Case of ketoacidosis by a sodium-glucose cotransporter 2 inhibitor in a diabetic patient with a low-carbohydrate diet
VerifiedAdded on 2023/05/29
|4
|2436
|398
AI Summary
The case report describes a 32-year-old diabetic woman with Prader–Willi syndrome who developed severe ketoacidosis caused by a sodium-glucose cotransporter 2 (SGLT2) inhibitor, a novel class of antihyperglycemic agents, during a strict low-carbohydrate diet. It is the first report of ketoacidosis caused by a SGLT2 inhibitor. The report suggests that it is necessary to pay attention when using a SGLT2 inhibitor in patients following a low-carbohydrate diet, but also to start a low-carbohydrate diet in patients treated with a SGLT2 inhibitor because of a high risk for developing ketoacidosis.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Case of ketoacidosis by a sodium-glucose
cotransporter 2 inhibitor in a diabetic patient
with a low-carbohydrate diet
Tomohide Hayami1
, Yoshiro Kato1
*,Hideki Kamiya1, Masaki Kondo1, Ena Naito1, Yukako Sugiura1, Chika Kojima1,
Sami Sato1, Yuichiro Yamada1, Rina Kasagi1, Toshihito Ando1, Saeko Noda1, Hiromi Nakai1, Eriko Takada1
, Emi Asano1,
Mikio Motegi1, Atsuko Watarai2, Koichi Kato3, Jiro Nakamura1
1Division of Diabetes,Department of InternalMedicine,AichiMedicalUniversity Schoolof Medicine,Nagakute,2
Center for Preventive Medicine,Chubu RosaiHospital,and3
Labora-
tory of Medicine,AichiGakuin University Schoolof Pharmacy,Nagoya,Aichi,Japan
Keywords
Ketoacidosis,Low-carbohydrate diet,
Sodium-glucose cotransporter 2
inhibitor
*Correspondence
Yoshiro Kato
Tel.:+81-561-63-1683
Fax:+81-561-63-1276
E-mailaddress:ykato4@aichi-
med-u.ac.jp
J Diabetes Invest 2015;6:587–590
doi: 10.1111/jdi.12330
ABSTRACT
We present a case of a 32-year-old diabetic woman with Prader–Willisyndrome who
developed severe ketoacidosis caused by a sodium-glucose cotransporter 2 (SG
tor,a novelclass of antihyperglycemic agents,during a strict low-carbohydrate diet.At
admission,a serum glucose levelof 191 mg/dL was relatively low,though laboratory eval-
uations showed severe ketoacidosis.This is the first report of ketoacidosis caused by a
SGLT2 inhibitor.It is necessary to not only pay attention when using a SGLT2 inhib
patients following a low-carbohydrate diet,but also to start a low-carbohydrate diet in
patients treated with a SGLT2 inhibitor because of a high risk for developing ke
INTRODUCTION
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a novel
class ofantihyperglycemic agents thatinhibitglucose reuptake
in the kidney1. A low-carbohydrate diet is believed to be effec-
tive in weightloss when producing ketosis2. However,a few
casesof ketoacidosiswithouthavingdiabetesmellituswere
reported during a low-carbohydrate diet3,4
.
We recently encountered a case of severe ketoacidosis caused
by a SGLT2 inhibitor during a low-carbohydrate diet.Here we
presentthis case,because,to our knowledge,there have been
no prior reports on the disease conditions shown in this case.
CASE REPORT
A 32-year-old woman with Prader–Willisyndrome was diag-
nosed with diabetesat the age of10 years,and wasrecom-
mended to starta strictlow-carbohydrate dietby herfamily
because ofpoorly-controlled glycemia atthe age of21 years.
Before starting a strictlow-carbohydrate diet,her bodyweight
was 54 kg,butgradually increased as a resultof an excessive
intake ofprotein and lipid.It recently increased up to 67 kg.
She received glimepiride(2 mg/day),metformin (2,250 mg/
day) and linagliptin (5 mg/day),but these oralmedicines were
switched to a SGLT2 inhibitor,ipragliflozin (50 mg/day) alone
13 daysbefore admission.Immediately aftertaking a SGLT2
inhibitor,polyuriadevelopedand the patient’sbodyweight
decreased byapproximately3 kg for 10 days.Epigastralgia
developed 2 daysbeforeadmission,and waterand dietary
intake decreased.Tachypnea developed 1 day before admissio
As her symptomsbecame worse,she visited the emergency
room ofAichi MedicalUniversity Hospital,Nagakute,Aichi,
Japan.Laboratory evaluation showed severe acidosis,ketonuria,
ketonemia and a normallevelof lactate (Table 1).Chest X-ray,
electrocardiogram,abdominalcomputed tomography and uri-
nary sediments showed no abnormalities,indicating the unlike-
lihood ofinfectiousdiseases.We diagnosed the patientwith
ketoacidosis.Immediately after admission,continuous intrave-
nous insulin,Ringer’s solution and glucose infusion was initi-
ated in an intensive care unit.Ketoacidosis was improved,and
the patient’sbodyweightincreased by approximately 2 kg on
the second day afteradmission (Figure 1).Antiglutamic acid
Received 22 August 2014;revised 4 December 2014;accepted 8 January 2015
ª 2015 The Authors.Journalof Diabetes Investigation published by Asian Association of the Study of Diabetes (AASD) and Wiley Publishing Asia Pty LtdJ Diabetes Invest Vol.6 No.5 September 2015 587
This is an open accessarticle underthe termsof the Creative CommonsAttribution-NonCommercialLicense,which permits use,distribution and
reproduction in any medium,provided the originalwork is properly cited and is not used for commercialpurposes.
CASE REPO RT
cotransporter 2 inhibitor in a diabetic patient
with a low-carbohydrate diet
Tomohide Hayami1
, Yoshiro Kato1
*,Hideki Kamiya1, Masaki Kondo1, Ena Naito1, Yukako Sugiura1, Chika Kojima1,
Sami Sato1, Yuichiro Yamada1, Rina Kasagi1, Toshihito Ando1, Saeko Noda1, Hiromi Nakai1, Eriko Takada1
, Emi Asano1,
Mikio Motegi1, Atsuko Watarai2, Koichi Kato3, Jiro Nakamura1
1Division of Diabetes,Department of InternalMedicine,AichiMedicalUniversity Schoolof Medicine,Nagakute,2
Center for Preventive Medicine,Chubu RosaiHospital,and3
Labora-
tory of Medicine,AichiGakuin University Schoolof Pharmacy,Nagoya,Aichi,Japan
Keywords
Ketoacidosis,Low-carbohydrate diet,
Sodium-glucose cotransporter 2
inhibitor
*Correspondence
Yoshiro Kato
Tel.:+81-561-63-1683
Fax:+81-561-63-1276
E-mailaddress:ykato4@aichi-
med-u.ac.jp
J Diabetes Invest 2015;6:587–590
doi: 10.1111/jdi.12330
ABSTRACT
We present a case of a 32-year-old diabetic woman with Prader–Willisyndrome who
developed severe ketoacidosis caused by a sodium-glucose cotransporter 2 (SG
tor,a novelclass of antihyperglycemic agents,during a strict low-carbohydrate diet.At
admission,a serum glucose levelof 191 mg/dL was relatively low,though laboratory eval-
uations showed severe ketoacidosis.This is the first report of ketoacidosis caused by a
SGLT2 inhibitor.It is necessary to not only pay attention when using a SGLT2 inhib
patients following a low-carbohydrate diet,but also to start a low-carbohydrate diet in
patients treated with a SGLT2 inhibitor because of a high risk for developing ke
INTRODUCTION
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a novel
class ofantihyperglycemic agents thatinhibitglucose reuptake
in the kidney1. A low-carbohydrate diet is believed to be effec-
tive in weightloss when producing ketosis2. However,a few
casesof ketoacidosiswithouthavingdiabetesmellituswere
reported during a low-carbohydrate diet3,4
.
We recently encountered a case of severe ketoacidosis caused
by a SGLT2 inhibitor during a low-carbohydrate diet.Here we
presentthis case,because,to our knowledge,there have been
no prior reports on the disease conditions shown in this case.
CASE REPORT
A 32-year-old woman with Prader–Willisyndrome was diag-
nosed with diabetesat the age of10 years,and wasrecom-
mended to starta strictlow-carbohydrate dietby herfamily
because ofpoorly-controlled glycemia atthe age of21 years.
Before starting a strictlow-carbohydrate diet,her bodyweight
was 54 kg,butgradually increased as a resultof an excessive
intake ofprotein and lipid.It recently increased up to 67 kg.
She received glimepiride(2 mg/day),metformin (2,250 mg/
day) and linagliptin (5 mg/day),but these oralmedicines were
switched to a SGLT2 inhibitor,ipragliflozin (50 mg/day) alone
13 daysbefore admission.Immediately aftertaking a SGLT2
inhibitor,polyuriadevelopedand the patient’sbodyweight
decreased byapproximately3 kg for 10 days.Epigastralgia
developed 2 daysbeforeadmission,and waterand dietary
intake decreased.Tachypnea developed 1 day before admissio
As her symptomsbecame worse,she visited the emergency
room ofAichi MedicalUniversity Hospital,Nagakute,Aichi,
Japan.Laboratory evaluation showed severe acidosis,ketonuria,
ketonemia and a normallevelof lactate (Table 1).Chest X-ray,
electrocardiogram,abdominalcomputed tomography and uri-
nary sediments showed no abnormalities,indicating the unlike-
lihood ofinfectiousdiseases.We diagnosed the patientwith
ketoacidosis.Immediately after admission,continuous intrave-
nous insulin,Ringer’s solution and glucose infusion was initi-
ated in an intensive care unit.Ketoacidosis was improved,and
the patient’sbodyweightincreased by approximately 2 kg on
the second day afteradmission (Figure 1).Antiglutamic acid
Received 22 August 2014;revised 4 December 2014;accepted 8 January 2015
ª 2015 The Authors.Journalof Diabetes Investigation published by Asian Association of the Study of Diabetes (AASD) and Wiley Publishing Asia Pty LtdJ Diabetes Invest Vol.6 No.5 September 2015 587
This is an open accessarticle underthe termsof the Creative CommonsAttribution-NonCommercialLicense,which permits use,distribution and
reproduction in any medium,provided the originalwork is properly cited and is not used for commercialpurposes.
CASE REPO RT
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.
decarboxylaseantibody,isletantigen-2 antibody and insulin
autoantibody were negative.The serum C-peptide was 0.4 ng/
mL and the urinary C-peptide was undetectable atadmission,
but increased up to 40.2 lg/day on the sixth day after admis-
sion.At admission,the plasma glucose,glycated hemoglobin
(HbA1c)and glycated albumin levelswere 191 mg/dL,9.3%
and 19.6%,respectively.After ketoacidosiswas improved,
basal–bolus insulin therapy was continued for a while.At dis-
charge,the patientwas treated with insulin glargine (24 units/
day),lixisenatide (20 lg/day)and metformin (2,250 mg/day).
A nutritionist instructed the patient to follow a 1,500-kcaldiet,
containing 65 g ofprotein,45 g offatand 210 g ofcarbohy-
drate.One month later,HbA1c and glycated albumin levels
were remarkably improved to 6.8% and 13.7%,respectively.
DISCUSSION
In the presentreport,we have described a case ofsevere ke-
toacidosis caused by a SGLT2 inhibitor during a low-carbohy-
drate diet.The most widely used diagnostic criteria for diabetic
ketoacidosisincludeblood glucose>250 mg/dL,arterialpH
<7.3,serum bicarbonate <15 mEq/L and a moderate degree of
ketonemia and/or ketonuria5. The conditions of this patient are
consistent with the diagnostic criteria,except for the blood glu-
cose level.The factthatthe lactate levelwas normaland that
the patient did notconsume alcoholindicated thather condi-
tionswereneitherlacticacidosisnor alcoholicketoacidosis.
Through thefactthatshe did not consumeexcessivesoft
drinks,soft drink ketosis was unlikely.There seems to be a dis-
crepancy between the values ofglycated albumin and HbA1c.
The glycated albumin level of 19.6% at admission was rela
low compared with the HbA1c levelof 9.3%,showing that the
blood glucoseleveldeclined with theadministration ofa
SGLT2 inhibitorfor approximately2 weeksbeforesevere
ketoacidosis developed.The characteristic feature of this patient
wasthatthe blood glucose levelof 191 mg/dL atadmission
was relatively low despite severe ketoacidosis.
The urinary C-peptide thatwasundetectable atadmission
increased up to 40.2 lg/day after the start of a diet contain
210 g carbohydrate/day.It has been suggested that endogenous
insulin secretion issuppressed during ketosisor ketoacidosis
and improvesaftertreatmentin patientswith non-insulin-
dependent diabetes6. Therefore,the insulin secretory capacity of
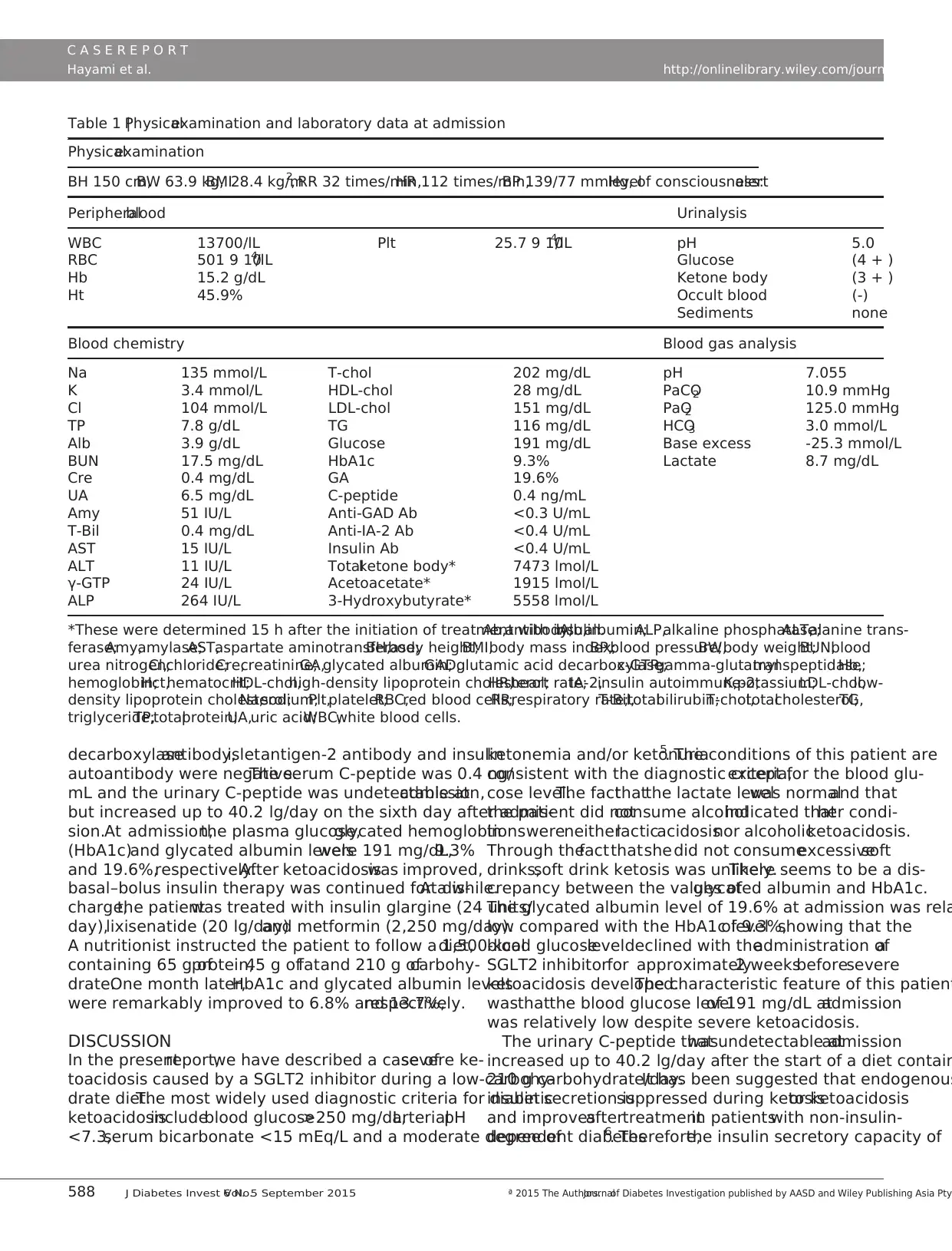
Table 1 |Physicalexamination and laboratory data at admission
Physicalexamination
BH 150 cm,BW 63.9 kg,BMI28.4 kg/m2, RR 32 times/min,HR 112 times/min,BP 139/77 mmHg,levelof consciousness:alert
Peripheralblood Urinalysis
WBC 13700/lL Plt 25.7 9 104/lL pH 5.0
RBC 501 9 104/lL Glucose (4 + )
Hb 15.2 g/dL Ketone body (3 + )
Ht 45.9% Occult blood (-)
Sediments none
Blood chemistry Blood gas analysis
Na 135 mmol/L T-chol 202 mg/dL pH 7.055
K 3.4 mmol/L HDL-chol 28 mg/dL PaCO2 10.9 mmHg
Cl 104 mmol/L LDL-chol 151 mg/dL PaO2 125.0 mmHg
TP 7.8 g/dL TG 116 mg/dL HCO3 3.0 mmol/L
Alb 3.9 g/dL Glucose 191 mg/dL Base excess -25.3 mmol/L
BUN 17.5 mg/dL HbA1c 9.3% Lactate 8.7 mg/dL
Cre 0.4 mg/dL GA 19.6%
UA 6.5 mg/dL C-peptide 0.4 ng/mL
Amy 51 IU/L Anti-GAD Ab <0.3 U/mL
T-Bil 0.4 mg/dL Anti-IA-2 Ab <0.4 U/mL
AST 15 IU/L Insulin Ab <0.4 U/mL
ALT 11 IU/L Totalketone body* 7473 lmol/L
γ-GTP 24 IU/L Acetoacetate* 1915 lmol/L
ALP 264 IU/L 3-Hydroxybutyrate* 5558 lmol/L
*These were determined 15 h after the initiation of treatment with insulin.Ab,antibody;Alb,albumin;ALP,alkaline phosphatase;ALT,alanine trans-
ferase;Amy,amylase;AST,aspartate aminotransferase;BH,body height;BMI,body mass index;BP,blood pressure;BW,body weight;BUN,blood
urea nitrogen;Cl,chloride;Cre,creatinine;GA,glycated albumin;GAD,glutamic acid decarboxylase;c-GTP,gamma-glutamyltranspeptidase;Hb,
hemoglobin;Hct,hematocrit;HDL-chol,high-density lipoprotein cholesterol;HR,heart rate;IA-2,insulin autoimmune-2;K,potassium;LDL-chol,low-
density lipoprotein cholesterol;Na,sodium;Plt,platelet;RBC,red blood cells;RR,respiratory rate;T-Bil,totalbilirubin;T-chol,totalcholesterol;TG,
triglyceride;TP,totalprotein;UA,uric acid;WBC,white blood cells.
588 J Diabetes Invest Vol.6 No.5 September 2015 ª 2015 The Authors.Journalof Diabetes Investigation published by AASD and Wiley Publishing Asia Pty
C A S E R E P O R T
Hayami et al. http://onlinelibrary.wiley.com/journal/jdi
autoantibody were negative.The serum C-peptide was 0.4 ng/
mL and the urinary C-peptide was undetectable atadmission,
but increased up to 40.2 lg/day on the sixth day after admis-
sion.At admission,the plasma glucose,glycated hemoglobin
(HbA1c)and glycated albumin levelswere 191 mg/dL,9.3%
and 19.6%,respectively.After ketoacidosiswas improved,
basal–bolus insulin therapy was continued for a while.At dis-
charge,the patientwas treated with insulin glargine (24 units/
day),lixisenatide (20 lg/day)and metformin (2,250 mg/day).
A nutritionist instructed the patient to follow a 1,500-kcaldiet,
containing 65 g ofprotein,45 g offatand 210 g ofcarbohy-
drate.One month later,HbA1c and glycated albumin levels
were remarkably improved to 6.8% and 13.7%,respectively.
DISCUSSION
In the presentreport,we have described a case ofsevere ke-
toacidosis caused by a SGLT2 inhibitor during a low-carbohy-
drate diet.The most widely used diagnostic criteria for diabetic
ketoacidosisincludeblood glucose>250 mg/dL,arterialpH
<7.3,serum bicarbonate <15 mEq/L and a moderate degree of
ketonemia and/or ketonuria5. The conditions of this patient are
consistent with the diagnostic criteria,except for the blood glu-
cose level.The factthatthe lactate levelwas normaland that
the patient did notconsume alcoholindicated thather condi-
tionswereneitherlacticacidosisnor alcoholicketoacidosis.
Through thefactthatshe did not consumeexcessivesoft
drinks,soft drink ketosis was unlikely.There seems to be a dis-
crepancy between the values ofglycated albumin and HbA1c.
The glycated albumin level of 19.6% at admission was rela
low compared with the HbA1c levelof 9.3%,showing that the
blood glucoseleveldeclined with theadministration ofa
SGLT2 inhibitorfor approximately2 weeksbeforesevere
ketoacidosis developed.The characteristic feature of this patient
wasthatthe blood glucose levelof 191 mg/dL atadmission
was relatively low despite severe ketoacidosis.
The urinary C-peptide thatwasundetectable atadmission
increased up to 40.2 lg/day after the start of a diet contain
210 g carbohydrate/day.It has been suggested that endogenous
insulin secretion issuppressed during ketosisor ketoacidosis
and improvesaftertreatmentin patientswith non-insulin-
dependent diabetes6. Therefore,the insulin secretory capacity of
Table 1 |Physicalexamination and laboratory data at admission
Physicalexamination
BH 150 cm,BW 63.9 kg,BMI28.4 kg/m2, RR 32 times/min,HR 112 times/min,BP 139/77 mmHg,levelof consciousness:alert
Peripheralblood Urinalysis
WBC 13700/lL Plt 25.7 9 104/lL pH 5.0
RBC 501 9 104/lL Glucose (4 + )
Hb 15.2 g/dL Ketone body (3 + )
Ht 45.9% Occult blood (-)
Sediments none
Blood chemistry Blood gas analysis
Na 135 mmol/L T-chol 202 mg/dL pH 7.055
K 3.4 mmol/L HDL-chol 28 mg/dL PaCO2 10.9 mmHg
Cl 104 mmol/L LDL-chol 151 mg/dL PaO2 125.0 mmHg
TP 7.8 g/dL TG 116 mg/dL HCO3 3.0 mmol/L
Alb 3.9 g/dL Glucose 191 mg/dL Base excess -25.3 mmol/L
BUN 17.5 mg/dL HbA1c 9.3% Lactate 8.7 mg/dL
Cre 0.4 mg/dL GA 19.6%
UA 6.5 mg/dL C-peptide 0.4 ng/mL
Amy 51 IU/L Anti-GAD Ab <0.3 U/mL
T-Bil 0.4 mg/dL Anti-IA-2 Ab <0.4 U/mL
AST 15 IU/L Insulin Ab <0.4 U/mL
ALT 11 IU/L Totalketone body* 7473 lmol/L
γ-GTP 24 IU/L Acetoacetate* 1915 lmol/L
ALP 264 IU/L 3-Hydroxybutyrate* 5558 lmol/L
*These were determined 15 h after the initiation of treatment with insulin.Ab,antibody;Alb,albumin;ALP,alkaline phosphatase;ALT,alanine trans-
ferase;Amy,amylase;AST,aspartate aminotransferase;BH,body height;BMI,body mass index;BP,blood pressure;BW,body weight;BUN,blood
urea nitrogen;Cl,chloride;Cre,creatinine;GA,glycated albumin;GAD,glutamic acid decarboxylase;c-GTP,gamma-glutamyltranspeptidase;Hb,
hemoglobin;Hct,hematocrit;HDL-chol,high-density lipoprotein cholesterol;HR,heart rate;IA-2,insulin autoimmune-2;K,potassium;LDL-chol,low-
density lipoprotein cholesterol;Na,sodium;Plt,platelet;RBC,red blood cells;RR,respiratory rate;T-Bil,totalbilirubin;T-chol,totalcholesterol;TG,
triglyceride;TP,totalprotein;UA,uric acid;WBC,white blood cells.
588 J Diabetes Invest Vol.6 No.5 September 2015 ª 2015 The Authors.Journalof Diabetes Investigation published by AASD and Wiley Publishing Asia Pty
C A S E R E P O R T
Hayami et al. http://onlinelibrary.wiley.com/journal/jdi
this patient would be preserved enough to prevent ketoacidosis
under the former treatment.
Investigation ofthe patient’s eating habits showed thatthe
totalcalories ofher daily meals was 1,860 kcal,consisting of
27.4% protein, 55.1% fat and 14.3% carbohydrate. Her est
carbohydrate intake wasjust66 g/day.Therefore,the patient
would have been chronically prone to ketosis on a strictlow-
carbohydrate diet.It was recently shown that a SGLT2 inhibitor
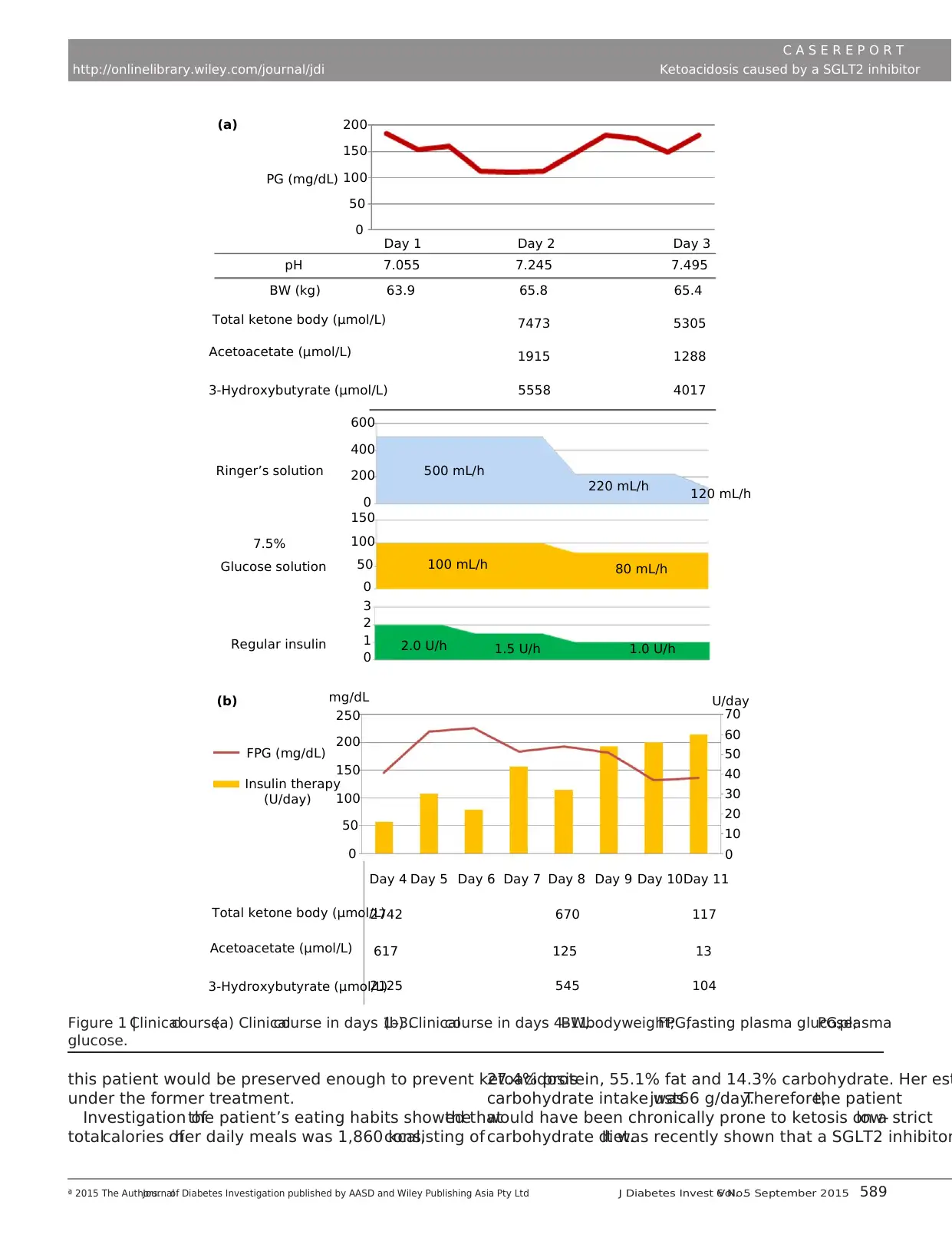
Total ketone body (μmol/L)
FPG (mg/dL)
mg/dL
250
200
150
50
0
100
Insulin therapy
(U/day)
Day 4
2742
617
2125
670
125
545
117
13
104
Day 5 Day 6 Day 7 Day 8 Day 9 Day 10Day 11
0
10
20
30
40
50
60
70
U/day
Acetoacetate (μmol/L)
3-Hydroxybutyrate (μmol/L)
200(a)
(b)
150
100
50
0
PG (mg/dL)
Day 1
pH
BW (kg) 63.9 65.8 65.4
53057473
1915 1288
40175558
600
500 mL/h
100 mL/h 80 mL/h
1.0 U/h1.5 U/h2.0 U/h
220 mL/h 120 mL/h
400
200
0
150
100
50
0
3
2
1
0
Total ketone body (μmol/L)
Acetoacetate (μmol/L)
3-Hydroxybutyrate (μmol/L)
Ringer’s solution
Glucose solution
Regular insulin
7.5%
7.055 7.245 7.495
Day 2 Day 3
Figure 1 |Clinicalcourse.(a) Clinicalcourse in days 1–3.(b) Clinicalcourse in days 4–11.BW,bodyweight;FPG,fasting plasma glucose;PG,plasma
glucose.
ª 2015 The Authors.Journalof Diabetes Investigation published by AASD and Wiley Publishing Asia Pty Ltd J Diabetes Invest Vol.6 No.5 September 2015 589
C A S E R E P O R T
http://onlinelibrary.wiley.com/journal/jdi Ketoacidosis caused by a SGLT2 inhibitor
under the former treatment.
Investigation ofthe patient’s eating habits showed thatthe
totalcalories ofher daily meals was 1,860 kcal,consisting of
27.4% protein, 55.1% fat and 14.3% carbohydrate. Her est
carbohydrate intake wasjust66 g/day.Therefore,the patient
would have been chronically prone to ketosis on a strictlow-
carbohydrate diet.It was recently shown that a SGLT2 inhibitor
Total ketone body (μmol/L)
FPG (mg/dL)
mg/dL
250
200
150
50
0
100
Insulin therapy
(U/day)
Day 4
2742
617
2125
670
125
545
117
13
104
Day 5 Day 6 Day 7 Day 8 Day 9 Day 10Day 11
0
10
20
30
40
50
60
70
U/day
Acetoacetate (μmol/L)
3-Hydroxybutyrate (μmol/L)
200(a)
(b)
150
100
50
0
PG (mg/dL)
Day 1
pH
BW (kg) 63.9 65.8 65.4
53057473
1915 1288
40175558
600
500 mL/h
100 mL/h 80 mL/h
1.0 U/h1.5 U/h2.0 U/h
220 mL/h 120 mL/h
400
200
0
150
100
50
0
3
2
1
0
Total ketone body (μmol/L)
Acetoacetate (μmol/L)
3-Hydroxybutyrate (μmol/L)
Ringer’s solution
Glucose solution
Regular insulin
7.5%
7.055 7.245 7.495
Day 2 Day 3
Figure 1 |Clinicalcourse.(a) Clinicalcourse in days 1–3.(b) Clinicalcourse in days 4–11.BW,bodyweight;FPG,fasting plasma glucose;PG,plasma
glucose.
ª 2015 The Authors.Journalof Diabetes Investigation published by AASD and Wiley Publishing Asia Pty Ltd J Diabetes Invest Vol.6 No.5 September 2015 589
C A S E R E P O R T
http://onlinelibrary.wiley.com/journal/jdi Ketoacidosis caused by a SGLT2 inhibitor

increased endogenous glucose production,serum glucagon level
and serum ketone bodies7–9
. Acceleration ofurinary glucose
excretion by a SGLT2 inhibitor,and discontinuation ofthe
former treatmentwith a sulfonylurea,metformin and dipept-
idylpeptidase-4 inhibitor that managed to maintain the insulin
action enough not to cause ketoacidosis would lead to the “no
carbohydrate available” state and the completely insulin deficient
condition, resulting in exacerbating ketosis and finally in ketoac-
idosis. In addition, dehydration would contribute to the develop-
mentof ketoacidosis.Even in patientswith enough insulin
secretory capacity,therefore,a combination of a strict low-car-
bohydrate diet and a SGLT2 inhibitor might cause ketoacidosis.
In conclusion,we for the first time described a case of severe
ketoacidosis caused by the administration of a SGLT2 inhibitor
during a low-carbohydrate diet.It is necessary to not only pay
attention to the use of a SGLT2 inhibitor in patients following
a low-carbohydrate diet,butalso to starta low-carbohydrate
dietin patients treated with a SGLT2 inhibitorbecause ofa
high risk for developing ketoacidosis.
DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
1. Fujita Y,InagakiN.Renalsodium glucose cotransporter 2
inhibitors as a noveltherapeutic approach to treatment of
type 2 diabetes:clinicaldata and mechanism of action.
J Diabetes Investig 2014;5:265–275.
2. Bravata DM,Sanders L,Huang J,et al.Efficacy and safety of
low-carbohydrate diets:a systematic review.JAMA 2003;289:
1837–1850.
3. Chen TY,Smith W,Rosenstock JL,et al.A life-threatening
complication of Atkins diet.Lancet 2006;367:958.
4. Shah P,Isley WL.Ketoacidosis during a low-carbohydrate
diet.N EnglJ Med 2006;354:97–98.
5. KitabchiAE,Umpierrez GE,Murphy MB,et al.Management
of hyperglycemic crises in patients with diabetes.Diabetes
Care 2001;24:131–153.
6. Tanaka K,Moriya T,KanamoriA,et al.Analysis and a
long-term follow up of ketosis-onset Japanese
NIDDM patients.Diabetes Res Clin Pract 1999;44:
137–146.
7. MerovciA,Solis-Herrera C,Daniele G,et al.Dapagliflozin
improves muscle insulin sensitivity but enhances
endogenous glucose production.J Clin Invest 2014;124:
509–514.
8. Seino Y.Luseogliflozin for the treatment of type 2 diabete
Expert Opin Pharmacother 2014;15:2741–2749.
9. Mudaliar S,Henry RR,Boden G,et al.Changes in insulin
sensitivity and insulin secretion with the sodium glucose
cotransporter 2 inhibitor dapagliflozin.Diabetes TechnolTher
2014;16:137–144.
590 J Diabetes Invest Vol.6 No.5 September 2015 ª 2015 The Authors.Journalof Diabetes Investigation published by AASD and Wiley Publishing Asia Pty
C A S E R E P O R T
Hayami et al. http://onlinelibrary.wiley.com/journal/jdi
and serum ketone bodies7–9
. Acceleration ofurinary glucose
excretion by a SGLT2 inhibitor,and discontinuation ofthe
former treatmentwith a sulfonylurea,metformin and dipept-
idylpeptidase-4 inhibitor that managed to maintain the insulin
action enough not to cause ketoacidosis would lead to the “no
carbohydrate available” state and the completely insulin deficient
condition, resulting in exacerbating ketosis and finally in ketoac-
idosis. In addition, dehydration would contribute to the develop-
mentof ketoacidosis.Even in patientswith enough insulin
secretory capacity,therefore,a combination of a strict low-car-
bohydrate diet and a SGLT2 inhibitor might cause ketoacidosis.
In conclusion,we for the first time described a case of severe
ketoacidosis caused by the administration of a SGLT2 inhibitor
during a low-carbohydrate diet.It is necessary to not only pay
attention to the use of a SGLT2 inhibitor in patients following
a low-carbohydrate diet,butalso to starta low-carbohydrate
dietin patients treated with a SGLT2 inhibitorbecause ofa
high risk for developing ketoacidosis.
DISCLOSURE
The authors declare no conflict of interest.
REFERENCES
1. Fujita Y,InagakiN.Renalsodium glucose cotransporter 2
inhibitors as a noveltherapeutic approach to treatment of
type 2 diabetes:clinicaldata and mechanism of action.
J Diabetes Investig 2014;5:265–275.
2. Bravata DM,Sanders L,Huang J,et al.Efficacy and safety of
low-carbohydrate diets:a systematic review.JAMA 2003;289:
1837–1850.
3. Chen TY,Smith W,Rosenstock JL,et al.A life-threatening
complication of Atkins diet.Lancet 2006;367:958.
4. Shah P,Isley WL.Ketoacidosis during a low-carbohydrate
diet.N EnglJ Med 2006;354:97–98.
5. KitabchiAE,Umpierrez GE,Murphy MB,et al.Management
of hyperglycemic crises in patients with diabetes.Diabetes
Care 2001;24:131–153.
6. Tanaka K,Moriya T,KanamoriA,et al.Analysis and a
long-term follow up of ketosis-onset Japanese
NIDDM patients.Diabetes Res Clin Pract 1999;44:
137–146.
7. MerovciA,Solis-Herrera C,Daniele G,et al.Dapagliflozin
improves muscle insulin sensitivity but enhances
endogenous glucose production.J Clin Invest 2014;124:
509–514.
8. Seino Y.Luseogliflozin for the treatment of type 2 diabete
Expert Opin Pharmacother 2014;15:2741–2749.
9. Mudaliar S,Henry RR,Boden G,et al.Changes in insulin
sensitivity and insulin secretion with the sodium glucose
cotransporter 2 inhibitor dapagliflozin.Diabetes TechnolTher
2014;16:137–144.
590 J Diabetes Invest Vol.6 No.5 September 2015 ª 2015 The Authors.Journalof Diabetes Investigation published by AASD and Wiley Publishing Asia Pty
C A S E R E P O R T
Hayami et al. http://onlinelibrary.wiley.com/journal/jdi
1 out of 4
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.