Laboratory Report: Microbial Analysis of Food Products and Samples
VerifiedAdded on 2020/05/28
|22
|4653
|250
Report
AI Summary
This laboratory report details two experiments focused on microbial analysis of food products. The first experiment investigates the microflora of raw fish samples, utilizing nutrient agar, nutrient agar with salt, and PSB agar to assess bacterial growth. The experiment employs serial dilutions and plate counting techniques to identify and quantify various microorganisms, including Micrococcus, Pseudomonas, Bacillus, Staphylococcus, and Escherichia coli. The results, presented in tables, show the percentage of bacterial growth across different fish types (oily fish, white fish, and prawns) and dilutions. The second experiment analyzes three different sandwich samples, employing similar techniques and media, including TSA, VRBGA, Baird Parker Medium, Kanamycin Aesculin Azide Agar Base, Malt Extract Agar, and XLD Medium, to determine the presence of total viable count, coliforms, Escherichia coli, Staphylococcus aureus, Streptococcocus feacalis, and yeast and molds. The report discusses the impact of heat on microbial reproduction, disinfection methods, and the importance of sanitation in food preparation, packaging, and consumption to prevent foodborne infections and spoilage. The report includes detailed methods, results tables, and a discussion of the findings, concluding with the successful fulfillment of the experiments' aims and objectives.

Laboratory Report Diary1
Laboratory Report Diary
By Name
Course lab report
Instructor
Institution
Location
Date
Laboratory Report Diary
By Name
Course lab report
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Laboratory Report Diary2
Laboratory Report Diary
Experiment 1: Microbial Analysis of Raw Fish – In Duplicate
Aim
To examine the micro flora of fish samples
Introduction
Fish consumption has been going on for so long in various cultures around the world.
There are several bacteria associated with the various fish types and a lot of research has been
going on (Asakura et al, 2014). Understanding the microbial of fish is very critical in improving
the sanitation levels in growing fish, preparation, packaging and consuming of fish. We usually
find fish in cold environments that may be polluted by soil particles or even faeces (Clarke et al,
2018). It is very obvious for someone to expect finding psychotropic organisms as well as
halophiles and coliforms such as the pseudomonas.
Microbiological analysis involves the identification of total viable aerobic count by
standard plate count method and probable number method was used for enumeration of fecal and
total coliform. Salmonella spp. and Vibrio cholerae are some of the most common bacteria
associated with fish-related infection. Fish is among the most preferred foods due to the high
nutritional value but contamination during handling, transport and processing is a big challenge.
The higher nutrition value of fish has made them a key export earning commodity in
several countries around the world. The various types of fish have a high protein content, low
amount of carbohydrates and zero unhealthy fats. At the various stages of handling,
transportation and processing the fish may be contaminated by pathogens that cause foodborne
infections. The contamination may be caused by the raw material, processing tools or leakages.
We used nutrient agar and PSB agar as a medium to support the growth and development
of a broad range of microbial organisms. The nutrient agar is constituted by peptone, beef or
yeast extracts, sodium chloride and distilled water. The purpose of the peptone in the medium is
to provide organic nitrogen while the water-soluble content of yeast or beef extracts provides
carbohydrates, nitrogen, vitamins and salts. The 0.5 % sodium in the medium contributes to the
mixture to have similar proportions to those in the cytoplasm of majority of the microbial
organisms. The distilled water provides a transport medium for various substances in the agar.
The pH of the medium is usually adjusted and the nutrient agar is sterilized before use.
Laboratory Report Diary
Experiment 1: Microbial Analysis of Raw Fish – In Duplicate
Aim
To examine the micro flora of fish samples
Introduction
Fish consumption has been going on for so long in various cultures around the world.
There are several bacteria associated with the various fish types and a lot of research has been
going on (Asakura et al, 2014). Understanding the microbial of fish is very critical in improving
the sanitation levels in growing fish, preparation, packaging and consuming of fish. We usually
find fish in cold environments that may be polluted by soil particles or even faeces (Clarke et al,
2018). It is very obvious for someone to expect finding psychotropic organisms as well as
halophiles and coliforms such as the pseudomonas.
Microbiological analysis involves the identification of total viable aerobic count by
standard plate count method and probable number method was used for enumeration of fecal and
total coliform. Salmonella spp. and Vibrio cholerae are some of the most common bacteria
associated with fish-related infection. Fish is among the most preferred foods due to the high
nutritional value but contamination during handling, transport and processing is a big challenge.
The higher nutrition value of fish has made them a key export earning commodity in
several countries around the world. The various types of fish have a high protein content, low
amount of carbohydrates and zero unhealthy fats. At the various stages of handling,
transportation and processing the fish may be contaminated by pathogens that cause foodborne
infections. The contamination may be caused by the raw material, processing tools or leakages.
We used nutrient agar and PSB agar as a medium to support the growth and development
of a broad range of microbial organisms. The nutrient agar is constituted by peptone, beef or
yeast extracts, sodium chloride and distilled water. The purpose of the peptone in the medium is
to provide organic nitrogen while the water-soluble content of yeast or beef extracts provides
carbohydrates, nitrogen, vitamins and salts. The 0.5 % sodium in the medium contributes to the
mixture to have similar proportions to those in the cytoplasm of majority of the microbial
organisms. The distilled water provides a transport medium for various substances in the agar.
The pH of the medium is usually adjusted and the nutrient agar is sterilized before use.

Laboratory Report Diary3
Provided Samples
Whole fish-oily 1 sample
Sample of white fish – 2 samples
Raw king prawn- 2samples
Method
1. Place the stomacher bag onto the balance and tare the weight.
2. Sterilise the scalpel and forceps by dipping them in methylated spirits and flaming carefully
to burn off the excess.
3. Weigh accurately, using an aseptic technique, 10 grams of food into the stomacher bag. Do
not handle the food, bag or implements.
4. Aseptically add 90 mls of MRD to the stomacher bag.
5. Place in the stomacher and stomach for 30 to 60 seconds.
6. Sit the bag in a beaker for use.
This is now a 1 in 10 (10-1) dilution of the sample- repeat for all three sandwiches.
Proceed to make two further dilutions of each using the standard technique. Dilutions will be 10-2
and 10-3. Make these with 1ml of the 10-1 and 9ml of MRD. Make sure that all the bottles are
labeled correctly.
Label 12 TSA plates with sandwich name, dilution, group initials, temperature of incubation have
2 plates for each. Repeat this for Baird Parker, MEA and KAA. Make up the XLD as per the
instructions on packaging and pour 12 plates.
Each group will be allocated a sandwich type and dilution pour 8 plates of VRBGA, 4 plates for
37°C and 4 plates at 44°C.
Once you have labelled and poured plates pipette sample (0.5ml) onto plates in duplicate and
incubate at recommended temperatures.
Results
Provided Samples
Whole fish-oily 1 sample
Sample of white fish – 2 samples
Raw king prawn- 2samples
Method
1. Place the stomacher bag onto the balance and tare the weight.
2. Sterilise the scalpel and forceps by dipping them in methylated spirits and flaming carefully
to burn off the excess.
3. Weigh accurately, using an aseptic technique, 10 grams of food into the stomacher bag. Do
not handle the food, bag or implements.
4. Aseptically add 90 mls of MRD to the stomacher bag.
5. Place in the stomacher and stomach for 30 to 60 seconds.
6. Sit the bag in a beaker for use.
This is now a 1 in 10 (10-1) dilution of the sample- repeat for all three sandwiches.
Proceed to make two further dilutions of each using the standard technique. Dilutions will be 10-2
and 10-3. Make these with 1ml of the 10-1 and 9ml of MRD. Make sure that all the bottles are
labeled correctly.
Label 12 TSA plates with sandwich name, dilution, group initials, temperature of incubation have
2 plates for each. Repeat this for Baird Parker, MEA and KAA. Make up the XLD as per the
instructions on packaging and pour 12 plates.
Each group will be allocated a sandwich type and dilution pour 8 plates of VRBGA, 4 plates for
37°C and 4 plates at 44°C.
Once you have labelled and poured plates pipette sample (0.5ml) onto plates in duplicate and
incubate at recommended temperatures.
Results
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Laboratory Report Diary4
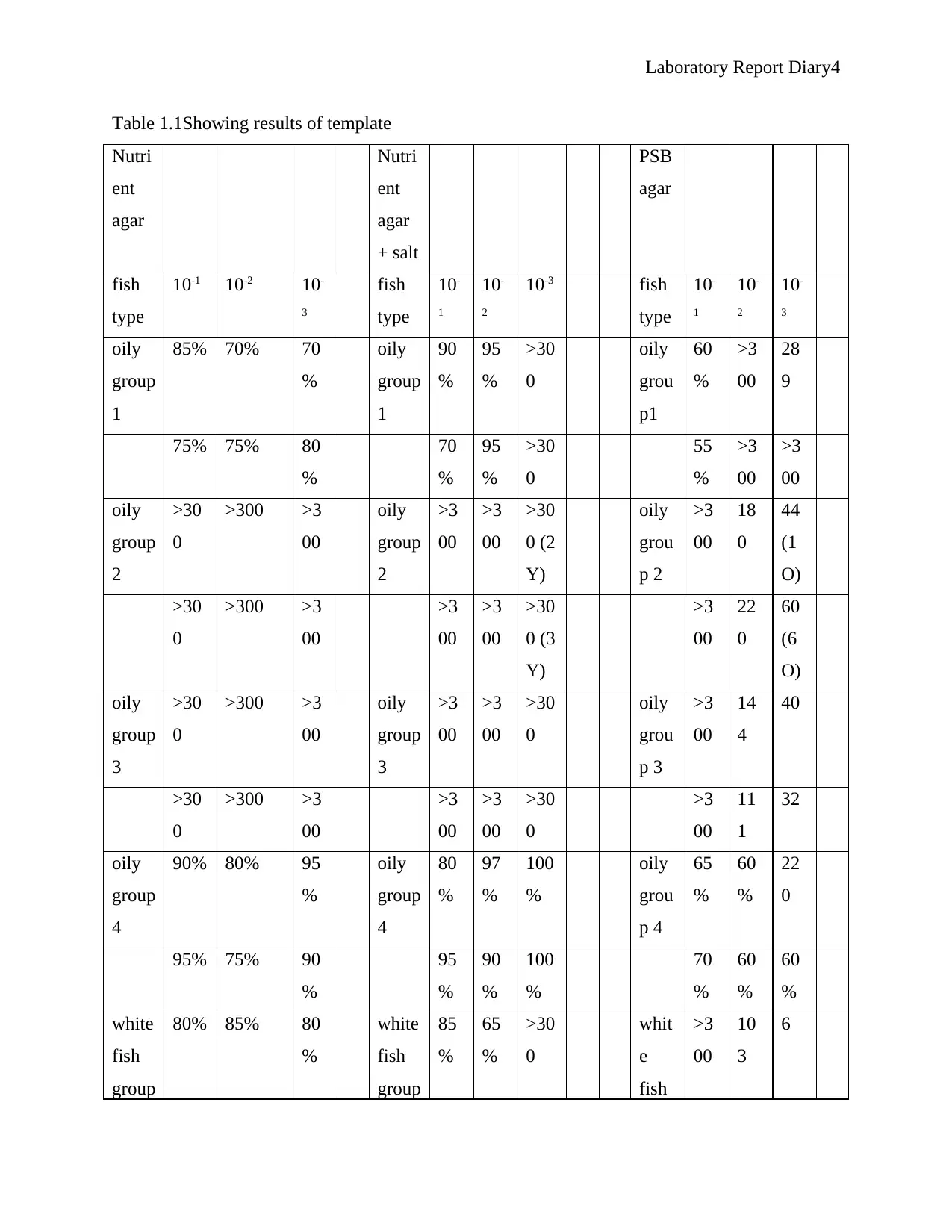
Table 1.1Showing results of template
Nutri
ent
agar
Nutri
ent
agar
+ salt
PSB
agar
fish
type
10-1 10-2 10-
3
fish
type
10-
1
10-
2
10-3 fish
type
10-
1
10-
2
10-
3
oily
group
1
85% 70% 70
%
oily
group
1
90
%
95
%
>30
0
oily
grou
p1
60
%
>3
00
28
9
75% 75% 80
%
70
%
95
%
>30
0
55
%
>3
00
>3
00
oily
group
2
>30
0
>300 >3
00
oily
group
2
>3
00
>3
00
>30
0 (2
Y)
oily
grou
p 2
>3
00
18
0
44
(1
O)
>30
0
>300 >3
00
>3
00
>3
00
>30
0 (3
Y)
>3
00
22
0
60
(6
O)
oily
group
3
>30
0
>300 >3
00
oily
group
3
>3
00
>3
00
>30
0
oily
grou
p 3
>3
00
14
4
40
>30
0
>300 >3
00
>3
00
>3
00
>30
0
>3
00
11
1
32
oily
group
4
90% 80% 95
%
oily
group
4
80
%
97
%
100
%
oily
grou
p 4
65
%
60
%
22
0
95% 75% 90
%
95
%
90
%
100
%
70
%
60
%
60
%
white
fish
group
80% 85% 80
%
white
fish
group
85
%
65
%
>30
0
whit
e
fish
>3
00
10
3
6
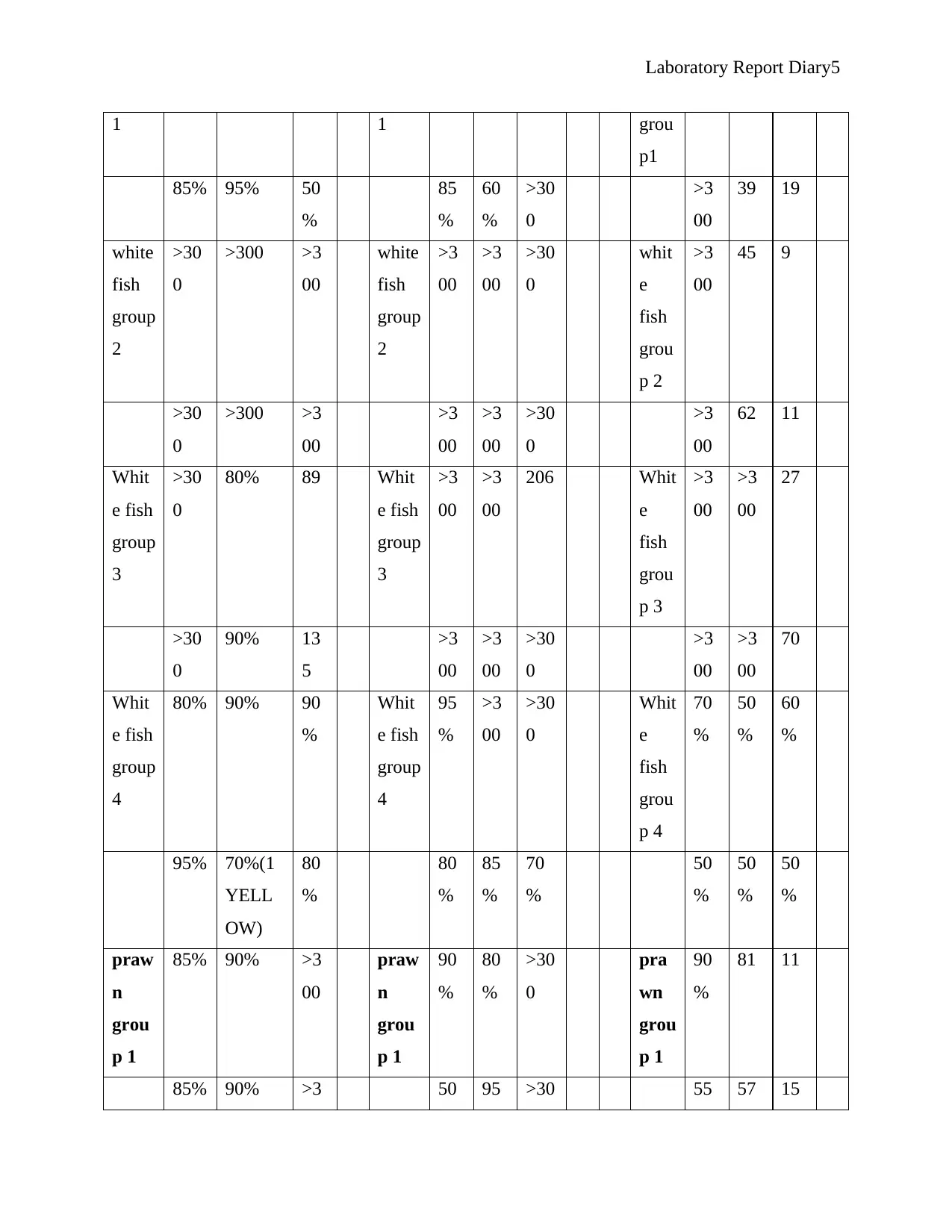
Table 1.1Showing results of template
Nutri
ent
agar
Nutri
ent
agar
+ salt
PSB
agar
fish
type
10-1 10-2 10-
3
fish
type
10-
1
10-
2
10-3 fish
type
10-
1
10-
2
10-
3
oily
group
1
85% 70% 70
%
oily
group
1
90
%
95
%
>30
0
oily
grou
p1
60
%
>3
00
28
9
75% 75% 80
%
70
%
95
%
>30
0
55
%
>3
00
>3
00
oily
group
2
>30
0
>300 >3
00
oily
group
2
>3
00
>3
00
>30
0 (2
Y)
oily
grou
p 2
>3
00
18
0
44
(1
O)
>30
0
>300 >3
00
>3
00
>3
00
>30
0 (3
Y)
>3
00
22
0
60
(6
O)
oily
group
3
>30
0
>300 >3
00
oily
group
3
>3
00
>3
00
>30
0
oily
grou
p 3
>3
00
14
4
40
>30
0
>300 >3
00
>3
00
>3
00
>30
0
>3
00
11
1
32
oily
group
4
90% 80% 95
%
oily
group
4
80
%
97
%
100
%
oily
grou
p 4
65
%
60
%
22
0
95% 75% 90
%
95
%
90
%
100
%
70
%
60
%
60
%
white
fish
group
80% 85% 80
%
white
fish
group
85
%
65
%
>30
0
whit
e
fish
>3
00
10
3
6
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Laboratory Report Diary5
1 1 grou
p1
85% 95% 50
%
85
%
60
%
>30
0
>3
00
39 19
white
fish
group
2
>30
0
>300 >3
00
white
fish
group
2
>3
00
>3
00
>30
0
whit
e
fish
grou
p 2
>3
00
45 9
>30
0
>300 >3
00
>3
00
>3
00
>30
0
>3
00
62 11
Whit
e fish
group
3
>30
0
80% 89 Whit
e fish
group
3
>3
00
>3
00
206 Whit
e
fish
grou
p 3
>3
00
>3
00
27
>30
0
90% 13
5
>3
00
>3
00
>30
0
>3
00
>3
00
70
Whit
e fish
group
4
80% 90% 90
%
Whit
e fish
group
4
95
%
>3
00
>30
0
Whit
e
fish
grou
p 4
70
%
50
%
60
%
95% 70%(1
YELL
OW)
80
%
80
%
85
%
70
%
50
%
50
%
50
%
praw
n
grou
p 1
85% 90% >3
00
praw
n
grou
p 1
90
%
80
%
>30
0
pra
wn
grou
p 1
90
%
81 11
85% 90% >3 50 95 >30 55 57 15
1 1 grou
p1
85% 95% 50
%
85
%
60
%
>30
0
>3
00
39 19
white
fish
group
2
>30
0
>300 >3
00
white
fish
group
2
>3
00
>3
00
>30
0
whit
e
fish
grou
p 2
>3
00
45 9
>30
0
>300 >3
00
>3
00
>3
00
>30
0
>3
00
62 11
Whit
e fish
group
3
>30
0
80% 89 Whit
e fish
group
3
>3
00
>3
00
206 Whit
e
fish
grou
p 3
>3
00
>3
00
27
>30
0
90% 13
5
>3
00
>3
00
>30
0
>3
00
>3
00
70
Whit
e fish
group
4
80% 90% 90
%
Whit
e fish
group
4
95
%
>3
00
>30
0
Whit
e
fish
grou
p 4
70
%
50
%
60
%
95% 70%(1
YELL
OW)
80
%
80
%
85
%
70
%
50
%
50
%
50
%
praw
n
grou
p 1
85% 90% >3
00
praw
n
grou
p 1
90
%
80
%
>30
0
pra
wn
grou
p 1
90
%
81 11
85% 90% >3 50 95 >30 55 57 15
Laboratory Report Diary6
00 % % 0 %
praw
n
group
2
>30
0
>300 >3
00
( y-
4,p
-6)
praw
n
group
2
>3
00
>3
00
>30
0
praw
n
grou
p 2
24
0
60 24,
all
p
>30
0
>300 >3
00
>3
00
>3
00
252 16
0
30 14,
all
p
praw
n
group
3
95% 95% 95
%
praw
n
group
3
95
%
>3
00
>30
0
praw
n
grou
p 3
>3
00
13
0
10
80% 95% 95
%
95
%
>3
00
>30
0
>3
00
11
8
23
praw
n
group
4
>30
0
>300 >3
00
praw
n
group
4
>3
00
>3
00
>300(28
YELLO
W)
praw
n
grou
p 4
15
5
81 29
>30
0
>300 >3
00
>3
00
>3
00
>300(2
YELLO
W)
>3
00
83 10
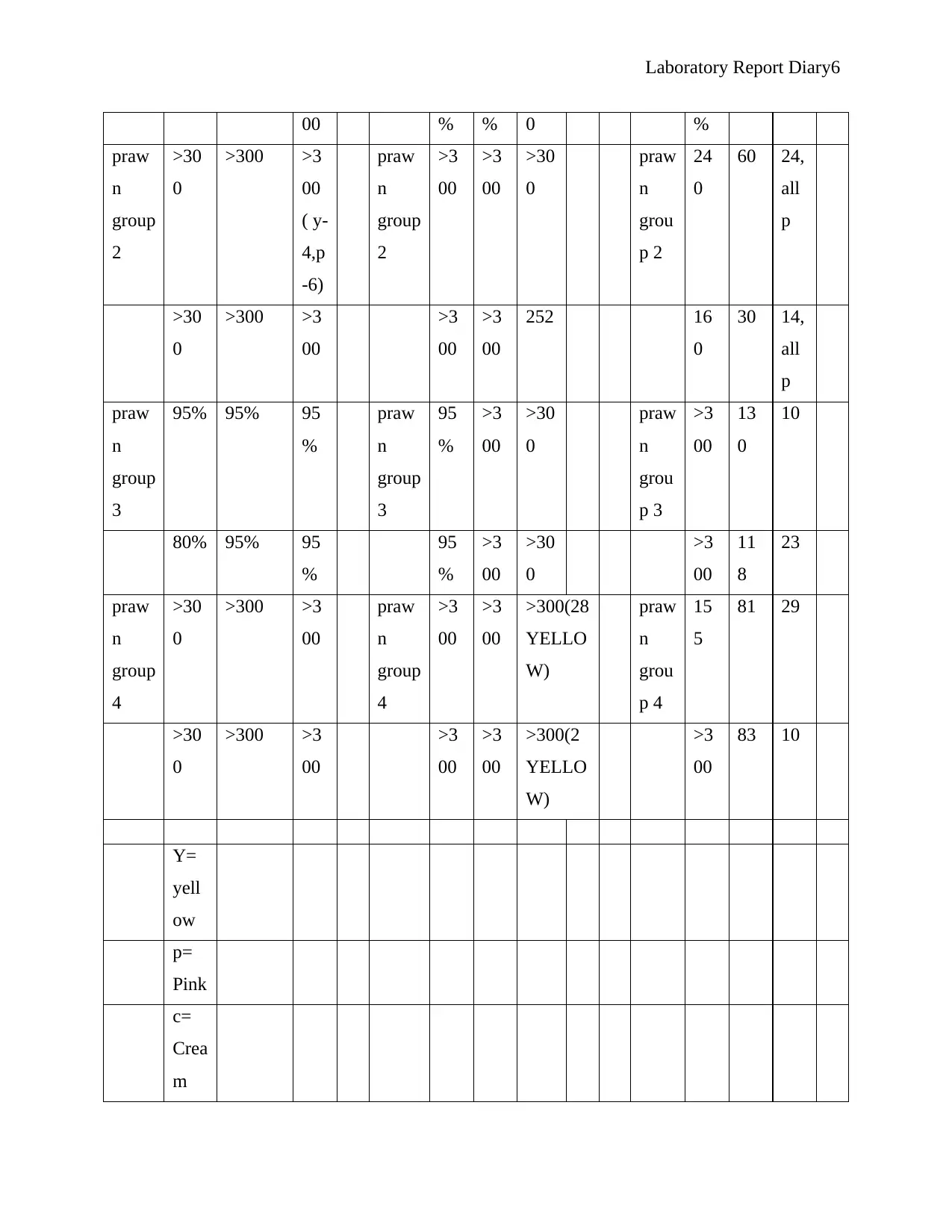
Y=
yell
ow
p=
Pink
c=
Crea
m
00 % % 0 %
praw
n
group
2
>30
0
>300 >3
00
( y-
4,p
-6)
praw
n
group
2
>3
00
>3
00
>30
0
praw
n
grou
p 2
24
0
60 24,
all
p
>30
0
>300 >3
00
>3
00
>3
00
252 16
0
30 14,
all
p
praw
n
group
3
95% 95% 95
%
praw
n
group
3
95
%
>3
00
>30
0
praw
n
grou
p 3
>3
00
13
0
10
80% 95% 95
%
95
%
>3
00
>30
0
>3
00
11
8
23
praw
n
group
4
>30
0
>300 >3
00
praw
n
group
4
>3
00
>3
00
>300(28
YELLO
W)
praw
n
grou
p 4
15
5
81 29
>30
0
>300 >3
00
>3
00
>3
00
>300(2
YELLO
W)
>3
00
83 10
Y=
yell
ow
p=
Pink
c=
Crea
m
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Laboratory Report Diary7
O = orange
Purple represent positive while pink represent negative results for the presence of the various
bacteria.
1. Micrococcus aurentiaca- purple
2. Micrococcus roseus – purple
3. Pseudomonas fluorescenes – pink
4. Bacillus subtilis – purple
5. Bacillus megaterium – purple
6. Staphelocious epidermidis – purple
7. Escherichia coli – purple
8. Micrococcus luteus- pink
Discussion
Heat has an effect on reproduction. Varying the temperatures specifically increasing the
temperature stopped reproduction. This is because heat causes denaturing of DWA and they are
killed off. The reaction is catalyzed by enzymes at moderate temperatures but for temperatures
above 40 0C the enzymes which are protein in nature get denatured and reaction drops
drastically. Moist of the dry plates react more effective. Steam burn worse than moist then dry
and this is because steam penetrates more making it more effective (Liguori et al, 2016).
Thermal death occurs at different temperature rates and affects the texture of the as well as the
flavor and this has serious cost implications. Heat resistance results to medium heat effects. At
acid pH thermal death occurs more rapidly. Dormant spores are special endospores that are
specifically meant for survival during environment stress.
Micrococcus aurentiaca, Micrococcus roseus, Bacillus subtilis, Bacillus megaterium,
Staphelocious epidermidis, Escherichia coli are the specific pathogens we identified in the fish
plates. Micrococcus luteus, Pseudomonas fluorescenes were both missing in the white fish, oily
fish and the prawn. To make the fish food safe for consumption disinfection should be done
(Bienart et al, 2018). Disinfection directly targets pathogens. Although it may not eliminate all
the pathogens, it could bring down the pathogens to a safe level. Disinfectants are used for
O = orange
Purple represent positive while pink represent negative results for the presence of the various
bacteria.
1. Micrococcus aurentiaca- purple
2. Micrococcus roseus – purple
3. Pseudomonas fluorescenes – pink
4. Bacillus subtilis – purple
5. Bacillus megaterium – purple
6. Staphelocious epidermidis – purple
7. Escherichia coli – purple
8. Micrococcus luteus- pink
Discussion
Heat has an effect on reproduction. Varying the temperatures specifically increasing the
temperature stopped reproduction. This is because heat causes denaturing of DWA and they are
killed off. The reaction is catalyzed by enzymes at moderate temperatures but for temperatures
above 40 0C the enzymes which are protein in nature get denatured and reaction drops
drastically. Moist of the dry plates react more effective. Steam burn worse than moist then dry
and this is because steam penetrates more making it more effective (Liguori et al, 2016).
Thermal death occurs at different temperature rates and affects the texture of the as well as the
flavor and this has serious cost implications. Heat resistance results to medium heat effects. At
acid pH thermal death occurs more rapidly. Dormant spores are special endospores that are
specifically meant for survival during environment stress.
Micrococcus aurentiaca, Micrococcus roseus, Bacillus subtilis, Bacillus megaterium,
Staphelocious epidermidis, Escherichia coli are the specific pathogens we identified in the fish
plates. Micrococcus luteus, Pseudomonas fluorescenes were both missing in the white fish, oily
fish and the prawn. To make the fish food safe for consumption disinfection should be done
(Bienart et al, 2018). Disinfection directly targets pathogens. Although it may not eliminate all
the pathogens, it could bring down the pathogens to a safe level. Disinfectants are used for
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Laboratory Report Diary8
inanimate objects while antiseptics are used to disinfect life surfaces. Disinfection has two
effects on microbes (Marsh et al, 2014). It may kill the microbes and this is an irreversible
process or it may reduce the reproduction and reaction of the microbes and this is a reversible
process. We found out that bactericidal kills bacteria.
The Mesophiles and pscrophiles identified at 30 0 C drastically reduce the quality of food
by tempering with its test. Several bacteria were identified in the food sample indicating the
importance of good sanitation for fast foods such as the sandwiches. The preparation, packaging
and consumption various types of fish should be controlled for several reasons. One of the
reasons is preventing pathogenic infection of the foods that mostly cause infections to the people
consuming these foods (Dufrene, 2015). Reducing food spoilage is also one of the reasons for
controlling the sanitation of fish. In order to prevent the growth of microorganism controlling
factors such as pH, temperature, time, nutrients and water can be regulated appropriately.
Conclusion
The experiment was a success as the aim and objectives were successfully met. However
there are some challenges that we encountered during the experiment that we would have to
avoid in case we were to undertake a similar experiment. The handling of the sample should be
done so keen to ensure that both the equipment and the sample are not infected. More samples
can also be incorporated in the next experiment to ensure that a wide range of microbial data is
obtained which will help researchers to narrow down into relevant and constructive conclusions.
Images of the agar used in the experiment
Image 1.1
inanimate objects while antiseptics are used to disinfect life surfaces. Disinfection has two
effects on microbes (Marsh et al, 2014). It may kill the microbes and this is an irreversible
process or it may reduce the reproduction and reaction of the microbes and this is a reversible
process. We found out that bactericidal kills bacteria.
The Mesophiles and pscrophiles identified at 30 0 C drastically reduce the quality of food
by tempering with its test. Several bacteria were identified in the food sample indicating the
importance of good sanitation for fast foods such as the sandwiches. The preparation, packaging
and consumption various types of fish should be controlled for several reasons. One of the
reasons is preventing pathogenic infection of the foods that mostly cause infections to the people
consuming these foods (Dufrene, 2015). Reducing food spoilage is also one of the reasons for
controlling the sanitation of fish. In order to prevent the growth of microorganism controlling
factors such as pH, temperature, time, nutrients and water can be regulated appropriately.
Conclusion
The experiment was a success as the aim and objectives were successfully met. However
there are some challenges that we encountered during the experiment that we would have to
avoid in case we were to undertake a similar experiment. The handling of the sample should be
done so keen to ensure that both the equipment and the sample are not infected. More samples
can also be incorporated in the next experiment to ensure that a wide range of microbial data is
obtained which will help researchers to narrow down into relevant and constructive conclusions.
Images of the agar used in the experiment
Image 1.1
Laboratory Report Diary9
Image 1.2
Image 1.2
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Laboratory Report Diary10
Experiment 2: Practical investigation into a range of fresh products
Aim
To prepare serial dilutions of three different sample sandwiches and analyze them
Introduction
Fast foods, the sandwiches included are ready to eat foods that can be prepared easily and
can be an easy take away package. The consumption of fast foods has become part and parcel of
daily food patterns all over the world including here in the United Kingdom. The consumption of
these foods such as the sandwiches, pizza and burgers has greatly been associated to several
health problems. This has necessitated the microbial analysis of three commonly consumed in
the United Kingdom to get a good insight and understanding of the sandwiches. This will boost
the prevention of food-borne diseases that result from microbial and sanitary quality of the
sandwiches.
Equipment and Material
Stomacher bag
Sterile scalpel and forceps
Methylated spirits
Bunsen (keep away from the above)
Stomacher
90 mls maximum recovery diluent (MRD)
9 ml maximum recovery diluent
Method
1. Label plates appropriately- white fish sample, prawn sample and oily fish sample. Also
include your initials, date, type of agar and temperature( 5°C), purchase date and dilution
2. Aseptically remove a sample of fish including skin if present 10g in weight, place in
bottle of 90 ml MRD and shake vigorously for 30 seconds and put in stomacher for 1
minute this will be a 10-1dilution.
3. Prepare two further dilutions in 9ml of MRD 10-2 and 10-3
4. Pipette 1ml of each dilution onto each Agar in duplicate. You should end up with 6
plates for each fish type on each of the three agars e.g.: Nutrient agar 6 plates oily fish 2
x 10-1, 2 x 10-2 and 2x 10-3
5. Each group will have 54 plates in total
Results
Table showing the tests and incubation temperatures
Name of Test Agar or Medium Used Incubation
Experiment 2: Practical investigation into a range of fresh products
Aim
To prepare serial dilutions of three different sample sandwiches and analyze them
Introduction
Fast foods, the sandwiches included are ready to eat foods that can be prepared easily and
can be an easy take away package. The consumption of fast foods has become part and parcel of
daily food patterns all over the world including here in the United Kingdom. The consumption of
these foods such as the sandwiches, pizza and burgers has greatly been associated to several
health problems. This has necessitated the microbial analysis of three commonly consumed in
the United Kingdom to get a good insight and understanding of the sandwiches. This will boost
the prevention of food-borne diseases that result from microbial and sanitary quality of the
sandwiches.
Equipment and Material
Stomacher bag
Sterile scalpel and forceps
Methylated spirits
Bunsen (keep away from the above)
Stomacher
90 mls maximum recovery diluent (MRD)
9 ml maximum recovery diluent
Method
1. Label plates appropriately- white fish sample, prawn sample and oily fish sample. Also
include your initials, date, type of agar and temperature( 5°C), purchase date and dilution
2. Aseptically remove a sample of fish including skin if present 10g in weight, place in
bottle of 90 ml MRD and shake vigorously for 30 seconds and put in stomacher for 1
minute this will be a 10-1dilution.
3. Prepare two further dilutions in 9ml of MRD 10-2 and 10-3
4. Pipette 1ml of each dilution onto each Agar in duplicate. You should end up with 6
plates for each fish type on each of the three agars e.g.: Nutrient agar 6 plates oily fish 2
x 10-1, 2 x 10-2 and 2x 10-3
5. Each group will have 54 plates in total
Results
Table showing the tests and incubation temperatures
Name of Test Agar or Medium Used Incubation
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
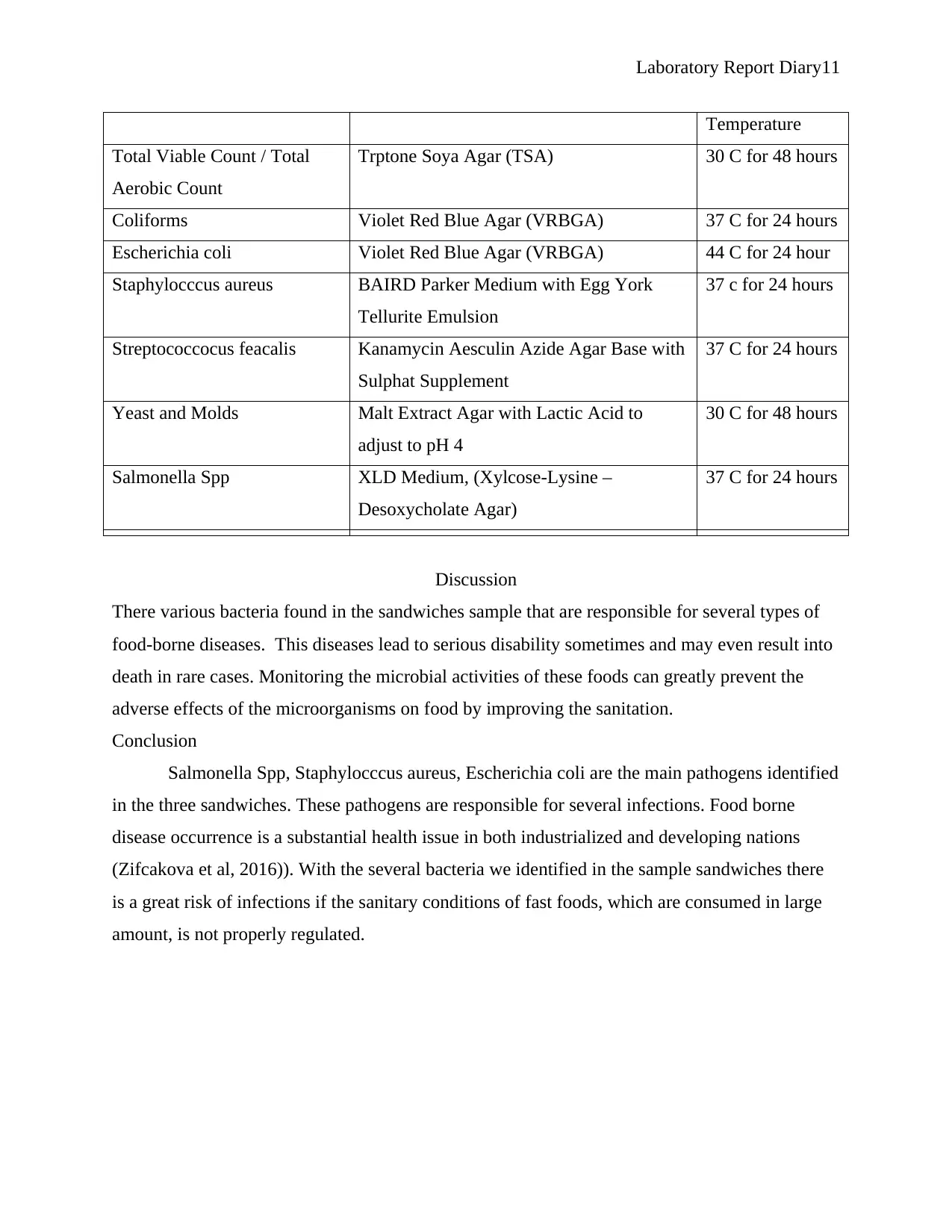
Laboratory Report Diary11
Temperature
Total Viable Count / Total
Aerobic Count
Trptone Soya Agar (TSA) 30 C for 48 hours
Coliforms Violet Red Blue Agar (VRBGA) 37 C for 24 hours
Escherichia coli Violet Red Blue Agar (VRBGA) 44 C for 24 hour
Staphylocccus aureus BAIRD Parker Medium with Egg York
Tellurite Emulsion
37 c for 24 hours
Streptococcocus feacalis Kanamycin Aesculin Azide Agar Base with
Sulphat Supplement
37 C for 24 hours
Yeast and Molds Malt Extract Agar with Lactic Acid to
adjust to pH 4
30 C for 48 hours
Salmonella Spp XLD Medium, (Xylcose-Lysine –
Desoxycholate Agar)
37 C for 24 hours
Discussion
There various bacteria found in the sandwiches sample that are responsible for several types of
food-borne diseases. This diseases lead to serious disability sometimes and may even result into
death in rare cases. Monitoring the microbial activities of these foods can greatly prevent the
adverse effects of the microorganisms on food by improving the sanitation.
Conclusion
Salmonella Spp, Staphylocccus aureus, Escherichia coli are the main pathogens identified
in the three sandwiches. These pathogens are responsible for several infections. Food borne
disease occurrence is a substantial health issue in both industrialized and developing nations
(Zifcakova et al, 2016)). With the several bacteria we identified in the sample sandwiches there
is a great risk of infections if the sanitary conditions of fast foods, which are consumed in large
amount, is not properly regulated.
Temperature
Total Viable Count / Total
Aerobic Count
Trptone Soya Agar (TSA) 30 C for 48 hours
Coliforms Violet Red Blue Agar (VRBGA) 37 C for 24 hours
Escherichia coli Violet Red Blue Agar (VRBGA) 44 C for 24 hour
Staphylocccus aureus BAIRD Parker Medium with Egg York
Tellurite Emulsion
37 c for 24 hours
Streptococcocus feacalis Kanamycin Aesculin Azide Agar Base with
Sulphat Supplement
37 C for 24 hours
Yeast and Molds Malt Extract Agar with Lactic Acid to
adjust to pH 4
30 C for 48 hours
Salmonella Spp XLD Medium, (Xylcose-Lysine –
Desoxycholate Agar)
37 C for 24 hours
Discussion
There various bacteria found in the sandwiches sample that are responsible for several types of
food-borne diseases. This diseases lead to serious disability sometimes and may even result into
death in rare cases. Monitoring the microbial activities of these foods can greatly prevent the
adverse effects of the microorganisms on food by improving the sanitation.
Conclusion
Salmonella Spp, Staphylocccus aureus, Escherichia coli are the main pathogens identified
in the three sandwiches. These pathogens are responsible for several infections. Food borne
disease occurrence is a substantial health issue in both industrialized and developing nations
(Zifcakova et al, 2016)). With the several bacteria we identified in the sample sandwiches there
is a great risk of infections if the sanitary conditions of fast foods, which are consumed in large
amount, is not properly regulated.

Laboratory Report Diary12
Images of the agar used in the experiment
Image 2.1
Images of the agar used in the experiment
Image 2.1
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 22
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
