Immunology Report: ELISA-Based Gliadin Quantification in Bread Samples
VerifiedAdded on 2023/04/06
|8
|1888
|279
Report
AI Summary
This report details an Enzyme-Linked Immunosorbent Assay (ELISA) experiment conducted to quantify gliadin levels in various bread samples. The introduction provides background on ELISA as a biochemical assay and gliadin, a component of gluten. The aim was to establish an ELISA standard curve, determine gliadin concentrations in unknown bread samples, and assess which samples could be labeled as gluten-free or low-gluten. The results section presents a calibration curve and gliadin concentration values for five bread samples and a positive control. The discussion interprets the findings, highlighting the importance of ELISA in gluten detection, particularly for individuals with celiac disease. It also discusses the FDA's stance on gluten-free labeling. The conclusion summarizes the study's key findings, emphasizing the successful application of ELISA for gliadin quantification and its relevance to understanding celiac disease. The study successfully used ELISA to quantify gliadin levels in bread, providing insights into gluten content and implications for consumers and food labeling.

Immunology
1
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Table of Contents
Introduction 3
Aims 4
Results 4
Discussion 5
Conclusion 7
References 7
2
Introduction 3
Aims 4
Results 4
Discussion 5
Conclusion 7
References 7
2

Introduction:
The enzyme-linked immunosorbent assay (ELISA) is the most commonly used biochemical
assay technique. ELISA uses solid-phase enzyme immunoassay (EIA) for the detection of
ligand in a liquid sample through antibodies directed against protein. ELISA is being used in
medicine as a diagnostic tool and quality control tool in biochemical industries. In ELISA,
antigens are attached to solid surface like polystyrene microtiter plate followed by antibodies
added to bind to the antigens. This detects antibody form complex with the unknown
antigens. Detection antibody are usually attached to enzyme or it can be detected by
secondary antibody which is attached to enzyme through bioconjugation (Konstantinou,
2017). After completion of each step, ELISA plate needs to be washed with detergent to
remove unbound protein and enzymes. Final step comprises of addition of enzyme substrate.
End point in ELISA is colour change which is usually measured using UV-Visible detection.
Based on the sequence of bonding of analytes and antibodies, ELISA are categorised into
different types like Direct ELISA, Sandwich ELISA, Competitive ELISA and reverse ELISA.
ELISA is being used for the determination of serum antibody concentrations in HIV test, for
detection of food allergens and in serological blood test for coeliac disease (Hornbeck, 2015).
Gliadin is a class of prolamin protein available in wheat and other cereals from grass genus
Triticum. Gliadins are heterogenous mixture of single chain polypeptides which are soluble in
70 % alcohol and these contain intramolecular disulphide bonds. Gliadins are components of
gluten which is useful to rise bread properly during baking. Gliadin is water-insoluble and
glutenin is water soluble. Gliadin exists in three forms like α, β, γ, and ω (Urade et al., 2018).
α gliadins are with fastest mobility while ω gliadins are with slowest mobility. Body is
intolerant to gliadin in coeliac (or celiac) disease (CD). Gliadin has the ability to cross
intestinal epithelium. Gliadins are responsible for precipitating coeliac disease while
glutenins are nontoxic in nature. Peptides derived from the gliadins are immunostimulatory in
coeliac disease (Kelly et al., 2015). Both gliadin and glutenin play role in the formation of
gluten. Gliadins are responsible for food derived pathogenesis. ELISA assays are usually
sensitive, specific and relatively easy to perform for the estimation of gluten content in food
products including breads. However, it is necessary to use different extraction solvents for
different types of foods for the estimation of gliadin using ELISA. ELISA assay was
implemented for the estimation of α-gliadin and whole gliadin in the wheat using polyclonal
rabbit or mouse enzyme labelled antisera. Literature mentioned that minimum 0.4 ng quantity
of gliadin in food products can also be estimated using ELISA. ELISA assay is useful in
3
The enzyme-linked immunosorbent assay (ELISA) is the most commonly used biochemical
assay technique. ELISA uses solid-phase enzyme immunoassay (EIA) for the detection of
ligand in a liquid sample through antibodies directed against protein. ELISA is being used in
medicine as a diagnostic tool and quality control tool in biochemical industries. In ELISA,
antigens are attached to solid surface like polystyrene microtiter plate followed by antibodies
added to bind to the antigens. This detects antibody form complex with the unknown
antigens. Detection antibody are usually attached to enzyme or it can be detected by
secondary antibody which is attached to enzyme through bioconjugation (Konstantinou,
2017). After completion of each step, ELISA plate needs to be washed with detergent to
remove unbound protein and enzymes. Final step comprises of addition of enzyme substrate.
End point in ELISA is colour change which is usually measured using UV-Visible detection.
Based on the sequence of bonding of analytes and antibodies, ELISA are categorised into
different types like Direct ELISA, Sandwich ELISA, Competitive ELISA and reverse ELISA.
ELISA is being used for the determination of serum antibody concentrations in HIV test, for
detection of food allergens and in serological blood test for coeliac disease (Hornbeck, 2015).
Gliadin is a class of prolamin protein available in wheat and other cereals from grass genus
Triticum. Gliadins are heterogenous mixture of single chain polypeptides which are soluble in
70 % alcohol and these contain intramolecular disulphide bonds. Gliadins are components of
gluten which is useful to rise bread properly during baking. Gliadin is water-insoluble and
glutenin is water soluble. Gliadin exists in three forms like α, β, γ, and ω (Urade et al., 2018).
α gliadins are with fastest mobility while ω gliadins are with slowest mobility. Body is
intolerant to gliadin in coeliac (or celiac) disease (CD). Gliadin has the ability to cross
intestinal epithelium. Gliadins are responsible for precipitating coeliac disease while
glutenins are nontoxic in nature. Peptides derived from the gliadins are immunostimulatory in
coeliac disease (Kelly et al., 2015). Both gliadin and glutenin play role in the formation of
gluten. Gliadins are responsible for food derived pathogenesis. ELISA assays are usually
sensitive, specific and relatively easy to perform for the estimation of gluten content in food
products including breads. However, it is necessary to use different extraction solvents for
different types of foods for the estimation of gliadin using ELISA. ELISA assay was
implemented for the estimation of α-gliadin and whole gliadin in the wheat using polyclonal
rabbit or mouse enzyme labelled antisera. Literature mentioned that minimum 0.4 ng quantity
of gliadin in food products can also be estimated using ELISA. ELISA assay is useful in
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
measuring immunogenicity of gliadin for the exacerbations of the CD. ELISA assay is also
useful for the cooked and processed food because antibody provided in the ELISA is directed
against epitopes in ω gliadin which remain stable after cooking (Barak et al., 2015; Lexhaller
et al., 2017). Hence, ELISA assay is appropriate for the estimation of gliadin in the
commercially available food products including bread.
Aim:
To establish an ELISA (Enzyme Linked Immunosorbant Assay) standard curve from
standard samples containing known concentrations of gliadin.
To determine the exact concentration of gliadin in ‘unknown’ samples by calculation
from the standard curve.
To determine which bread extracts can be legitimately advertised as ‘gluten-free’ or
‘low gluten’.
Results:
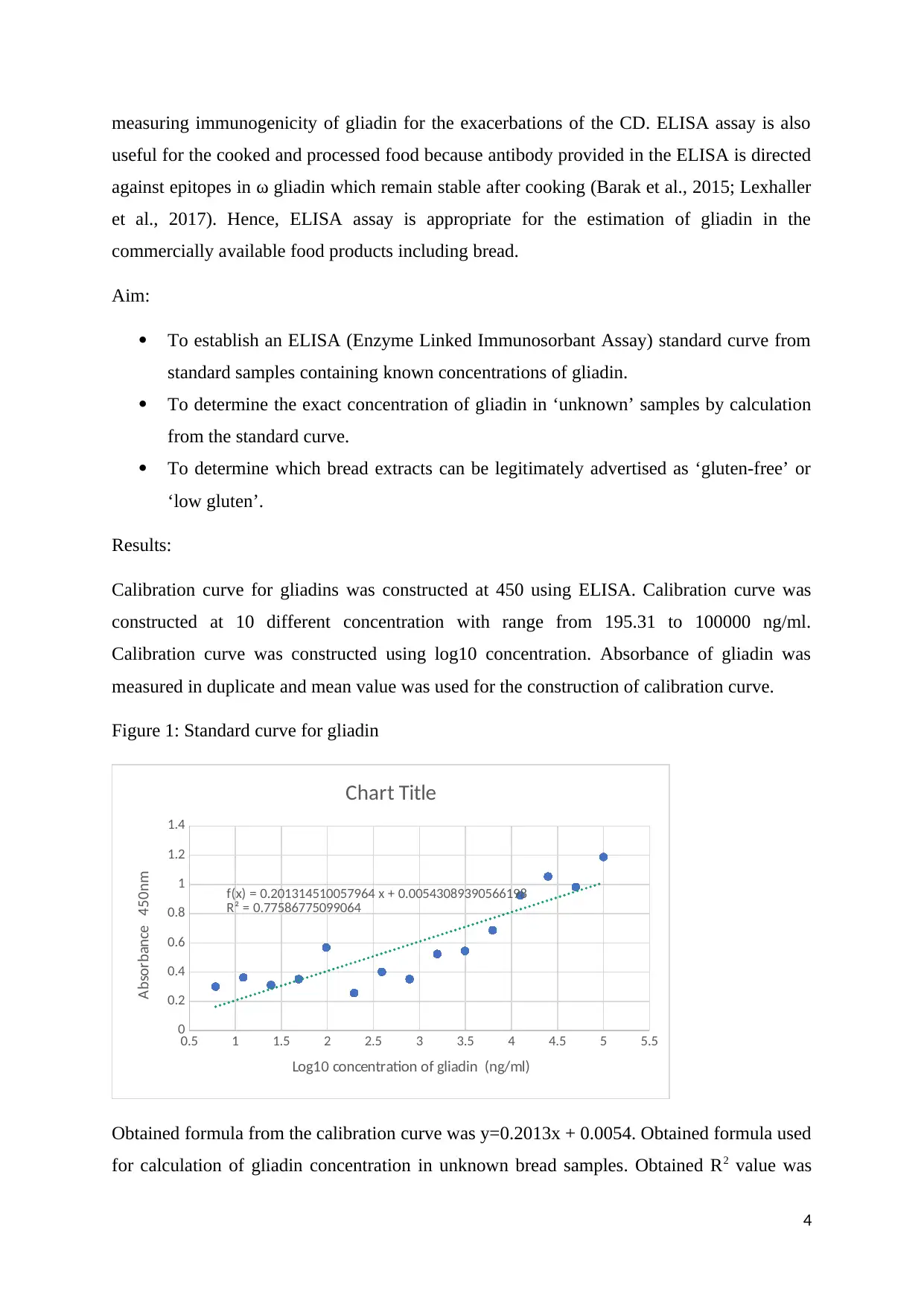
Calibration curve for gliadins was constructed at 450 using ELISA. Calibration curve was
constructed at 10 different concentration with range from 195.31 to 100000 ng/ml.
Calibration curve was constructed using log10 concentration. Absorbance of gliadin was
measured in duplicate and mean value was used for the construction of calibration curve.
Figure 1: Standard curve for gliadin
0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5
0
0.2
0.4
0.6
0.8
1
1.2
1.4
f(x) = 0.201314510057964 x + 0.00543089390566198
R² = 0.77586775099064
Chart Title
Log10 concentration of gliadin (ng/ml)
Absorbance 450nm
Obtained formula from the calibration curve was y=0.2013x + 0.0054. Obtained formula used
for calculation of gliadin concentration in unknown bread samples. Obtained R2 value was
4
useful for the cooked and processed food because antibody provided in the ELISA is directed
against epitopes in ω gliadin which remain stable after cooking (Barak et al., 2015; Lexhaller
et al., 2017). Hence, ELISA assay is appropriate for the estimation of gliadin in the
commercially available food products including bread.
Aim:
To establish an ELISA (Enzyme Linked Immunosorbant Assay) standard curve from
standard samples containing known concentrations of gliadin.
To determine the exact concentration of gliadin in ‘unknown’ samples by calculation
from the standard curve.
To determine which bread extracts can be legitimately advertised as ‘gluten-free’ or
‘low gluten’.
Results:
Calibration curve for gliadins was constructed at 450 using ELISA. Calibration curve was
constructed at 10 different concentration with range from 195.31 to 100000 ng/ml.
Calibration curve was constructed using log10 concentration. Absorbance of gliadin was
measured in duplicate and mean value was used for the construction of calibration curve.
Figure 1: Standard curve for gliadin
0.5 1 1.5 2 2.5 3 3.5 4 4.5 5 5.5
0
0.2
0.4
0.6
0.8
1
1.2
1.4
f(x) = 0.201314510057964 x + 0.00543089390566198
R² = 0.77586775099064
Chart Title
Log10 concentration of gliadin (ng/ml)
Absorbance 450nm
Obtained formula from the calibration curve was y=0.2013x + 0.0054. Obtained formula used
for calculation of gliadin concentration in unknown bread samples. Obtained R2 value was
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
0.7759. Five bread samples were evaluated for the gliadin content. In addition to this, bread
sample with gluten content was used as positive control. Gliadin content estimated in
different bread samples like Br1, Br2, Br3, Br4 and Br5 were 23120, 164, 11040, 606 and
258 respectively. Estimated gliadin content in the bread sample of positive control found to
be 1137 ng/ml. Hence, samples Br2 and Br5 were with low gliadin content and sample Br4 is
considered as gluten-free. Gliadin content found in the samples Br1 and Br3 were more as
compared to the positive control sample.
Table 1: Gliadin concentration in unknown bread samples and positive control sample
Sample Duplicate
E
Duplicate
F
Mean
OD
Actual
Mean
OD
Concentration
(ng/ml)
Br1 1.1007 0.7951 0.9479 0.8838 23,120
Br2 0.2960 0.7348 0.5154 0.4513 164.097
Br3 1.0250 0.7416 0.8833 0.8192 11,040
Br4 0.3293 0.1405 0.2349 0.1708 6.6374
Br5 0.6207 0.4893 0.555 0.4909 258.23
positive
control or
Br+
0.7916 0.5776 0.6846 0.6205 1137
Table 2: Gliadin concentration, % of gliadin depletion and gluten levels in unknown bread
samples and positive control sample
Unknown bread
sample
(Gliadin) ng/ml % of Gliadin
depletion
Gluten level
Br1 23120 0 High-gluten
Br2 164 86% Low-gluten
Br3 11040 0 High- gluten
Br4 6.6 99% Gluten-free
Br5 258 77% Low gluten
Br+ 1137 0 Gluten
Discussion:
Literature reported that few studies are available for the estimation of gluten in the food
products through ELISA. ELISA is being considered as the gold standard method for the
estimation gluten in the food products. However, this method is not a reliable method for
gluten estimation after consumption in individuals and populations. Gluten estimation using
5
sample with gluten content was used as positive control. Gliadin content estimated in
different bread samples like Br1, Br2, Br3, Br4 and Br5 were 23120, 164, 11040, 606 and
258 respectively. Estimated gliadin content in the bread sample of positive control found to
be 1137 ng/ml. Hence, samples Br2 and Br5 were with low gliadin content and sample Br4 is
considered as gluten-free. Gliadin content found in the samples Br1 and Br3 were more as
compared to the positive control sample.
Table 1: Gliadin concentration in unknown bread samples and positive control sample
Sample Duplicate
E
Duplicate
F
Mean
OD
Actual
Mean
OD
Concentration
(ng/ml)
Br1 1.1007 0.7951 0.9479 0.8838 23,120
Br2 0.2960 0.7348 0.5154 0.4513 164.097
Br3 1.0250 0.7416 0.8833 0.8192 11,040
Br4 0.3293 0.1405 0.2349 0.1708 6.6374
Br5 0.6207 0.4893 0.555 0.4909 258.23
positive
control or
Br+
0.7916 0.5776 0.6846 0.6205 1137
Table 2: Gliadin concentration, % of gliadin depletion and gluten levels in unknown bread
samples and positive control sample
Unknown bread
sample
(Gliadin) ng/ml % of Gliadin
depletion
Gluten level
Br1 23120 0 High-gluten
Br2 164 86% Low-gluten
Br3 11040 0 High- gluten
Br4 6.6 99% Gluten-free
Br5 258 77% Low gluten
Br+ 1137 0 Gluten
Discussion:
Literature reported that few studies are available for the estimation of gluten in the food
products through ELISA. ELISA is being considered as the gold standard method for the
estimation gluten in the food products. However, this method is not a reliable method for
gluten estimation after consumption in individuals and populations. Gluten estimation using
5

ELISA method is useful in establishing link between the gluten levels and CD presentation.
Estimation of gluten in food products is useful in both clinical and research application
(Infantino et al., 2016; Rzychon et al., 2017; Lexhaller et al., 2016). Aim of this study was to
evaluate gluten in different bread samples using gold standard ELISA method. Results
indicated that bread sample Br4 is gluten-free which can be highly recommended in CD
patients. Moreover, bread samples Br2 and Br5 are with low gluten levels. Hence, Br2 and
Br5 should also be recommended in the CD patients with regular monitoring. Samples Br1
and Br3 contain highest levels of gluten; hence, these should not be recommended in the CD
patients.
CD is an autoimmune enteropathy which is marked by small intestine damage. It has been
demonstrated that gliadin is the trigger factor for CD. However, it should be valued that it is
difficult to consume completely gluten-free diet because in both natural and labelled gluten
free diet, trace amount of gluten would be available. CD is an inflammatory disease in which
interaction occur between T cells and gluten peptides in the context of human leukocyte
antigen (HLA) DQ2 or HLA-DQ8 molecules. CD is highly heritable disease. There is no
viable treatment available for CD except gluten free diet (Kaur et al., 2017). CD patients on
gluten-free diet for approximately six months exhibited abolished symptoms and serum
antibodies. Administration of gluten-free diet proved helpful in the restoration of normal
architecture of intestinal villi. Abnormal intestinal permeability is mainly responsible for the
occurrence of cascade of mechanisms for occurrence of CD. Consumption of gluten
containing food results in the small intestine destruction lining. CD is associated with small
intestine lesion which is characterised by illous blunting, crypt cell hyperplasi, and infiltration
of leukocytes, involves the activation of gluten-reactive CD4 (cluster of differentiation 4) T
cells (Kaur et al., 2017; Murch, 2016).
National Institute of Health (NIH) produced “Celiac Disease” brochure which advised people
to consume gluten-free diet. Gluten free wheat and wheat derivatives are mainly used as main
ingredients in the processed foods. Consumption of gluten free diet can be effectively
improved through checking labels of the processed foods (Lee et al., 2014).
The U.S. Food and Drug Administration (FDA) stated the term “gluten-free” for voluntarily
labelling on the food products. Food products with the label gluten-free should be within
range of FDA’s gluten-free labelling rule. Since; FDA rules are applicable worldwide, CD
patients can consume foods with more confidence. According to FDA amount of gluten in the
6
Estimation of gluten in food products is useful in both clinical and research application
(Infantino et al., 2016; Rzychon et al., 2017; Lexhaller et al., 2016). Aim of this study was to
evaluate gluten in different bread samples using gold standard ELISA method. Results
indicated that bread sample Br4 is gluten-free which can be highly recommended in CD
patients. Moreover, bread samples Br2 and Br5 are with low gluten levels. Hence, Br2 and
Br5 should also be recommended in the CD patients with regular monitoring. Samples Br1
and Br3 contain highest levels of gluten; hence, these should not be recommended in the CD
patients.
CD is an autoimmune enteropathy which is marked by small intestine damage. It has been
demonstrated that gliadin is the trigger factor for CD. However, it should be valued that it is
difficult to consume completely gluten-free diet because in both natural and labelled gluten
free diet, trace amount of gluten would be available. CD is an inflammatory disease in which
interaction occur between T cells and gluten peptides in the context of human leukocyte
antigen (HLA) DQ2 or HLA-DQ8 molecules. CD is highly heritable disease. There is no
viable treatment available for CD except gluten free diet (Kaur et al., 2017). CD patients on
gluten-free diet for approximately six months exhibited abolished symptoms and serum
antibodies. Administration of gluten-free diet proved helpful in the restoration of normal
architecture of intestinal villi. Abnormal intestinal permeability is mainly responsible for the
occurrence of cascade of mechanisms for occurrence of CD. Consumption of gluten
containing food results in the small intestine destruction lining. CD is associated with small
intestine lesion which is characterised by illous blunting, crypt cell hyperplasi, and infiltration
of leukocytes, involves the activation of gluten-reactive CD4 (cluster of differentiation 4) T
cells (Kaur et al., 2017; Murch, 2016).
National Institute of Health (NIH) produced “Celiac Disease” brochure which advised people
to consume gluten-free diet. Gluten free wheat and wheat derivatives are mainly used as main
ingredients in the processed foods. Consumption of gluten free diet can be effectively
improved through checking labels of the processed foods (Lee et al., 2014).
The U.S. Food and Drug Administration (FDA) stated the term “gluten-free” for voluntarily
labelling on the food products. Food products with the label gluten-free should be within
range of FDA’s gluten-free labelling rule. Since; FDA rules are applicable worldwide, CD
patients can consume foods with more confidence. According to FDA amount of gluten in the
6
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

food should be less than 20 ppm. FDA’s rule related to gluten is helpful in assessment gluten
safety. This rule is applicable to all FDA-regulated packaged foods including dietary
supplements. According to FDA rules, manufactured food should be either completely gluten
free or its should gluten content should be less than 20 ppm. FDA’s regulatory definition
gluten-free would helpful in reducing confusion of the consumers about the consumption of
food (Lee et al., 2014).
Conclusion:
In this experiment, a simple and sensitive ELISA method was used for the estimation of
gliadin in the bread samples. Standard curve was prepared using standard gliadin and it was
used for the estimation of gliadin in the unknown samples. Positive control was used for
grading the unknown samples. Samples were graded as gluten-free, low gluten and high
gluten based in the comparison with positive control sample. This method can be successfully
implemented for the estimation of gliadin in other food products. Application of this method
for the estimation of gluten is helpful in adopting practical gluten consumption. This assay
needs to be repeated at least seven times to get the robust results. In summary, this method is
applicable in understanding biology of the CD.
References:
Barak, S., Mudgil, D., and Khatkar, B.S. (2015) Biochemical and functional properties of
wheat gliadins: a review. Critical Reviews in Food Science and Nutrition, 55(3), pp. 357-68.
Hornbeck, P.V. (2015) Enzyme-Linked Immunosorbent Assays. Current Protocols in
Immunology, 110(2), pp. 1-23.
Infantino, M., Meacci, F., Grossi, V., Macchia, D., and Manfredi, M. (2016) Anti-gliadin
antibodies in non-celiac gluten sensitivity. Minerva Gastroenterologica e Dietologica, 63(1),
pp. 1-4.
Kaur, A., Shimoni, O., and Wallach, M. (2017) Celiac disease: from etiological factors to
evolving diagnostic approaches. Journal of Gastroenterology, 52(9), pp. 1001-1012.
Kelly, C.P., Bai, J.C., Liu, E., and Leffler, D.A. (2015) Advances in diagnosis and
management of celiac disease. Gastroenterology, 148(6), pp. 1175-86.
Konstantinou, G.N. (2017) Enzyme-Linked Immunosorbent Assay (ELISA). Methods in
Molecular Biology, 1592, pp. 79-94.
Lee, H.J., Anderson, Z., and Ryu, D. (2014) Anderson Z, Ryu D. Gluten contamination in
foods labeled as "gluten free" in the United States. Journal of Food Protection, 77(10)14, pp.
1830-3.
7
safety. This rule is applicable to all FDA-regulated packaged foods including dietary
supplements. According to FDA rules, manufactured food should be either completely gluten
free or its should gluten content should be less than 20 ppm. FDA’s regulatory definition
gluten-free would helpful in reducing confusion of the consumers about the consumption of
food (Lee et al., 2014).
Conclusion:
In this experiment, a simple and sensitive ELISA method was used for the estimation of
gliadin in the bread samples. Standard curve was prepared using standard gliadin and it was
used for the estimation of gliadin in the unknown samples. Positive control was used for
grading the unknown samples. Samples were graded as gluten-free, low gluten and high
gluten based in the comparison with positive control sample. This method can be successfully
implemented for the estimation of gliadin in other food products. Application of this method
for the estimation of gluten is helpful in adopting practical gluten consumption. This assay
needs to be repeated at least seven times to get the robust results. In summary, this method is
applicable in understanding biology of the CD.
References:
Barak, S., Mudgil, D., and Khatkar, B.S. (2015) Biochemical and functional properties of
wheat gliadins: a review. Critical Reviews in Food Science and Nutrition, 55(3), pp. 357-68.
Hornbeck, P.V. (2015) Enzyme-Linked Immunosorbent Assays. Current Protocols in
Immunology, 110(2), pp. 1-23.
Infantino, M., Meacci, F., Grossi, V., Macchia, D., and Manfredi, M. (2016) Anti-gliadin
antibodies in non-celiac gluten sensitivity. Minerva Gastroenterologica e Dietologica, 63(1),
pp. 1-4.
Kaur, A., Shimoni, O., and Wallach, M. (2017) Celiac disease: from etiological factors to
evolving diagnostic approaches. Journal of Gastroenterology, 52(9), pp. 1001-1012.
Kelly, C.P., Bai, J.C., Liu, E., and Leffler, D.A. (2015) Advances in diagnosis and
management of celiac disease. Gastroenterology, 148(6), pp. 1175-86.
Konstantinou, G.N. (2017) Enzyme-Linked Immunosorbent Assay (ELISA). Methods in
Molecular Biology, 1592, pp. 79-94.
Lee, H.J., Anderson, Z., and Ryu, D. (2014) Anderson Z, Ryu D. Gluten contamination in
foods labeled as "gluten free" in the United States. Journal of Food Protection, 77(10)14, pp.
1830-3.
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Lexhaller, B., Tompos, C., and Scherf, K.A. (2016) Comparative analysis of prolamin and
glutelin fractions from wheat, rye, and barley with five sandwich ELISA test kits. Analytical
and Bioanalytical Chemistry, 408(22), pp. 6093-104.
Lexhaller, B., Tompos, C., and Scherf, K.A. (2017) Fundamental study on reactivities of
gluten protein types from wheat, rye and barley with five sandwich ELISA test kits. Food
Chemistry, 237, pp. 320-330.
Murch, S. (2016) Recent Advances in Celiac Disease. Indian Journal of Pediatrics, 83(12 -
13), pp. 1428-1435.
Rzychon, M., Brohée, M., Cordeiro, F., Haraszi, R., Ulberth, F., and O'Connor, G. (2017)
The feasibility of harmonizing gluten ELISA measurements’, Food Chemistry, 234, pp. 144-
154.
Urade, R., Sato, N., and Sugiyama, M. (2018) Gliadins from wheat grain: an overview, from
primary structure to nanostructures of aggregates. Biophysical Reviews, 10(2), pp. 435-443.
8
glutelin fractions from wheat, rye, and barley with five sandwich ELISA test kits. Analytical
and Bioanalytical Chemistry, 408(22), pp. 6093-104.
Lexhaller, B., Tompos, C., and Scherf, K.A. (2017) Fundamental study on reactivities of
gluten protein types from wheat, rye and barley with five sandwich ELISA test kits. Food
Chemistry, 237, pp. 320-330.
Murch, S. (2016) Recent Advances in Celiac Disease. Indian Journal of Pediatrics, 83(12 -
13), pp. 1428-1435.
Rzychon, M., Brohée, M., Cordeiro, F., Haraszi, R., Ulberth, F., and O'Connor, G. (2017)
The feasibility of harmonizing gluten ELISA measurements’, Food Chemistry, 234, pp. 144-
154.
Urade, R., Sato, N., and Sugiyama, M. (2018) Gliadins from wheat grain: an overview, from
primary structure to nanostructures of aggregates. Biophysical Reviews, 10(2), pp. 435-443.
8
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

