated ICP due to
trauma and not responding to the standard
treatment showed it to be effective in reducing
the ICP and CPP. With the aim of reducing the
ICP to below 20 mmHg, Peterson, et al.(21)
administered a 3% saline infusion to 68
children with trauma who did not respond to
standard treatment. They found serum-Na
concentrations of 150-170 mEq/L and a serum
osmolarity of 300-330 mOsm/L to correlate
with better prognosis. Our study is a
retrospective analysis of two years period with
a patient population consisting of children.
Our cases and etiologic factors are different
from other studies. In our study the etiologic
factors included infection, hemorrhage,
anoxia, and metabolic factors. ICP measure-
ment could not be conducted in our study, so
treatment continued considering the serum-Na
concentration and osmolarity until clinical
improvement was achieved. We have shown
better results in Group II and Group III with
no significant side effects. The main dis-
advantage is the fact that our study is
retrospective and no ICP measurement was
conducted. However, compared with
mannitol, the clinical efficacy has also been
confirmed by mortality assessment.
Hypertonic saline has been used more
frequently in trauma, intracerebral hemorr-
hage, burn and stroke patients. Our patient
group was different in their etiology of brain
edema. Rationale of use of mannitol and HS is
similiar in both traumatic and non traumatic
cerebral edema because all cerebral edemas
with varied etiologies usually have vasogenic
mechanism. In addition, HS is more effective
and safer than mannitol. Greater efficacy of
HS compared to mannitol can be speculated
by different reflection coefficients of these
agents(2-4). Because the reflection coefficient
for Na and Cl was 1.0, such a side effect, in
saline treatment may not be expected. To our
knowledge, there is no report of HS treatment
in edema of anoxic, infectious, and metabolic
encephalopathy in the literature.
In terms of the efficacy and side effect
profile of the saline treatment in brain edema,
the optimum serum-Na concentration and
osmolarity are not known. In a retrospective
study Peterson, et al.(21) though making
no comparative analysis, suggested that
prognosis in patients with serum-Na
concentration within the range of 150-170
mEq/L and serum osmolarity of 300-340
mOsm/L seems to be better. Also, some
studies report an inverse relationship between
serum-Na concentration and ICP(18-21).
There was no significant difference in our
patients with Na level of 150-160 and 160-170
mEq/L in terms of duration of the comatose
state and mortality. As during the hyper-
osmolar state, to maintain the osmotic
balance, idiogenic hyperosmoles form within
the cells in 72-96 hours(25-28). So after the
termination of HS, serum-Na concentration
should be gradually reduced over a period
more than 2 days.
Potential side effects of hypertonic saline
have been reported(29): Myelinolysis, acute
tubular necrosis and renal failure, subdural
hematoma or effusion, heart failure, pulmo-
nary edema, hypokalemia, hyperchloremic
metabolic acidosis, coagulopathy, intra-
vascular hemolysis, and rebound cerebral
edema may occur. Myelinolysis occur more
frequently if there is a rapid transition from
YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
Comparative studies with mannitol have also
been conducted and published. In a pros-
pective, randomized study, Vialet, et al.(23)
showed that HS at 7.5% concentration admi-
nistered as an isovolemic bolus (2 mL/kg) was
more effective than 20% mannitol in reducing
the ICP in trauma patients. Another pros-
pective study conducted by Horn, et al.
(24) using 7.5% saline administered as bolus
infusion to patients with elevated ICP due to
trauma and not responding to the standard
treatment showed it to be effective in reducing
the ICP and CPP. With the aim of reducing the
ICP to below 20 mmHg, Peterson, et al.(21)
administered a 3% saline infusion to 68
children with trauma who did not respond to
standard treatment. They found serum-Na
concentrations of 150-170 mEq/L and a serum
osmolarity of 300-330 mOsm/L to correlate
with better prognosis. Our study is a
retrospective analysis of two years period with
a patient population consisting of children.
Our cases and etiologic factors are different
from other studies. In our study the etiologic
factors included infection, hemorrhage,
anoxia, and metabolic factors. ICP measure-
ment could not be conducted in our study, so
treatment continued considering the serum-Na
concentration and osmolarity until clinical
improvement was achieved. We have shown
better results in Group II and Group III with
no significant side effects. The main dis-
advantage is the fact that our study is
retrospective and no ICP measurement was
conducted. However, compared with
mannitol, the clinical efficacy has also been
confirmed by mortality assessment.
Hypertonic saline has been used more
frequently in trauma, intracerebral hemorr-
hage, burn and stroke patients. Our patient
group was different in their etiology of brain
edema. Rationale of use of mannitol and HS is
similiar in both traumatic and non traumatic
cerebral edema because all cerebral edemas
with varied etiologies usually have vasogenic
mechanism. In addition, HS is more effective
and safer than mannitol. Greater efficacy of
HS compared to mannitol can be speculated
by different reflection coefficients of these
agents(2-4). Because the reflection coefficient
for Na and Cl was 1.0, such a side effect, in
saline treatment may not be expected. To our
knowledge, there is no report of HS treatment
in edema of anoxic, infectious, and metabolic
encephalopathy in the literature.
In terms of the efficacy and side effect
profile of the saline treatment in brain edema,
the optimum serum-Na concentration and
osmolarity are not known. In a retrospective
study Peterson, et al.(21) though making
no comparative analysis, suggested that
prognosis in patients with serum-Na
concentration within the range of 150-170
mEq/L and serum osmolarity of 300-340
mOsm/L seems to be better. Also, some
studies report an inverse relationship between
serum-Na concentration and ICP(18-21).
There was no significant difference in our
patients with Na level of 150-160 and 160-170
mEq/L in terms of duration of the comatose
state and mortality. As during the hyper-
osmolar state, to maintain the osmotic
balance, idiogenic hyperosmoles form within
the cells in 72-96 hours(25-28). So after the
termination of HS, serum-Na concentration
should be gradually reduced over a period
more than 2 days.
Potential side effects of hypertonic saline
have been reported(29): Myelinolysis, acute
tubular necrosis and renal failure, subdural
hematoma or effusion, heart failure, pulmo-
nary edema, hypokalemia, hyperchloremic
metabolic acidosis, coagulopathy, intra-
vascular hemolysis, and rebound cerebral
edema may occur. Myelinolysis occur more
frequently if there is a rapid transition from
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
INDIAN PEDIATRICS 777 VOLUME 43__SEPTEMBER 17, 2006
hyponatremia to hypernatremia. For myelino-
lysis to occur a daily serum-Na concentration
load of 35-40 mEq/L is required(30). The
region most susceptible to myelinolysis is the
pontine white matter with visualized MRI and
central pontine myelinolysis is manifested
clinically as lethargy and quadriplegia/paresia.
In 6 of our patients hyponatremia was present
initially. However, no patient developed daily
serum-Na rise exceeding 20-30 mEq/L. MRI
study of 22 patients, performed 1-5 days after
the termination of saline treatment in group III
revealed no signs of pontine myelinolysis.
Also, we have not observed acute flaccid
paralysis/plegia after saline treatment.
Especially, in patients with significant degrees
of cerebral injury, the clinical assessment of
this complication could not be detected
possibly by the fact that they had blunted
mental status.
Renal failure, congestive heart failure,
pulmonary edema, hypokalemia and phlebitis
were not observed in any of our patients.
There was no significant tendency for hemo-
lysis or hemorrhage associated with acute fall
in the hematocrit level. In 4 patients, hyper-
chloremic metabolic acidosis developed but
resolved with proper treatment.
In conclusion, in the treatment of cerebral
edema of infectious, anoxic, hemorrhagic and
metabolic origin, administration of HS is prob-
ably more effective and safer than mannitol.
However, to determine just when to initiate
treatment, how long to continue treatment, and
target serum-Na concentration requires moni-
toring of the intracranial pressure. Further stud-
ies are required to resolve these concerns.
Contributors: DY contributed to patient
management, designed, coordinated and supervised
the study, interpreted the results and drafted the
manuscript; SA, OH contributed to patient
management and helped in drafting the final paper;
UC contributed to collection and analysis of the data
and literature search.
Funding: None.
Competing interests: None.
REFERENCES
1. Wise BL, Chater N. Use of hypertonic mannitol
solutions to lower cerebrospinal fluid pressure
and decrease brain bulk in man. Surg Forum
1961; 12:398-399.
2. Betz AL. Sodium transport in capillaries isolated
from rat brain. J Neurochem 1983; 41: 1150-
1157.
3. Betz AL. Sodium transport from blood to brain:
Inhibition by furosemide and amiloride. J
Neurochem 1983; 41: 1158-1164.
4. Fenstermacher JD, Johnson JA. Filtration and
reflection coefficients of the rabbit blood-brain
barrier. Am J Physiol 1966; 211: 311-346.
5. Zornow MH, Scheller MS, Shackford SR. Effect
of a hypertonic lactated Ringer's solution on
intracranial pressure and cerebral water content
in a model of traumatic brain injury. J Trauma
1989; 29: 484-488.
6. Todd MM, Tommasino C, Moore S. Cerebral
effects of isovolemic hemodilution with a
Key Messages
• Hypertonic saline treatment is effective and safe in the treatment of infectious,anoxic,
hemorrhagic and metabolic origin.
• Serum sodium should be maintained between 150-160 mEq/L while treating with hypertonic
saline.
• We recommend bolus dose of 1 mL/kg at 3% saline for 4-6 times in 15 minutes and then
infusion dose of 0.5-2 mL/kg/hr.
INDIAN PEDIATRICS 777 VOLUME 43__SEPTEMBER 17, 2006
hyponatremia to hypernatremia. For myelino-
lysis to occur a daily serum-Na concentration
load of 35-40 mEq/L is required(30). The
region most susceptible to myelinolysis is the
pontine white matter with visualized MRI and
central pontine myelinolysis is manifested
clinically as lethargy and quadriplegia/paresia.
In 6 of our patients hyponatremia was present
initially. However, no patient developed daily
serum-Na rise exceeding 20-30 mEq/L. MRI
study of 22 patients, performed 1-5 days after
the termination of saline treatment in group III
revealed no signs of pontine myelinolysis.
Also, we have not observed acute flaccid
paralysis/plegia after saline treatment.
Especially, in patients with significant degrees
of cerebral injury, the clinical assessment of
this complication could not be detected
possibly by the fact that they had blunted
mental status.
Renal failure, congestive heart failure,
pulmonary edema, hypokalemia and phlebitis
were not observed in any of our patients.
There was no significant tendency for hemo-
lysis or hemorrhage associated with acute fall
in the hematocrit level. In 4 patients, hyper-
chloremic metabolic acidosis developed but
resolved with proper treatment.
In conclusion, in the treatment of cerebral
edema of infectious, anoxic, hemorrhagic and
metabolic origin, administration of HS is prob-
ably more effective and safer than mannitol.
However, to determine just when to initiate
treatment, how long to continue treatment, and
target serum-Na concentration requires moni-
toring of the intracranial pressure. Further stud-
ies are required to resolve these concerns.
Contributors: DY contributed to patient
management, designed, coordinated and supervised
the study, interpreted the results and drafted the
manuscript; SA, OH contributed to patient
management and helped in drafting the final paper;
UC contributed to collection and analysis of the data
and literature search.
Funding: None.
Competing interests: None.
REFERENCES
1. Wise BL, Chater N. Use of hypertonic mannitol
solutions to lower cerebrospinal fluid pressure
and decrease brain bulk in man. Surg Forum
1961; 12:398-399.
2. Betz AL. Sodium transport in capillaries isolated
from rat brain. J Neurochem 1983; 41: 1150-
1157.
3. Betz AL. Sodium transport from blood to brain:
Inhibition by furosemide and amiloride. J
Neurochem 1983; 41: 1158-1164.
4. Fenstermacher JD, Johnson JA. Filtration and
reflection coefficients of the rabbit blood-brain
barrier. Am J Physiol 1966; 211: 311-346.
5. Zornow MH, Scheller MS, Shackford SR. Effect
of a hypertonic lactated Ringer's solution on
intracranial pressure and cerebral water content
in a model of traumatic brain injury. J Trauma
1989; 29: 484-488.
6. Todd MM, Tommasino C, Moore S. Cerebral
effects of isovolemic hemodilution with a
Key Messages
• Hypertonic saline treatment is effective and safe in the treatment of infectious,anoxic,
hemorrhagic and metabolic origin.
• Serum sodium should be maintained between 150-160 mEq/L while treating with hypertonic
saline.
• We recommend bolus dose of 1 mL/kg at 3% saline for 4-6 times in 15 minutes and then
infusion dose of 0.5-2 mL/kg/hr.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

INDIAN PEDIATRICS 778 VOLUME 43__SEPTEMBER 17, 2006
YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
hypertonic saline solution. J Neurosurg 1985; 63:
944-948.
7. Qureshi AI, Wilson DA, Traystman RJ.
Treatment of elevated intracranial pressure in
experimental intracerebral hemorrhage: Com-
parison between mannitol and hypertonic saline.
Neurosurgery 1999; 44: 1055-1064.
8. Zornow MH, Scheller MS, Shackford SR. Effect
of a hypertonic lactated Ringer's solution on
intracranial pressure and cerebral water content
in a model of traumatic brain injury. J Trauma
1989; 29: 484-488.
9. Stack C. Trauma. In: Stack C, Dobbs P eds.
Essentials of Pediatric Intensive Care, 1st edn.
London: Greenwich Medical Media Limited,
2004: 155-161.
10. Pollay M. Blood-Brain Barrier; Cerebral edema.
In:Wilkins RH, Rengachary SS eds. Neuro-
surgery, 2nd edn. New York: McGraw-Hill,
1996: 335-344.
11. Weed LH, McKibben PS. Experimental
alteration of brain bulk. Am J Physiol 1919; 48:
531-555.
12. Zornow MH. Hypertonic saline as a safe and
efficacious treatment of intracranial hyper-
tension. J Neurosurg Anesth 1996; 8:
175-177.
13. Ducey JP, Mozingo DW, Lamiell JM, Okerburg
J, Guellre GE. A comparison of the cerebral and
cardiovascular effects of complete reuscitation
with isotonic and hypertonic saline, hetastarch,
and whole blood following hemorrhage. J
Trauma 1989; 29: 1518-1519.
14. Schmoker J, Zhuang J, Schackford S. Hypertonic
fluid resuscitation improves cerebral oxygen deli-
very and reduces intracranial pressure after hemor-
rhagic shock. J Trauma 1991; 31: 1607-1613.
15. Prough DS, Whitley J, Taylor CL, Deal DD,
DeWitt DS. Regional cerebral blood flow
following resuscitation from hemorrhagic shock
with hypertonic saline. Anesthesiology 1991; 75:
319-327.
16. Worthley LI, Cooper DJ, Jones N. Treatment of
resistant intracranial hypertension with
hypertonic saline: Report of two cases. J
Neurosurg 1988; 68: 478-481.
17. Weinstabl C, Mayer N, Germann P, Wisner D.
Hypertonic, hyperoncotic hydroxyethyl starch
decreases intracarnial pressure following
neurotrauma. Anesthesiology 1992; 75: 201.
18. Qureshi AL, Suarez JL, Bhardwaj A, et al.
Use of hypertonic (3%) saline/acetate infusion in
the treatment of cerebral edema: Effect on
intracranial pressure and lateral displace-
ment of the brain. Crit Care Med 1998; 26: 440-
446.
19. Suarez JI, Qureshi AI, Bhardwaj A, Williams
MA, Schnittzer MS, Mriski M, et al. Treatment
of refractory intracranial hypertension with
23.4% saline. Crit Care Med 1998; 26: 1118-
1122.
20. Mazzola CA, Adelson PD. Critical care
mangement of head trauma in children. Crit care
Med 2002;30(11 Suppl): S393-S401.
21. Peterson B, Khanna S, Fisher B, Marshall L.
Prolonged hypernatremia controls elevated
intracranial pressure in head-injured pediatric
patients. Crit Care Med 2000; 28: 1136-1143.
22. Khanna S, Davis D, Peterson B, Fisher B, Tung
H, Oquigley J, et al. Use of hypertonic saline in
the treatment of severe refractory posttraumatic
intracranial hypertension in pediatric traumatic
brain injury. Crit Care Med 2000; 28: 1144-
1151.
23. Vialet R, Albanese J, Thomachot L, Antonini F,
Bourgoun A, Aliez B, et al. Isovolemic
hypertonic solutes (sodium chloride and
mannitol) in the treatment of refractory
posttraumatic intracranial hypertension: 2 mL/kg
7.5% saline is more effective than 2 mL/kg
20% mannitol. Crit Care Med 2003; 31: 1683-
1687.
24. Horn P, Munch E, Vajkoczy P, Herrman P,
Quintel M, Schilling L, et al. Hypertonic saline
solution for control of elevated intracranial
pressure in patients with exhausted response to
mannitol and barbiturates. Neurol Res 1999; 21:
758-764.
25. Videen JS, MicHaelis T, Pinto P, Ross BD.
Human cerebral osmolytes during chronic
hyponatremia. A proton magnetic resonance
spectroscopy study. J Clin Invest 1995; 95: 788-
793.
YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
hypertonic saline solution. J Neurosurg 1985; 63:
944-948.
7. Qureshi AI, Wilson DA, Traystman RJ.
Treatment of elevated intracranial pressure in
experimental intracerebral hemorrhage: Com-
parison between mannitol and hypertonic saline.
Neurosurgery 1999; 44: 1055-1064.
8. Zornow MH, Scheller MS, Shackford SR. Effect
of a hypertonic lactated Ringer's solution on
intracranial pressure and cerebral water content
in a model of traumatic brain injury. J Trauma
1989; 29: 484-488.
9. Stack C. Trauma. In: Stack C, Dobbs P eds.
Essentials of Pediatric Intensive Care, 1st edn.
London: Greenwich Medical Media Limited,
2004: 155-161.
10. Pollay M. Blood-Brain Barrier; Cerebral edema.
In:Wilkins RH, Rengachary SS eds. Neuro-
surgery, 2nd edn. New York: McGraw-Hill,
1996: 335-344.
11. Weed LH, McKibben PS. Experimental
alteration of brain bulk. Am J Physiol 1919; 48:
531-555.
12. Zornow MH. Hypertonic saline as a safe and
efficacious treatment of intracranial hyper-
tension. J Neurosurg Anesth 1996; 8:
175-177.
13. Ducey JP, Mozingo DW, Lamiell JM, Okerburg
J, Guellre GE. A comparison of the cerebral and
cardiovascular effects of complete reuscitation
with isotonic and hypertonic saline, hetastarch,
and whole blood following hemorrhage. J
Trauma 1989; 29: 1518-1519.
14. Schmoker J, Zhuang J, Schackford S. Hypertonic
fluid resuscitation improves cerebral oxygen deli-
very and reduces intracranial pressure after hemor-
rhagic shock. J Trauma 1991; 31: 1607-1613.
15. Prough DS, Whitley J, Taylor CL, Deal DD,
DeWitt DS. Regional cerebral blood flow
following resuscitation from hemorrhagic shock
with hypertonic saline. Anesthesiology 1991; 75:
319-327.
16. Worthley LI, Cooper DJ, Jones N. Treatment of
resistant intracranial hypertension with
hypertonic saline: Report of two cases. J
Neurosurg 1988; 68: 478-481.
17. Weinstabl C, Mayer N, Germann P, Wisner D.
Hypertonic, hyperoncotic hydroxyethyl starch
decreases intracarnial pressure following
neurotrauma. Anesthesiology 1992; 75: 201.
18. Qureshi AL, Suarez JL, Bhardwaj A, et al.
Use of hypertonic (3%) saline/acetate infusion in
the treatment of cerebral edema: Effect on
intracranial pressure and lateral displace-
ment of the brain. Crit Care Med 1998; 26: 440-
446.
19. Suarez JI, Qureshi AI, Bhardwaj A, Williams
MA, Schnittzer MS, Mriski M, et al. Treatment
of refractory intracranial hypertension with
23.4% saline. Crit Care Med 1998; 26: 1118-
1122.
20. Mazzola CA, Adelson PD. Critical care
mangement of head trauma in children. Crit care
Med 2002;30(11 Suppl): S393-S401.
21. Peterson B, Khanna S, Fisher B, Marshall L.
Prolonged hypernatremia controls elevated
intracranial pressure in head-injured pediatric
patients. Crit Care Med 2000; 28: 1136-1143.
22. Khanna S, Davis D, Peterson B, Fisher B, Tung
H, Oquigley J, et al. Use of hypertonic saline in
the treatment of severe refractory posttraumatic
intracranial hypertension in pediatric traumatic
brain injury. Crit Care Med 2000; 28: 1144-
1151.
23. Vialet R, Albanese J, Thomachot L, Antonini F,
Bourgoun A, Aliez B, et al. Isovolemic
hypertonic solutes (sodium chloride and
mannitol) in the treatment of refractory
posttraumatic intracranial hypertension: 2 mL/kg
7.5% saline is more effective than 2 mL/kg
20% mannitol. Crit Care Med 2003; 31: 1683-
1687.
24. Horn P, Munch E, Vajkoczy P, Herrman P,
Quintel M, Schilling L, et al. Hypertonic saline
solution for control of elevated intracranial
pressure in patients with exhausted response to
mannitol and barbiturates. Neurol Res 1999; 21:
758-764.
25. Videen JS, MicHaelis T, Pinto P, Ross BD.
Human cerebral osmolytes during chronic
hyponatremia. A proton magnetic resonance
spectroscopy study. J Clin Invest 1995; 95: 788-
793.

YILDIZDAS D., ET AL. HYPERTONIC SALINE TREATMENT OF CEREBRAL EDEMA
INDIAN PEDIATRICS 779 VOLUME 43__SEPTEMBER 17, 2006
26. Haussinger D, Laubenberger J, Vom Dahl S,
Ernst T, Bayer S, Larger M, et al. Proton
magnetic resonance spectroscopy studies on
human brain myo-inositol in hypo-osmolarity
and hepatic encephalopathy. Gastroenterology
1994; 107: 1475-1480.
27. Lien YH, Shapiro JI, Chan L. Study of brain
electrolytes and organic osmolytes during
correction of chronic hyponatremia. Implica-
tions for the pathogenesis of central pontine
myelinolysis. J Clin Invest 1991; 88: 303-
309.
28. Olson JE, Goldfinger MD. Aminoacid content of
rat cerebral astrocytes adapted to hyperosmotic
medium in vitro. J Neurosci Res 1990; 27: 241-
246.
29. Bhardwaj A, Ulatowski A. Hypertonic saline
solutions in brain injury. Curr Opin Crit Care
2004; 10: 126-131.
30. Soupart A, Pennickx R, Namias B, Stenuit A,
Perier O, Decaun G, et al. Brain myelinolysis
following hypernatremia in rats. J Neuropathol
Exp Neurol 1996; 55: 106-113.
INDIAN PEDIATRICS 779 VOLUME 43__SEPTEMBER 17, 2006
26. Haussinger D, Laubenberger J, Vom Dahl S,
Ernst T, Bayer S, Larger M, et al. Proton
magnetic resonance spectroscopy studies on
human brain myo-inositol in hypo-osmolarity
and hepatic encephalopathy. Gastroenterology
1994; 107: 1475-1480.
27. Lien YH, Shapiro JI, Chan L. Study of brain
electrolytes and organic osmolytes during
correction of chronic hyponatremia. Implica-
tions for the pathogenesis of central pontine
myelinolysis. J Clin Invest 1991; 88: 303-
309.
28. Olson JE, Goldfinger MD. Aminoacid content of
rat cerebral astrocytes adapted to hyperosmotic
medium in vitro. J Neurosci Res 1990; 27: 241-
246.
29. Bhardwaj A, Ulatowski A. Hypertonic saline
solutions in brain injury. Curr Opin Crit Care
2004; 10: 126-131.
30. Soupart A, Pennickx R, Namias B, Stenuit A,
Perier O, Decaun G, et al. Brain myelinolysis
following hypernatremia in rats. J Neuropathol
Exp Neurol 1996; 55: 106-113.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.