Inorganic Chemistry Assignment: Detailed Question Solutions, Answers
VerifiedAdded on 2023/01/20
|13
|1462
|69
Homework Assignment
AI Summary
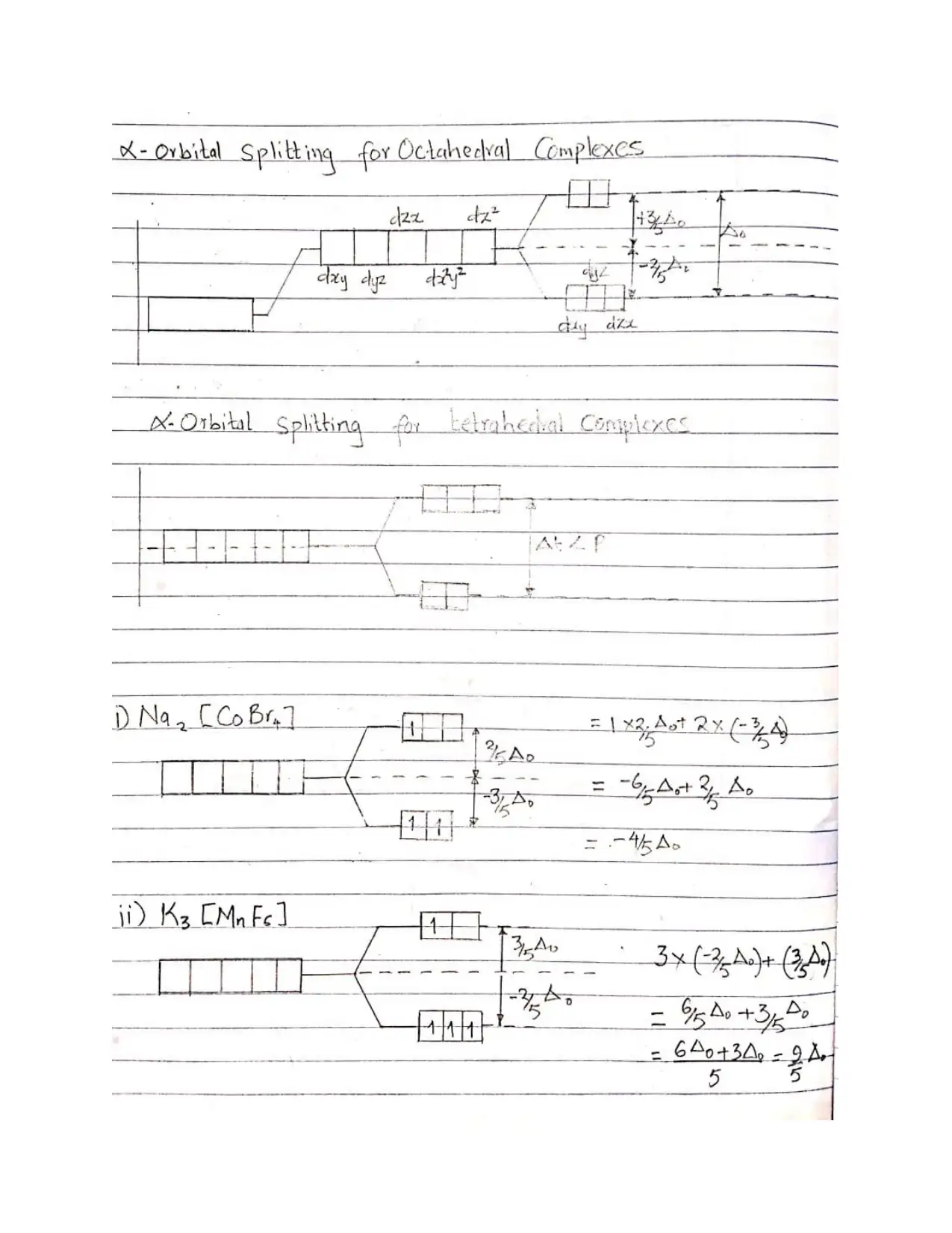
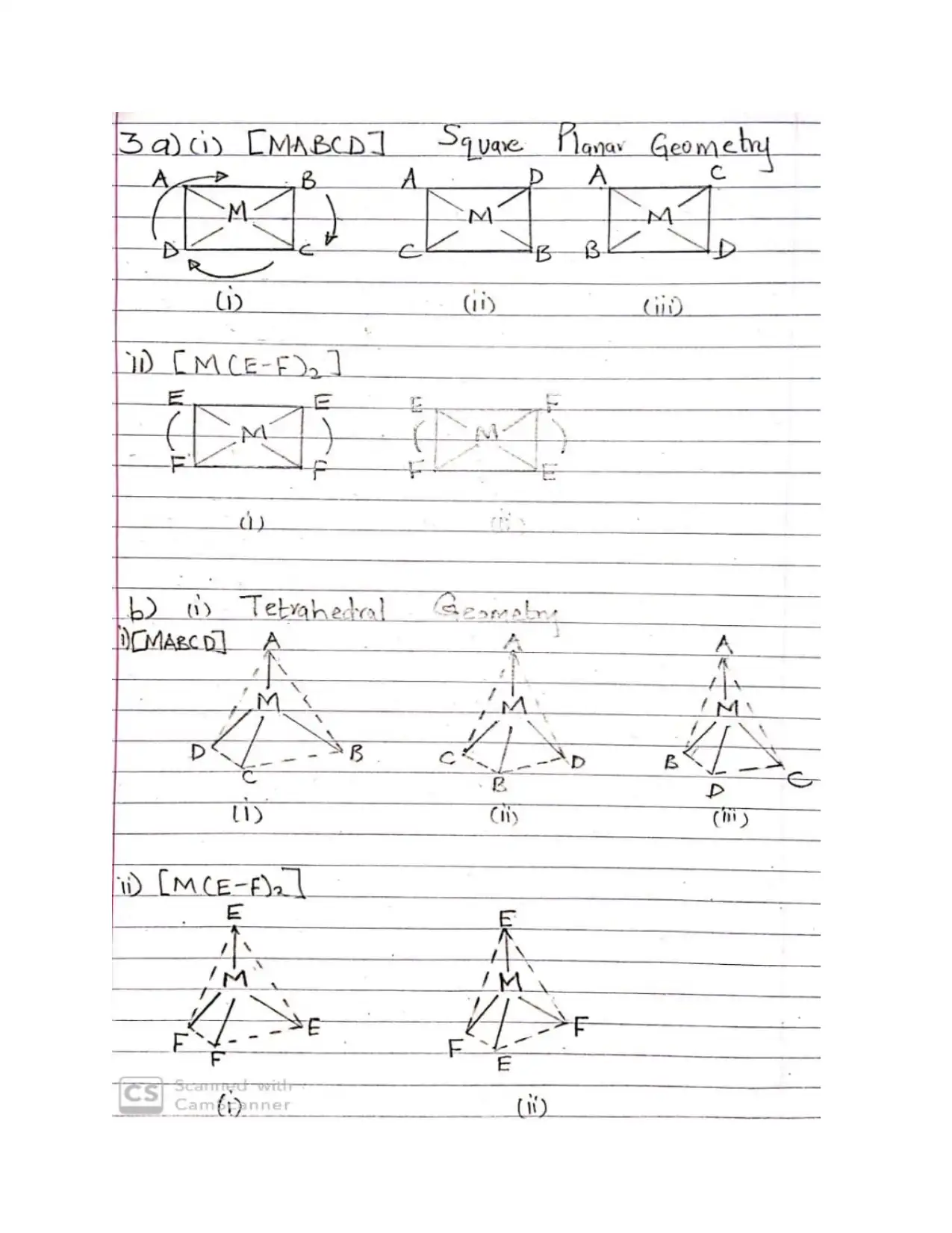
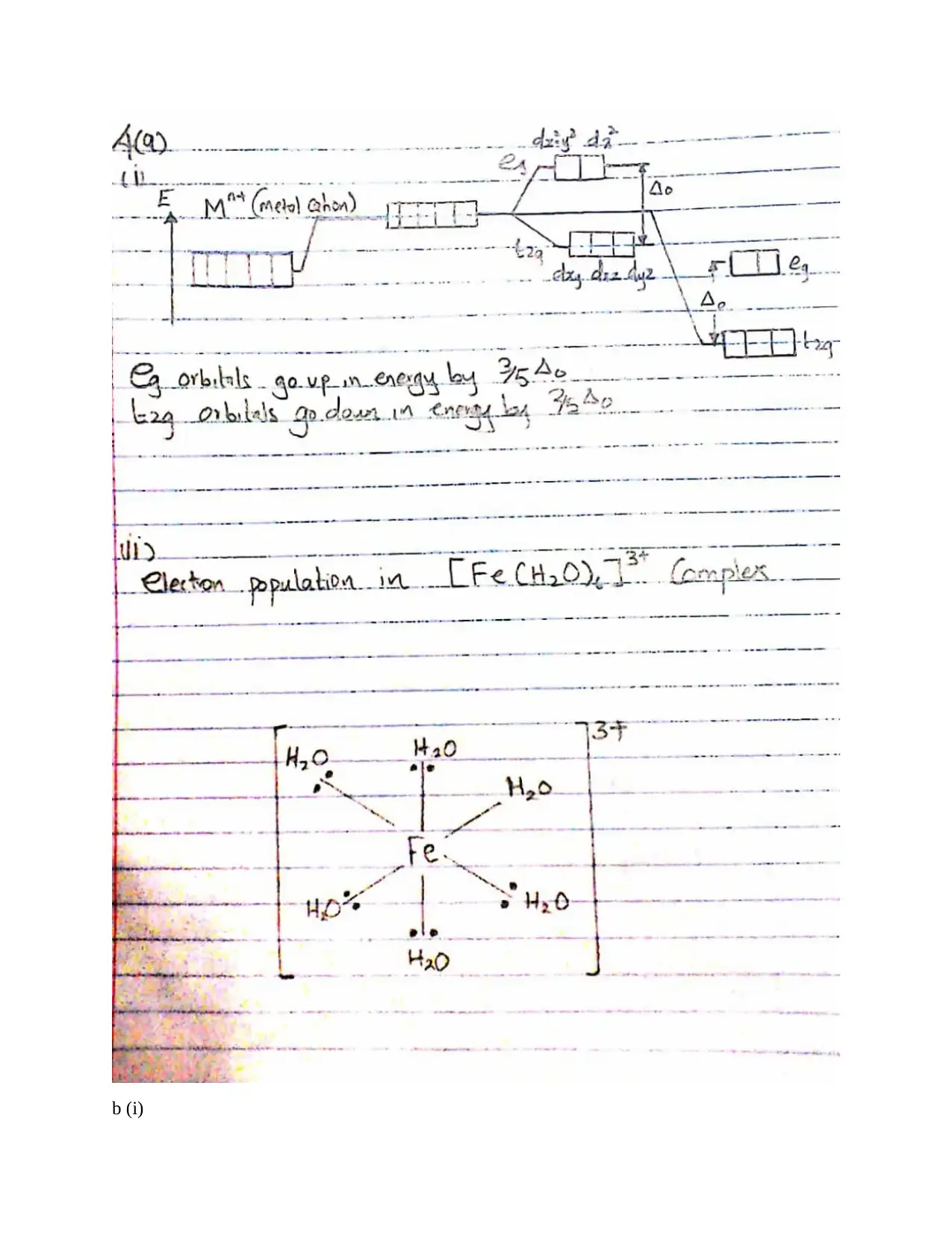
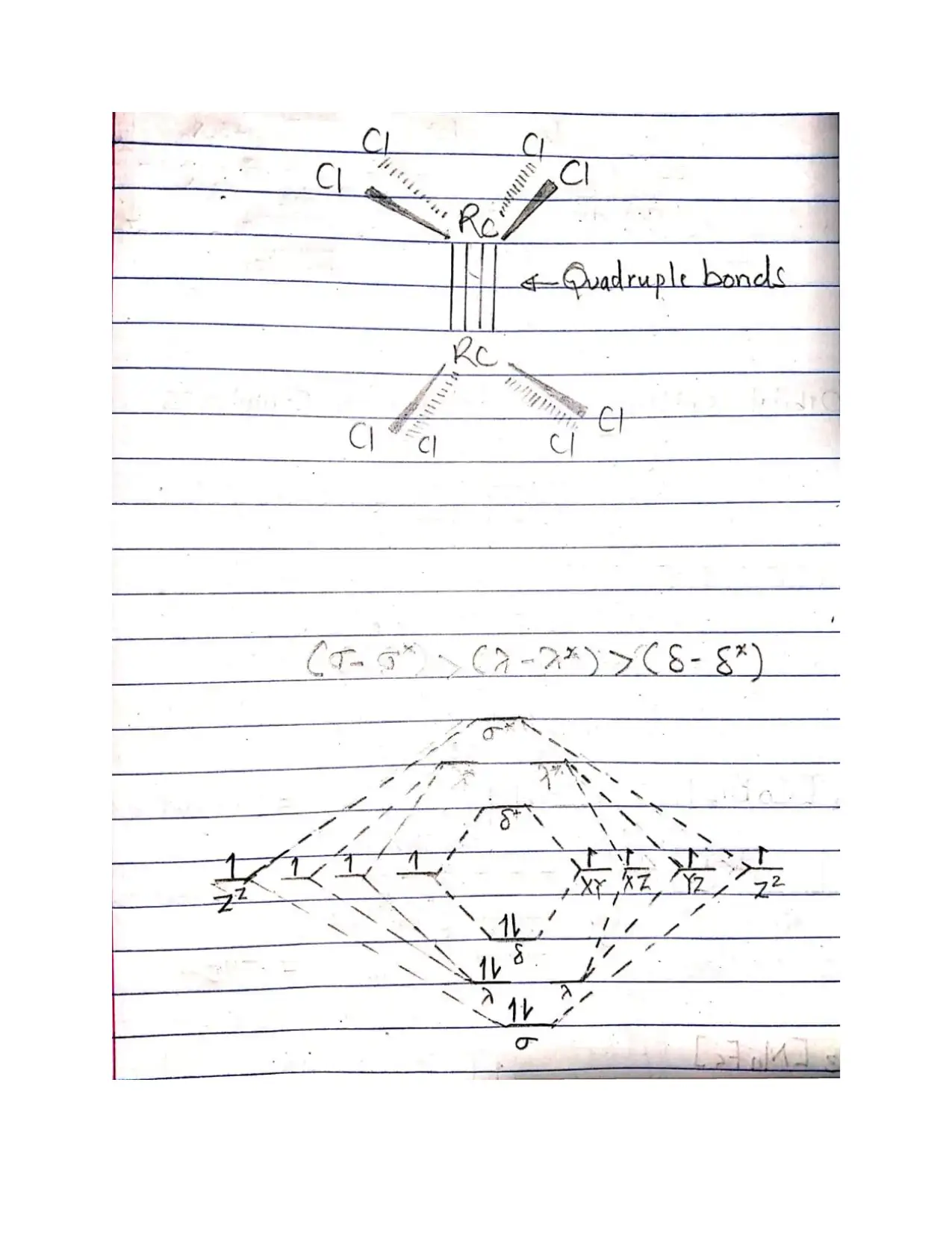
This document provides comprehensive solutions to an inorganic chemistry assignment, addressing various key concepts. The solutions cover reaction mechanisms, specifically inner-sphere and outer-sphere mechanisms, and the prediction of the most probable mechanism along with experimental evidence. It further explores electronic spectra observed for transition metal complexes, providing examples for each type, and delves into Crystal Field Theory, including its basis, assumptions, and orbital splitting diagrams for octahedral and tetrahedral complexes. Crystal Field Stabilization Energy calculations are also included. The assignment also uses Valence Bond Theory to analyze the variation in magnetic moments of manganese complexes, and examines the structures of different isomers. The document also details the preparation methods of cobalt (III) complexes, structures with seven coordination, the mechanisms for substitution reactions of octahedral metal complexes, the trans-effect, back bonding, and the Jahn-Teller effect. Finally, it explains why cobalt(II) forms tetrahedral complexes, while cobalt(III) forms octahedral complexes, and the square planar complex formation of Ni(II), Pd(II) and Pt(II).
1 out of 13










![[object Object]](/_next/static/media/star-bottom.7253800d.svg)