An Analysis of Kirchhoff's Law of Thermal Radiation in Physics
VerifiedAdded on 2023/06/04
|11
|1000
|428
Report
AI Summary
This report provides a comprehensive overview of Kirchhoff's Law of Thermal Radiation, beginning with its historical context and the contributions of Gustav Kirchhoff. It delves into the core principles, including the relationship between emissive power and absorption, and the concept of blackbody radiation. The report explains how the law applies to thermodynamic equilibrium and the transfer of heat through radiation, particularly in a vacuum like space. It discusses the behavior of materials at different wavelengths and temperatures, and how the law is essential for understanding energy conservation and the adsorption of incident radiation. Additionally, it references Planck's contribution, and the principles that govern thermal radiation, including the first law of thermodynamics, the radiation of blackbodies, and the role of temperature changes. The report concludes with a discussion of black-body radiation and the impact of temperature on the color of the body.

Name of the Student
Name of the professor
State/City
Date/Month/Year
Name of the professor
State/City
Date/Month/Year
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

History
The Kirchhoff law eventually named after the author Gustav Kirchhoff
who was a German physicist born on March 12, 1824, in Konigsberg,
Prussia. Before the recognition of Kirchhoff law, it had been
experimentally established that a good absorber is a good emitter and a
poor absorber is a poor emitter and this is the reason why emergency
thermal blankets are based on reflective metallic coatings thus losing
little heat through radiation. Kirchhoff great insight was to recognize the
universality and uniqueness of the function that describes the blackbody
emissive power but he did not know the precise form or character of that
universal function. Sir James Jeans and Lord Rayleigh 1900-1905 made
an attempt to describe it in classical terms resulting in Rayleigh-Jeans
law.
The Kirchhoff law eventually named after the author Gustav Kirchhoff
who was a German physicist born on March 12, 1824, in Konigsberg,
Prussia. Before the recognition of Kirchhoff law, it had been
experimentally established that a good absorber is a good emitter and a
poor absorber is a poor emitter and this is the reason why emergency
thermal blankets are based on reflective metallic coatings thus losing
little heat through radiation. Kirchhoff great insight was to recognize the
universality and uniqueness of the function that describes the blackbody
emissive power but he did not know the precise form or character of that
universal function. Sir James Jeans and Lord Rayleigh 1900-1905 made
an attempt to describe it in classical terms resulting in Rayleigh-Jeans
law.

Kirchhoff’s law of thermal radiation refers to wavelength-specific
radiative emission as well as absorption by a material body in
thermodynamic equilibrium including radiative exchange equilibrium. A
perfect black body in thermodynamics equilibrium absorbs all light that
strikes it and radiates energy according to a unique law of radiative
emissive power for temperature. Kirchhoff’s law simply states that for a
body of any arbitrary material emitting and absorbing thermal
electromagnetic radiation at every wavelength in thermodynamic
equilibrium, the ratio of its emissive power to its dimensionless
coefficient of absorption is equal to a universal function only of radiative
wavelength and temperature.
radiative emission as well as absorption by a material body in
thermodynamic equilibrium including radiative exchange equilibrium. A
perfect black body in thermodynamics equilibrium absorbs all light that
strikes it and radiates energy according to a unique law of radiative
emissive power for temperature. Kirchhoff’s law simply states that for a
body of any arbitrary material emitting and absorbing thermal
electromagnetic radiation at every wavelength in thermodynamic
equilibrium, the ratio of its emissive power to its dimensionless
coefficient of absorption is equal to a universal function only of radiative
wavelength and temperature.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Thermodynamics equilibrium with a certain amount of energy in a blackbody enclosure
contains electromagnetic radiation and this photon gas will have a Planck distribution of
energies and at each frequency. (Miller 2010, 432). A new second law of thermodynamics
where it is advocating that the radiation from the colder body never reaches the hotter
body claiming that the radiation was canceled out and the measurements of radiation
reaching the hotter body were fraudulent.
In space which is a vacuum, the experiments are simplified since there is no convection or
conduction of heat and this leaves radiation as the mechanism for the transfer of heat. In
radiation, the shape of the temperature curve is probably not surprising since the
temperature will decrease because the body is radiating heat with no incoming radiation to
balance out the loss, and there is no internal source of heat. When the temperature rate
gets lower, less heat is radiated every second.
contains electromagnetic radiation and this photon gas will have a Planck distribution of
energies and at each frequency. (Miller 2010, 432). A new second law of thermodynamics
where it is advocating that the radiation from the colder body never reaches the hotter
body claiming that the radiation was canceled out and the measurements of radiation
reaching the hotter body were fraudulent.
In space which is a vacuum, the experiments are simplified since there is no convection or
conduction of heat and this leaves radiation as the mechanism for the transfer of heat. In
radiation, the shape of the temperature curve is probably not surprising since the
temperature will decrease because the body is radiating heat with no incoming radiation to
balance out the loss, and there is no internal source of heat. When the temperature rate
gets lower, less heat is radiated every second.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

The materials which are black in particular wavelength bands and such
materials do not survive very high temperatures and bodies that are
opaque to thermal radiation that falls on them. The interface is not a
material body and can neither absorb or emit and the opaque body which
is considered to have a material interior that absorbs all and scatters or
transmits none of the radiation that reaches it through refraction at the
interface. The wall of the cavity can be made of opaque materials that
absorb significant amounts of radiation at all wavelengths. The effective
range of absorbing wavelength can be extended by the use of patches of
several differently absorbing materials. According to Planck, it was noted
that Kirchhoff’s do not occur in physical reality and Kirchhoff perfect
bodies absorb all the radiation that falls on them.
materials do not survive very high temperatures and bodies that are
opaque to thermal radiation that falls on them. The interface is not a
material body and can neither absorb or emit and the opaque body which
is considered to have a material interior that absorbs all and scatters or
transmits none of the radiation that reaches it through refraction at the
interface. The wall of the cavity can be made of opaque materials that
absorb significant amounts of radiation at all wavelengths. The effective
range of absorbing wavelength can be extended by the use of patches of
several differently absorbing materials. According to Planck, it was noted
that Kirchhoff’s do not occur in physical reality and Kirchhoff perfect
bodies absorb all the radiation that falls on them.

The opaque body is considered to have a material interior that absorbs all
and scatters or transmits none of the radiation that reaches it through
refraction at the interface. The material having the opaque body is black to
radiation that reaches it while the interface and the interior do not show
perfect blackness. A theoretical model considered by Plank consists of a
cavity with perfectly reflecting walls, initially with no material contents into
which small piece of carbon are put and without the small piece of carbon,
there is no way non-equilibrium radiation initially in the cavity to drift towards
thermodynamic equilibrium. Thermal radiation is simply an electromagnetic
radiation generated by the thermal motion of charged particles in matter. All
matter with a temperature greater than absolute zero emits thermal radiation
and the movement of particles result into charge-acceleration or dipole
oscillation which produces electromagnetic radiation.
and scatters or transmits none of the radiation that reaches it through
refraction at the interface. The material having the opaque body is black to
radiation that reaches it while the interface and the interior do not show
perfect blackness. A theoretical model considered by Plank consists of a
cavity with perfectly reflecting walls, initially with no material contents into
which small piece of carbon are put and without the small piece of carbon,
there is no way non-equilibrium radiation initially in the cavity to drift towards
thermodynamic equilibrium. Thermal radiation is simply an electromagnetic
radiation generated by the thermal motion of charged particles in matter. All
matter with a temperature greater than absolute zero emits thermal radiation
and the movement of particles result into charge-acceleration or dipole
oscillation which produces electromagnetic radiation.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Thermal radiation is a byproduct of the collision arising from vibrational
motions of atoms and thermal photons are emitted by the electrons of the
atoms as a result of collusion. Black-body radiation diffuses thermal
energy throughout a substance as the photons are absorbed by
neighboring atoms, transferring momentum in the process. The condition
of thermodynamic equilibrium is necessary because the equality of
emissivity and absorptivity often does not hold when the material of the
body is not in thermodynamic equilibrium. Radiation can pass from one
system to the other and a cavity with walls that are rigid and opaque and,
are reflected by any wavelength to be brought to any connection through
an optical filter with the blackbody enclosure both at the same
temperature. A black body emits a temperature dependent spectrum of
light and this thermal radiation from a black body is termed black-body
radiation.
motions of atoms and thermal photons are emitted by the electrons of the
atoms as a result of collusion. Black-body radiation diffuses thermal
energy throughout a substance as the photons are absorbed by
neighboring atoms, transferring momentum in the process. The condition
of thermodynamic equilibrium is necessary because the equality of
emissivity and absorptivity often does not hold when the material of the
body is not in thermodynamic equilibrium. Radiation can pass from one
system to the other and a cavity with walls that are rigid and opaque and,
are reflected by any wavelength to be brought to any connection through
an optical filter with the blackbody enclosure both at the same
temperature. A black body emits a temperature dependent spectrum of
light and this thermal radiation from a black body is termed black-body
radiation.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
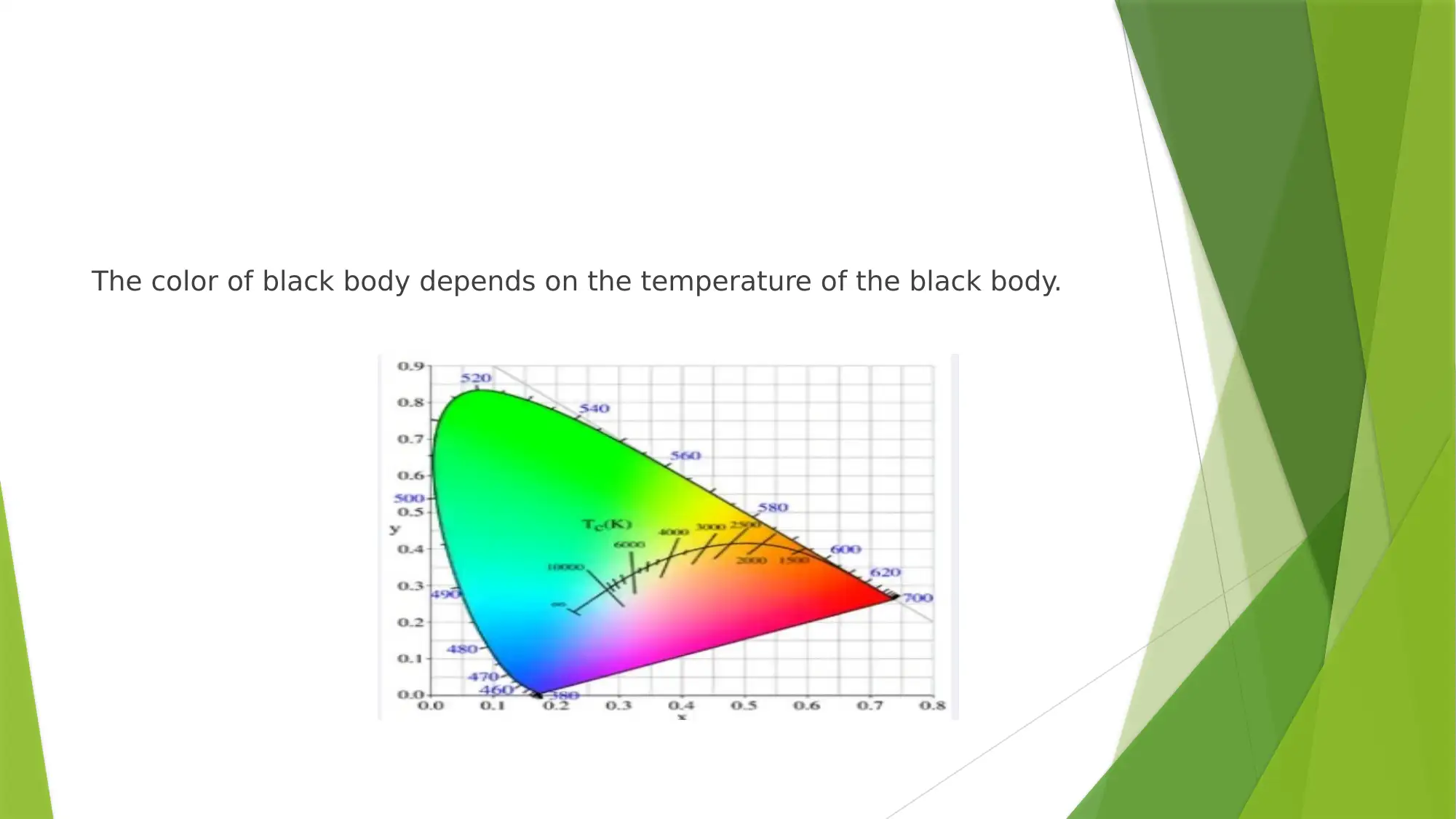
The color of black body depends on the temperature of the black body.
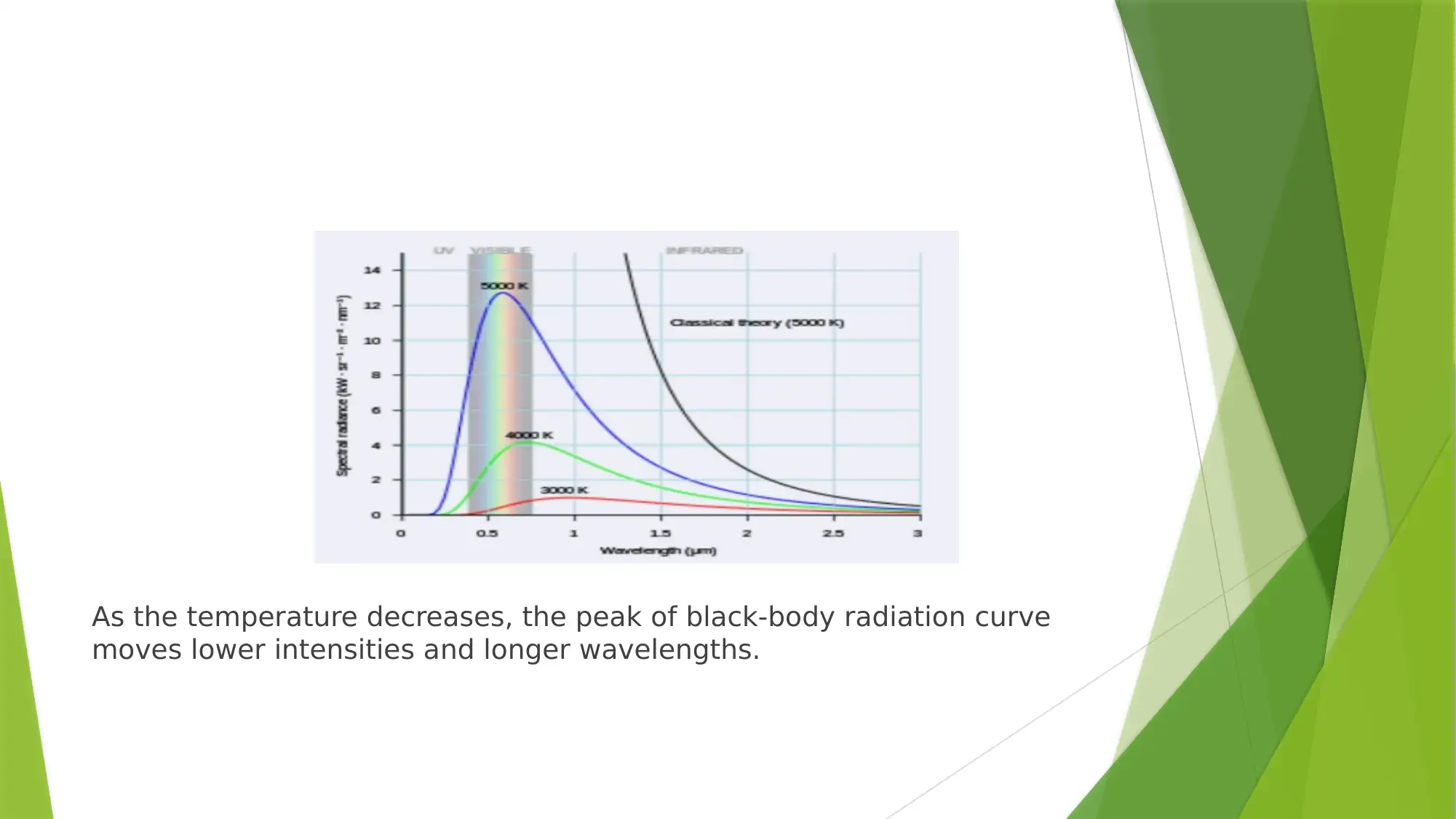
As the temperature decreases, the peak of black-body radiation curve
moves lower intensities and longer wavelengths.
moves lower intensities and longer wavelengths.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

The principles involved in thermal radiation include;
Energy conservation which is also called the first law of
thermodynamics.
The radiation of blackbodies.
The adsorption of incident radiation from one body to another.
The temperature changes according to dT=Q/mc.
Energy conservation which is also called the first law of
thermodynamics.
The radiation of blackbodies.
The adsorption of incident radiation from one body to another.
The temperature changes according to dT=Q/mc.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Bibliography
Miller, Frederic. 2010. Kirchhoff law of thermal radiation. Adventure Works Press.
Planck, Max. 2013. The theory of heat radiation. Scholastic.
Riedl, Max. 2009. Optical design fundamentals for infrared systems. OLMA Media Group.
Miller, Frederic. 2010. Kirchhoff law of thermal radiation. Adventure Works Press.
Planck, Max. 2013. The theory of heat radiation. Scholastic.
Riedl, Max. 2009. Optical design fundamentals for infrared systems. OLMA Media Group.
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


