University Chemistry Lab Report: Paracetamol Preparation and Analysis
VerifiedAdded on 2023/01/17
|7
|1486
|70
Report
AI Summary
This laboratory report details the synthesis and purification of paracetamol, a common organic compound, from p-aminophenol and acetic anhydride. The experiment involves the preparation of paracetamol, its isolation, and subsequent purification through recrystallization. The report includes the methodology, results, and discussion of the melting point determination for both benzoic acid and paracetamol to assess purity. Calculations for the theoretical and actual yield of paracetamol are presented, leading to a percentage yield analysis. The report also addresses potential sources of error that may have contributed to a lower-than-expected yield. Post-lab questions, including yield calculations for the preparation of cyclohexane-1,2-diol, are included. The report concludes with an assessment of the experiment's success in achieving its aims, highlighting the successful exploration of recrystallization and the assessment of paracetamol purity, while acknowledging the limitations of the yield obtained.

Laboratory Report on Preparation of Paracetamol
By(name)
Course
Tutor
Institution
City
Date
By(name)
Course
Tutor
Institution
City
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

INTRODUCTION
Background theory
Majority of the drugs used for medicinal purposes are usually small organic molecules.
Once designed by a chemist, the structure of the prepared chemical has to be tested to achieve
optimum conditions. Paracetamol is an organic compound made by reacting 4 aminophenols
with acetic anhydride (Lednicer, 2007). The reaction leads to the formation of the amide bond
and ethanoic acid as the by-product. Once the reaction is done, paracetamol is isolated before
being purified. The reaction below shows a simple paracetamol formation process.
Paracetamol synthesis can be subdivided into 3 different steps; mixing of the reactants to form
paracetamol, isolating the paracetamol to form ethanoic acid and recrystallization of the obtained
paracetamol.
In this study, nucleophile p-aminophenol is reacted with electrophile acetic anhydride.
The p-Aminophenol is nucleophilic at both the –OH and –NH2 though –NH2 is greater hence –
NH2 is the only group that has a significant reaction (Brown, Foote, Iverson, & Anslyn, 2010).
This results in paracetamol as shown in the above equation.
Aims
This experiment is aimed at producing and purifying paracetamol, purify it through
crystallization. The percentage yield will then have computed for the pure produce and its purity
ascertained by measuring the boiling point and then comparing the results to values in the
literature. In summary, the objectives of this experiment include:
To prepare paracetamol
To explore the concept of recrystallization
To determine the melting point of benzoic acid and paracetamol and use the melting
points to evaluate the percentage purity of the two substances.
Background theory
Majority of the drugs used for medicinal purposes are usually small organic molecules.
Once designed by a chemist, the structure of the prepared chemical has to be tested to achieve
optimum conditions. Paracetamol is an organic compound made by reacting 4 aminophenols
with acetic anhydride (Lednicer, 2007). The reaction leads to the formation of the amide bond
and ethanoic acid as the by-product. Once the reaction is done, paracetamol is isolated before
being purified. The reaction below shows a simple paracetamol formation process.
Paracetamol synthesis can be subdivided into 3 different steps; mixing of the reactants to form
paracetamol, isolating the paracetamol to form ethanoic acid and recrystallization of the obtained
paracetamol.
In this study, nucleophile p-aminophenol is reacted with electrophile acetic anhydride.
The p-Aminophenol is nucleophilic at both the –OH and –NH2 though –NH2 is greater hence –
NH2 is the only group that has a significant reaction (Brown, Foote, Iverson, & Anslyn, 2010).
This results in paracetamol as shown in the above equation.
Aims
This experiment is aimed at producing and purifying paracetamol, purify it through
crystallization. The percentage yield will then have computed for the pure produce and its purity
ascertained by measuring the boiling point and then comparing the results to values in the
literature. In summary, the objectives of this experiment include:
To prepare paracetamol
To explore the concept of recrystallization
To determine the melting point of benzoic acid and paracetamol and use the melting
points to evaluate the percentage purity of the two substances.

METHODOLOGY
Preparation of paracetamol
A solution of 6.2 g of anhydrous sodium acetate in 30ml of water was prepared in a
100mL beaker. 5.5 g of p-aminophenol was then weighed it into a 250ml conical flask. 25ml of
2M HCl (bench agent) was then added to the conical flask and swirled until it was completely
dissolved and then diluted with 80mL of water.
From a dispenser provided, 5.3mL of acetic anhydride was added to the p-aminophenol
solution in a conical flask prepared in the previous steps swirled until it mixed. The solution of
sodium acetate prepared in step 1 was then poured in a flask and then corked before it was
shaken vigorously until no more solid precipitate was observed.
Isolation of paracetamol.
Suction filtration apparatus was assembled on Buchner funnel and using the correct filter
size paper. Suction was applied to bed down the paper. The crystal of crude paracetamol was
then obtained on the Buchner funnel. The crystals were then washed using chilled water.
Purification of paracetamol
Paracetamol was recrystallized from water. This involved placing a sample of crude
paracetamol in a 100ml conical flask and adding 0.5g of ascorbic acid. 25ml of hot water was
then added for less than 2 minutes. The filtrate was then allowed to cool to room temperature to
reduce solubility. The crystals were then transferred into a clean filter paper and covered with
another filter paper before the sample was removed from the oven and allowed to cool at room
temperature.
Preparation of paracetamol
A solution of 6.2 g of anhydrous sodium acetate in 30ml of water was prepared in a
100mL beaker. 5.5 g of p-aminophenol was then weighed it into a 250ml conical flask. 25ml of
2M HCl (bench agent) was then added to the conical flask and swirled until it was completely
dissolved and then diluted with 80mL of water.
From a dispenser provided, 5.3mL of acetic anhydride was added to the p-aminophenol
solution in a conical flask prepared in the previous steps swirled until it mixed. The solution of
sodium acetate prepared in step 1 was then poured in a flask and then corked before it was
shaken vigorously until no more solid precipitate was observed.
Isolation of paracetamol.
Suction filtration apparatus was assembled on Buchner funnel and using the correct filter
size paper. Suction was applied to bed down the paper. The crystal of crude paracetamol was
then obtained on the Buchner funnel. The crystals were then washed using chilled water.
Purification of paracetamol
Paracetamol was recrystallized from water. This involved placing a sample of crude
paracetamol in a 100ml conical flask and adding 0.5g of ascorbic acid. 25ml of hot water was
then added for less than 2 minutes. The filtrate was then allowed to cool to room temperature to
reduce solubility. The crystals were then transferred into a clean filter paper and covered with
another filter paper before the sample was removed from the oven and allowed to cool at room
temperature.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
RESULTS AND DISCUSSION
a. Determination of melting points
MPR of benzoic acid (sample)=121.9-123.2oC
MP from literature=122.4oC (Smit, Bochkov, & Caple, 2011)
The melting point range=1.3oC. Normally, in practice, the melting point ranges between
0.5 to 1 oC for pure substance (Brown, Foote, Iverson, & Anslyn, 2010). From the above
results, it can be concluded that benzoic acid used in this case is not pure because it starts
to melt at a lower temperature and continues melting past the literature melting point over
a range of 1.3oC. Benzoic acid used in this experiment is thus not 100% pure. It has some
traces of impurities as shown by a melting point temperature range of 1.3oC
b. Preparation of paracetamol
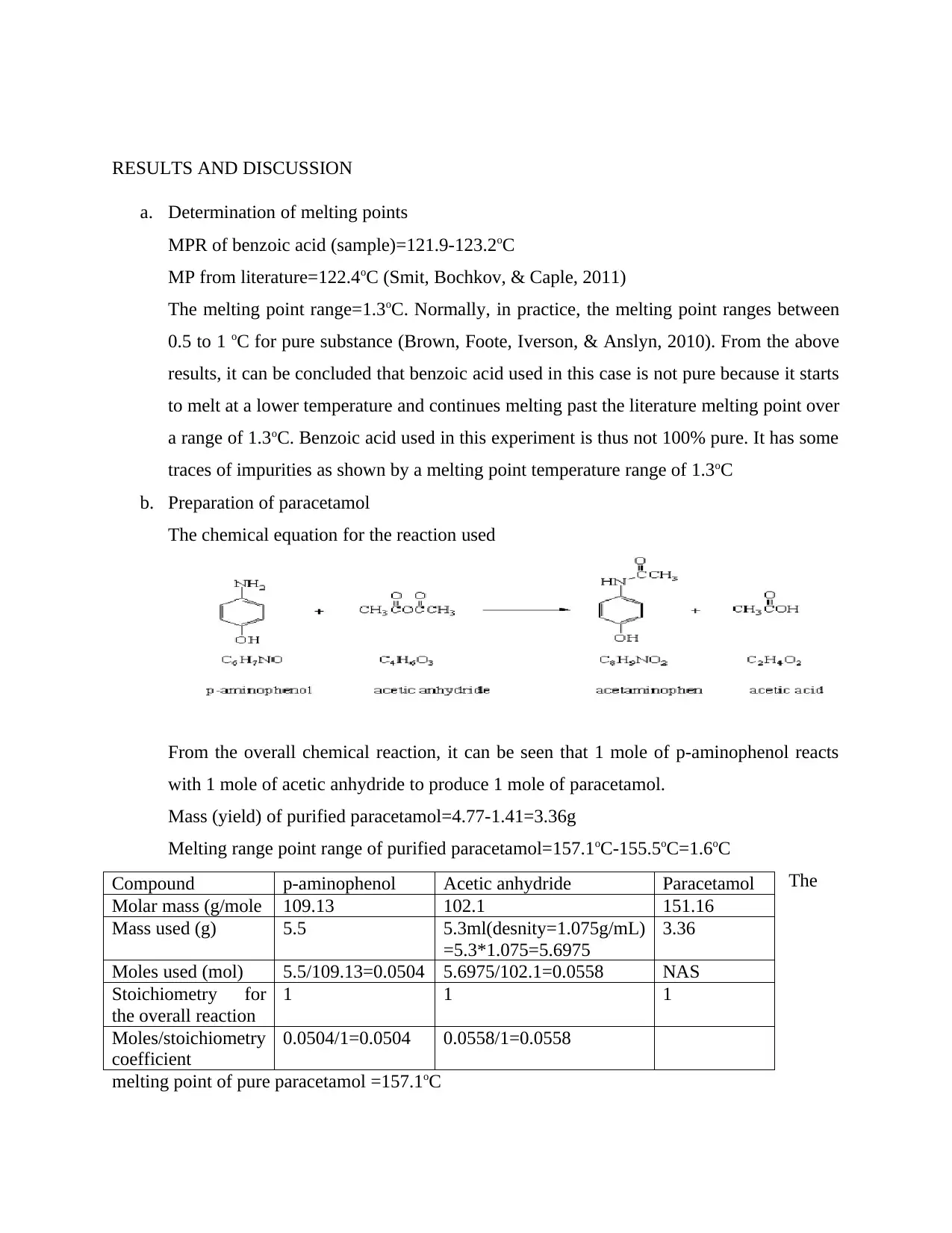
The chemical equation for the reaction used
From the overall chemical reaction, it can be seen that 1 mole of p-aminophenol reacts
with 1 mole of acetic anhydride to produce 1 mole of paracetamol.
Mass (yield) of purified paracetamol=4.77-1.41=3.36g
Melting range point range of purified paracetamol=157.1oC-155.5oC=1.6oC
The
melting point of pure paracetamol =157.1oC
Compound p-aminophenol Acetic anhydride Paracetamol
Molar mass (g/mole 109.13 102.1 151.16
Mass used (g) 5.5 5.3ml(desnity=1.075g/mL)
=5.3*1.075=5.6975
3.36
Moles used (mol) 5.5/109.13=0.0504 5.6975/102.1=0.0558 NAS
Stoichiometry for
the overall reaction
1 1 1
Moles/stoichiometry
coefficient
0.0504/1=0.0504 0.0558/1=0.0558
a. Determination of melting points
MPR of benzoic acid (sample)=121.9-123.2oC
MP from literature=122.4oC (Smit, Bochkov, & Caple, 2011)
The melting point range=1.3oC. Normally, in practice, the melting point ranges between
0.5 to 1 oC for pure substance (Brown, Foote, Iverson, & Anslyn, 2010). From the above
results, it can be concluded that benzoic acid used in this case is not pure because it starts
to melt at a lower temperature and continues melting past the literature melting point over
a range of 1.3oC. Benzoic acid used in this experiment is thus not 100% pure. It has some
traces of impurities as shown by a melting point temperature range of 1.3oC
b. Preparation of paracetamol
The chemical equation for the reaction used
From the overall chemical reaction, it can be seen that 1 mole of p-aminophenol reacts
with 1 mole of acetic anhydride to produce 1 mole of paracetamol.
Mass (yield) of purified paracetamol=4.77-1.41=3.36g
Melting range point range of purified paracetamol=157.1oC-155.5oC=1.6oC
The
melting point of pure paracetamol =157.1oC
Compound p-aminophenol Acetic anhydride Paracetamol
Molar mass (g/mole 109.13 102.1 151.16
Mass used (g) 5.5 5.3ml(desnity=1.075g/mL)
=5.3*1.075=5.6975
3.36
Moles used (mol) 5.5/109.13=0.0504 5.6975/102.1=0.0558 NAS
Stoichiometry for
the overall reaction
1 1 1
Moles/stoichiometry
coefficient
0.0504/1=0.0504 0.0558/1=0.0558
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

The melting point range=1.6oC. Normally, in practice, the melting point ranges between 0.5 to 1
oC for pure substance. The obtained range for the paracetamol manufactured is a relatively
narrow melting point range. Although paracetamol formed is not 100% pure because of a slight
range in melting point, the melting point from the literature falls within this range. The
percentage purity of the paracetamol formed is thus relatively high.
From the above moles per stoichiometry coefficient, the limiting reagent in the
production of paracetamol is=p-aminophenol.
Because it is the limiting reactant, p-aminophenol is used in calculating the theoretical
(100% efficiency). Consider the chemical equation shown below.
From the chemical equation, 1 mole of p-aminophenol reacts with 1 mole of acetic
anhydride to produce 1 mole of paracetamol.
The number of moles of paracetamol produced=1*0.0504=0.0504 moles.
Theoretical yield mass of paracetamol produced=moles *molar
mass=0.0504*151.16=7.618g
The actual yield of paracetamol=3.36g
The percentage yield of product=Actual yield/theoretical yield=3.36/7.618 *100=44%
Possible sources of low percentage yield.
The factors that might have influenced the 44% yield which is relatively a low yield. The
possible sources of errors which might have led to this low yield include loss of crystals during
glass transfers on the filter paper during filtration, competition among the various sides of
reaction, failure of the recrystallization process to be done in time before the filtration process.
Cases of mass loss in the purification of crude material is also another source of error in the
oC for pure substance. The obtained range for the paracetamol manufactured is a relatively
narrow melting point range. Although paracetamol formed is not 100% pure because of a slight
range in melting point, the melting point from the literature falls within this range. The
percentage purity of the paracetamol formed is thus relatively high.
From the above moles per stoichiometry coefficient, the limiting reagent in the
production of paracetamol is=p-aminophenol.
Because it is the limiting reactant, p-aminophenol is used in calculating the theoretical
(100% efficiency). Consider the chemical equation shown below.
From the chemical equation, 1 mole of p-aminophenol reacts with 1 mole of acetic
anhydride to produce 1 mole of paracetamol.
The number of moles of paracetamol produced=1*0.0504=0.0504 moles.
Theoretical yield mass of paracetamol produced=moles *molar
mass=0.0504*151.16=7.618g
The actual yield of paracetamol=3.36g
The percentage yield of product=Actual yield/theoretical yield=3.36/7.618 *100=44%
Possible sources of low percentage yield.
The factors that might have influenced the 44% yield which is relatively a low yield. The
possible sources of errors which might have led to this low yield include loss of crystals during
glass transfers on the filter paper during filtration, competition among the various sides of
reaction, failure of the recrystallization process to be done in time before the filtration process.
Cases of mass loss in the purification of crude material is also another source of error in the

experiment. The variations in the melting point could be attributed to the impurities and possible
human errors.
Post Laboratory question
Calculation of percentage yield of preparing cyclohexane-1,2-diol from the balanced equation
shown below.
If 10.0g of KMnO4 was added to 300ml of water and the resulting solution added to 34.6 ml of
density 0.81g/ml to produce 9.5g of cyclohexane 1, 2-diol
Mass of cyclohexane=34.6*0.81=28.026g
Moles of C6H10=28.026/(82.146)=0.34117
The molar mass of KMnO4=158.0339.
Moles of KMnO4=10/158.0339=0.0633 moles
From mole ratios, 2 moles of KMnO4 reacts with 3 moles of cyclohexane to produce 1 mole of
cyclohexane-1,2-diol
Hence 0.0633 would require 3/2 *0.0633=0.095moles thus KMnO4 was the limiting reagent.
From the mole ratios, moles of cyclohexane-1,2-diol produced=0.095 moles.
Molar mass of cyclohexane-1,2-diol =116.16
Theoretical mass produced=116.16*0.095=11.04g
The percentage yield of product=Actual yield/theoretical yield=9.5/11.04 *100=86.088%
CONCLUSION
The primary aim of this experiment was to prepare a pure sample of paracetamol. The
results obtained indicate that the experiment was a success since we were able to use the
concepts of recrystallization was explicitly explored. From the concepts of melting point and
temperature ranges, the results obtained show that the prepared paracetamol, though it had some
impurities, the obtained compound is almost pure because it has a narrow temperature range of
1.6oC. The purity of benzoic acid was also ascertained using the concept of melting point
temperature range. The percentage yield of prepared paracetamol was however relatively low.
human errors.
Post Laboratory question
Calculation of percentage yield of preparing cyclohexane-1,2-diol from the balanced equation
shown below.
If 10.0g of KMnO4 was added to 300ml of water and the resulting solution added to 34.6 ml of
density 0.81g/ml to produce 9.5g of cyclohexane 1, 2-diol
Mass of cyclohexane=34.6*0.81=28.026g
Moles of C6H10=28.026/(82.146)=0.34117
The molar mass of KMnO4=158.0339.
Moles of KMnO4=10/158.0339=0.0633 moles
From mole ratios, 2 moles of KMnO4 reacts with 3 moles of cyclohexane to produce 1 mole of
cyclohexane-1,2-diol
Hence 0.0633 would require 3/2 *0.0633=0.095moles thus KMnO4 was the limiting reagent.
From the mole ratios, moles of cyclohexane-1,2-diol produced=0.095 moles.
Molar mass of cyclohexane-1,2-diol =116.16
Theoretical mass produced=116.16*0.095=11.04g
The percentage yield of product=Actual yield/theoretical yield=9.5/11.04 *100=86.088%
CONCLUSION
The primary aim of this experiment was to prepare a pure sample of paracetamol. The
results obtained indicate that the experiment was a success since we were able to use the
concepts of recrystallization was explicitly explored. From the concepts of melting point and
temperature ranges, the results obtained show that the prepared paracetamol, though it had some
impurities, the obtained compound is almost pure because it has a narrow temperature range of
1.6oC. The purity of benzoic acid was also ascertained using the concept of melting point
temperature range. The percentage yield of prepared paracetamol was however relatively low.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

We were able to obtain a percent yield of 44%. This low yield is attributable to the sources of
errors discussed in the discussion section. In a nutshell, the experiment was a success since all
the aims were achieved.
Bibliography
Brown, W., Foote, C., Iverson, B., & Anslyn, E. (2010). Organic Chemistry, Enhanced Edition.
Boston, MA: Cengage Learning.
Lednicer, D. (2007). The Organic Chemistry of Drug Synthesis. Hoboken, NJ: John Wiley &
Sons.
Smit, W. A., Bochkov, A. F., & Caple, R. (2011). Organic Synthesis: The Science Behind the
Art. London, England: Royal Society of Chemistry.
errors discussed in the discussion section. In a nutshell, the experiment was a success since all
the aims were achieved.
Bibliography
Brown, W., Foote, C., Iverson, B., & Anslyn, E. (2010). Organic Chemistry, Enhanced Edition.
Boston, MA: Cengage Learning.
Lednicer, D. (2007). The Organic Chemistry of Drug Synthesis. Hoboken, NJ: John Wiley &
Sons.
Smit, W. A., Bochkov, A. F., & Caple, R. (2011). Organic Synthesis: The Science Behind the
Art. London, England: Royal Society of Chemistry.
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.
