Mass Spectrometry: Separation of Ions, Mass Range, Optimization, Ion Sources, Detection System, Recent Advancements, Advantages and Disadvantages
VerifiedAdded on 2023/04/24
|14
|3088
|376
AI Summary
This article discusses the principles of mass spectrometry, including the separation of ions, mass range, optimization, ion sources, detection system, recent advancements, advantages, and disadvantages. It also covers the use of time to flight mass spectrometry in biophysics and its applications in biomolecules, protein, peptide, sugars, and polymers.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: MASS SPECTROMETRY
Name of the Student:
Name of the University:
Author Note:
Name of the Student:
Name of the University:
Author Note:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

2
MASS SPECTROMETRY
Introduction:
In the twenty-first century, the contribution of mass spectrometry is revolutionary. It is a
mainstream chemical analysis technique used for separation of electrically charged molecules in
gas phases. The uniqueness of the mass spectrometry is that it enables direct identification of
molecular-based n the mass to charge ratios as well as fragmentation pattern. Thus, from decade
mass spectrometry is used for quantitative analysis of chemicals in the molecular level. Out of all
categories of mass spectrometry, time to flight mass spectrometry is one of the revolutionary
inventions in history. Buckwalter et al. (2016), highlighted that the time to flight mass
spectrometry is unique approach to obtain details of a broad molecular weight in which signals
are related to polar and non-polar compounds in a single sample. An electric field in this case is
used to accelerate the ions in the same potentials. This paper will illustrate the way of separation
of ion, mass range of analyzer, the way of optimizing analyzer, appropriate ion sources for use,
comment on the detection systems, recent research and advantage and disadvantages in
following paragraphs.
Discussion:
The way of mass analyzer separating ions:
The time to flight mass spectrometry is one revolutionary approach in biophysics which
taken into consideration a broad range of the molecular weight where range of signals associated
with polar and non-polar compounds in a single sample. The key principle of ion separation is
that the mass analyzer separate ions based on the mass to charge ratio. The mass charge to ratio
is determined by the time to flight measurements (Kathuria et al., 2015). The ions are accelerated
MASS SPECTROMETRY
Introduction:
In the twenty-first century, the contribution of mass spectrometry is revolutionary. It is a
mainstream chemical analysis technique used for separation of electrically charged molecules in
gas phases. The uniqueness of the mass spectrometry is that it enables direct identification of
molecular-based n the mass to charge ratios as well as fragmentation pattern. Thus, from decade
mass spectrometry is used for quantitative analysis of chemicals in the molecular level. Out of all
categories of mass spectrometry, time to flight mass spectrometry is one of the revolutionary
inventions in history. Buckwalter et al. (2016), highlighted that the time to flight mass
spectrometry is unique approach to obtain details of a broad molecular weight in which signals
are related to polar and non-polar compounds in a single sample. An electric field in this case is
used to accelerate the ions in the same potentials. This paper will illustrate the way of separation
of ion, mass range of analyzer, the way of optimizing analyzer, appropriate ion sources for use,
comment on the detection systems, recent research and advantage and disadvantages in
following paragraphs.
Discussion:
The way of mass analyzer separating ions:
The time to flight mass spectrometry is one revolutionary approach in biophysics which
taken into consideration a broad range of the molecular weight where range of signals associated
with polar and non-polar compounds in a single sample. The key principle of ion separation is
that the mass analyzer separate ions based on the mass to charge ratio. The mass charge to ratio
is determined by the time to flight measurements (Kathuria et al., 2015). The ions are accelerated
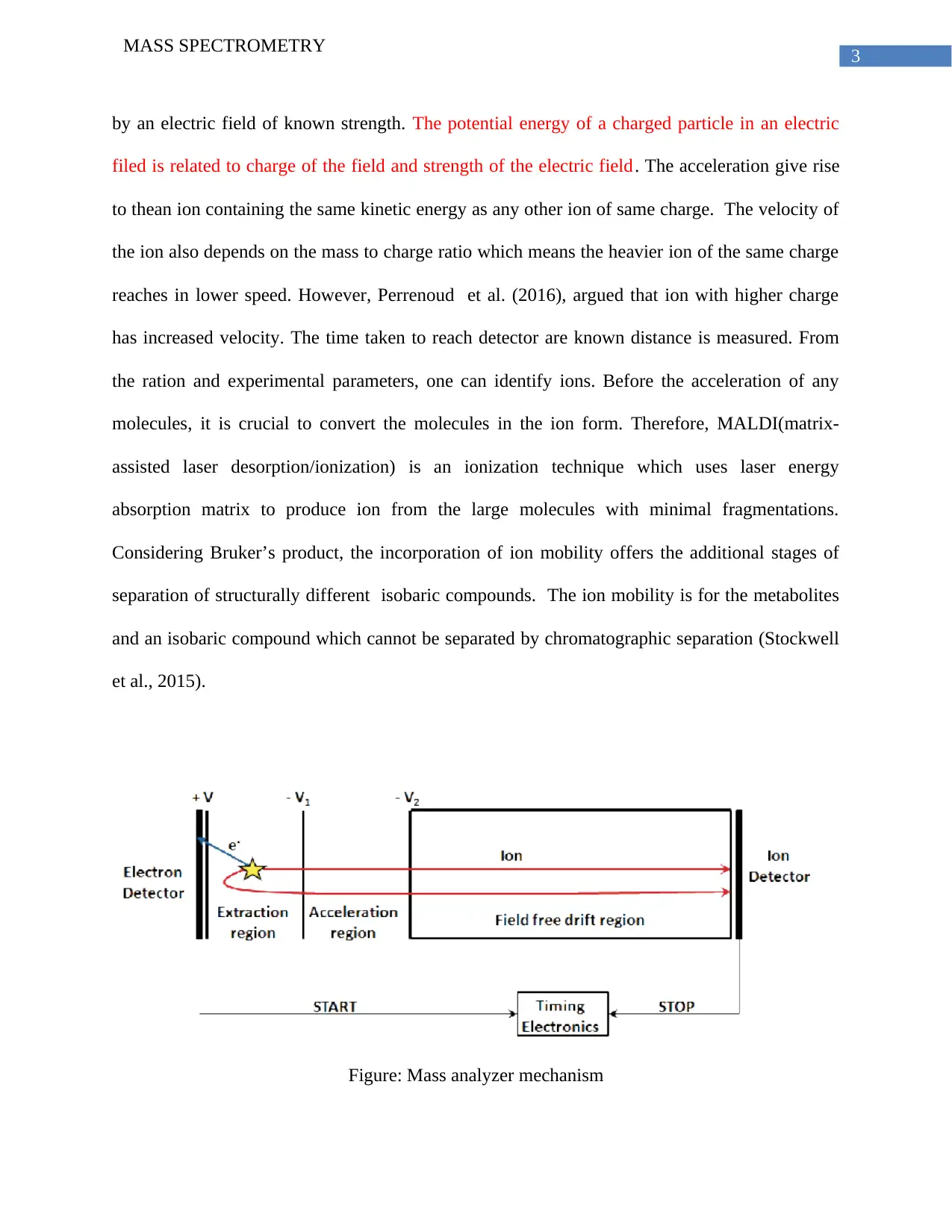
3
MASS SPECTROMETRY
by an electric field of known strength. The potential energy of a charged particle in an electric
filed is related to charge of the field and strength of the electric field. The acceleration give rise
to thean ion containing the same kinetic energy as any other ion of same charge. The velocity of
the ion also depends on the mass to charge ratio which means the heavier ion of the same charge
reaches in lower speed. However, Perrenoud et al. (2016), argued that ion with higher charge
has increased velocity. The time taken to reach detector are known distance is measured. From
the ration and experimental parameters, one can identify ions. Before the acceleration of any
molecules, it is crucial to convert the molecules in the ion form. Therefore, MALDI(matrix-
assisted laser desorption/ionization) is an ionization technique which uses laser energy
absorption matrix to produce ion from the large molecules with minimal fragmentations.
Considering Bruker’s product, the incorporation of ion mobility offers the additional stages of
separation of structurally different isobaric compounds. The ion mobility is for the metabolites
and an isobaric compound which cannot be separated by chromatographic separation (Stockwell
et al., 2015).
Figure: Mass analyzer mechanism
MASS SPECTROMETRY
by an electric field of known strength. The potential energy of a charged particle in an electric
filed is related to charge of the field and strength of the electric field. The acceleration give rise
to thean ion containing the same kinetic energy as any other ion of same charge. The velocity of
the ion also depends on the mass to charge ratio which means the heavier ion of the same charge
reaches in lower speed. However, Perrenoud et al. (2016), argued that ion with higher charge
has increased velocity. The time taken to reach detector are known distance is measured. From
the ration and experimental parameters, one can identify ions. Before the acceleration of any
molecules, it is crucial to convert the molecules in the ion form. Therefore, MALDI(matrix-
assisted laser desorption/ionization) is an ionization technique which uses laser energy
absorption matrix to produce ion from the large molecules with minimal fragmentations.
Considering Bruker’s product, the incorporation of ion mobility offers the additional stages of
separation of structurally different isobaric compounds. The ion mobility is for the metabolites
and an isobaric compound which cannot be separated by chromatographic separation (Stockwell
et al., 2015).
Figure: Mass analyzer mechanism

4
MASS SPECTROMETRY
Source: (Stockwell et al., 2015).
Mass range of time to flight analyzer:
Consider the mass range of the time to flight analyzer, the mass range is decided by an
m/z ratio. m stands for mass and Z stands for a charge of ions. In the mass analysis of in time to
flight, the electron is taken from molecules to create single charged ions. The mass range can be
which can be characterized by mass spectrometry with the specific resolution which is the
defined by the ability to distinguish adjacent peak. Considering the m/z ratio Mass Range
generally m/z 0-2000 or 0-4000 is considered as the conventional range. As highlighted by
Oviaño and Bou (2018), the mass range of time to flight for a 1 kDa to 500 kDa. The
conventional time to flight mass spectrometry can be applied in biomolecules involves, Protein,
peptide, sugars and polymer and other molecules which tend to be more fragmented when
ionized by conventional inanition method. Considering Bruker’ s time to flight mass
spectrometry, Doern and Butler-Wu (2016), highlighted that the mass range is 39.0229 to 148
kDa, even can detect 70 to 900 m/z. Therefore, because of the broad mass range, time to flight
mass spectrometer used for biomarker discovery, discovering drug metabolites, synthetic
chemical support and food, and water testing.
The way of optimizing resolution:
Considering time to flight mass spectrometry of Bruker, accurate mass measurements
require the highest possible mass resolution to contribute to the mass peak in question. Although
MASS SPECTROMETRY
Source: (Stockwell et al., 2015).
Mass range of time to flight analyzer:
Consider the mass range of the time to flight analyzer, the mass range is decided by an
m/z ratio. m stands for mass and Z stands for a charge of ions. In the mass analysis of in time to
flight, the electron is taken from molecules to create single charged ions. The mass range can be
which can be characterized by mass spectrometry with the specific resolution which is the
defined by the ability to distinguish adjacent peak. Considering the m/z ratio Mass Range
generally m/z 0-2000 or 0-4000 is considered as the conventional range. As highlighted by
Oviaño and Bou (2018), the mass range of time to flight for a 1 kDa to 500 kDa. The
conventional time to flight mass spectrometry can be applied in biomolecules involves, Protein,
peptide, sugars and polymer and other molecules which tend to be more fragmented when
ionized by conventional inanition method. Considering Bruker’ s time to flight mass
spectrometry, Doern and Butler-Wu (2016), highlighted that the mass range is 39.0229 to 148
kDa, even can detect 70 to 900 m/z. Therefore, because of the broad mass range, time to flight
mass spectrometer used for biomarker discovery, discovering drug metabolites, synthetic
chemical support and food, and water testing.
The way of optimizing resolution:
Considering time to flight mass spectrometry of Bruker, accurate mass measurements
require the highest possible mass resolution to contribute to the mass peak in question. Although
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

5
MASS SPECTROMETRY
the mass relation is defined as the fine separation between two peaks of equal height and width,
requires, mass resolving power of approximately 10 fold higher of equal width peaks whose peak
ratio is 100: 1 (Sanguinetti & Posteraro, 2017). The mathematical model of TOF analyzer
employs uniform electric field is present which allows exact calculation of the flight times as a
function of mass to charge ratio, initial velocity, position, applied voltage, and instrumental
geometry. The equation is based on a serious of thorough result derived which assists in
focusing conditions and resolution. In this case, to optimize the resolution, a situational decision
making is observed between ultimate resolution at a particular mass to charge ratio, resolution
and mass accuracy over dynamic range. In most practical application, the latter is prioritized for
better resolution (Gouriet et al., 2016). Therefore, calculations were compared with the
experimental result for a particular analyzer geometry, both at hypothetical optimal velocity
focus and calculation at optimum conditions where ultimate resolution is neglected for better
mass accuracy and broader range of relatively high resolution and.
MASS SPECTROMETRY
the mass relation is defined as the fine separation between two peaks of equal height and width,
requires, mass resolving power of approximately 10 fold higher of equal width peaks whose peak
ratio is 100: 1 (Sanguinetti & Posteraro, 2017). The mathematical model of TOF analyzer
employs uniform electric field is present which allows exact calculation of the flight times as a
function of mass to charge ratio, initial velocity, position, applied voltage, and instrumental
geometry. The equation is based on a serious of thorough result derived which assists in
focusing conditions and resolution. In this case, to optimize the resolution, a situational decision
making is observed between ultimate resolution at a particular mass to charge ratio, resolution
and mass accuracy over dynamic range. In most practical application, the latter is prioritized for
better resolution (Gouriet et al., 2016). Therefore, calculations were compared with the
experimental result for a particular analyzer geometry, both at hypothetical optimal velocity
focus and calculation at optimum conditions where ultimate resolution is neglected for better
mass accuracy and broader range of relatively high resolution and.
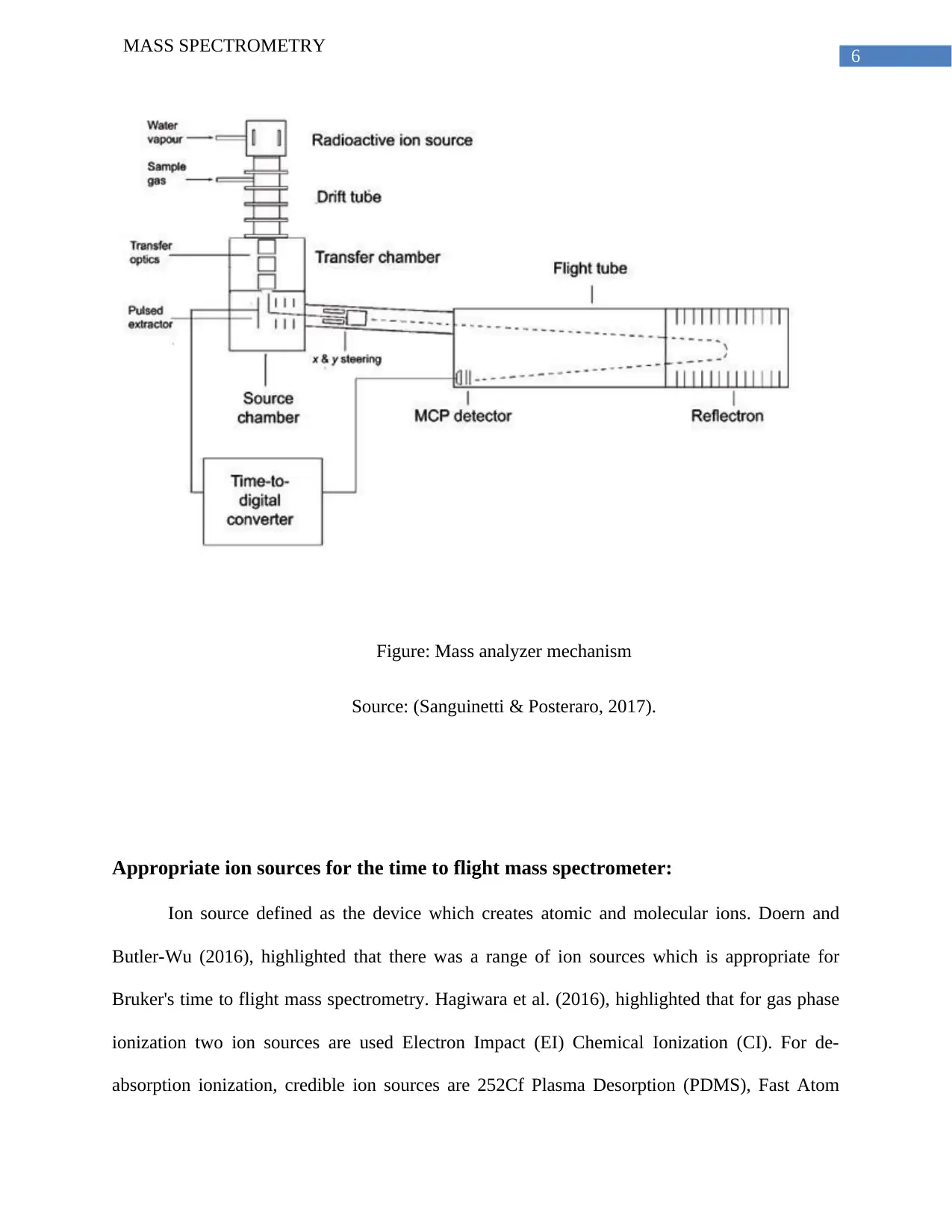
6
MASS SPECTROMETRY
Figure: Mass analyzer mechanism
Source: (Sanguinetti & Posteraro, 2017).
Appropriate ion sources for the time to flight mass spectrometer:
Ion source defined as the device which creates atomic and molecular ions. Doern and
Butler-Wu (2016), highlighted that there was a range of ion sources which is appropriate for
Bruker's time to flight mass spectrometry. Hagiwara et al. (2016), highlighted that for gas phase
ionization two ion sources are used Electron Impact (EI) Chemical Ionization (CI). For de-
absorption ionization, credible ion sources are 252Cf Plasma Desorption (PDMS), Fast Atom
MASS SPECTROMETRY
Figure: Mass analyzer mechanism
Source: (Sanguinetti & Posteraro, 2017).
Appropriate ion sources for the time to flight mass spectrometer:
Ion source defined as the device which creates atomic and molecular ions. Doern and
Butler-Wu (2016), highlighted that there was a range of ion sources which is appropriate for
Bruker's time to flight mass spectrometry. Hagiwara et al. (2016), highlighted that for gas phase
ionization two ion sources are used Electron Impact (EI) Chemical Ionization (CI). For de-
absorption ionization, credible ion sources are 252Cf Plasma Desorption (PDMS), Fast Atom

7
MASS SPECTROMETRY
Bombardment (FAB) or Secondary Ion MS (SIMS), Laser Desorption (LDMS), Matrix
Assisted Laser Desorption (MALDI). For Spray Ionization, the credible ion sources are
Thermospray (TSP), Atmospheric Pressure Chemical Ionization (APCI), Electrospray
(atmospheric pressure ionization) (ESI, API). Idelevich et al. (2018), highlighted for the most
appropriate ion sources for MALDI where sample for MALDI is uniformly mixed in a large
quantity of matric. Matrix absorption the UV light wavelength of 337 nm and convert it heat
energy, triggering de-absorption and finally, the analyzed molecules were protonated and
deprotonated. Bruker’ generally uses electrospray as ion source which has similar characteristics
of MALDI. The only difference between MALDI and ESI is ESI use a solvated sample which
is infused with into instruments, whereas MALDI uses solid state of the sample. The other
features ion source of Bruker's TOF is that it offers captivenanobooster, ion booster, APCII,
APPII, and GC-APCI (Bruker.com., 2019).
Figure: MALDI
Source :(Sanguinetti & Posteraro, 2017).
The detection system of time of flight:
The detection system of time to flight mass spectrometer typically consists of the micro
channel detector or faster secondary emission. The electrical signal from the detector is recorded
MASS SPECTROMETRY
Bombardment (FAB) or Secondary Ion MS (SIMS), Laser Desorption (LDMS), Matrix
Assisted Laser Desorption (MALDI). For Spray Ionization, the credible ion sources are
Thermospray (TSP), Atmospheric Pressure Chemical Ionization (APCI), Electrospray
(atmospheric pressure ionization) (ESI, API). Idelevich et al. (2018), highlighted for the most
appropriate ion sources for MALDI where sample for MALDI is uniformly mixed in a large
quantity of matric. Matrix absorption the UV light wavelength of 337 nm and convert it heat
energy, triggering de-absorption and finally, the analyzed molecules were protonated and
deprotonated. Bruker’ generally uses electrospray as ion source which has similar characteristics
of MALDI. The only difference between MALDI and ESI is ESI use a solvated sample which
is infused with into instruments, whereas MALDI uses solid state of the sample. The other
features ion source of Bruker's TOF is that it offers captivenanobooster, ion booster, APCII,
APPII, and GC-APCI (Bruker.com., 2019).
Figure: MALDI
Source :(Sanguinetti & Posteraro, 2017).
The detection system of time of flight:
The detection system of time to flight mass spectrometer typically consists of the micro
channel detector or faster secondary emission. The electrical signal from the detector is recorded
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8
MASS SPECTROMETRY
by means of time to flight digital converter. Microchannel detector is used as a detection system
in Bruker's Time to flight mass spectrometer. A Microchannel plate is a planner component used
for detection of the single particles such as an electron, ions, neutrons as well as low intensity
implementing radiation such as ultraviolet radiation and x-ray (Clark et al., 2018). It is closely
related to the electron multiplier as it intensifies both single and double by the multiplication of
electrons via secondary emission. However, because of the presence of the different slots, it can
provide spatial resolution. It acts an amplifier which turns the single particle into the cloud of
electrons. Therefore, the unique feature of Bruker's time to flight mass spectrometry that it gives
detailed and dynamic resolutions.
The recent technical advancement in technology:
Although the advantage of TOF mass spectrometry is the poor resolution compared to
others, The recent technical advancement Bruker’ s offer 2000- full sensitivity resolution for
gaining the finer details of the particles which higher indicated that it has the ability of the
separating two narrow spectral peaks (Li et al., 2016). It has a low picogram sensitivity which
means it can address the smallest differences in peak which can further change the reading of the
instrument. bruker’ s time to flight mass spectrometry offers advanced qualitative and
quantitative multitarget screening for forensic and doping control. Besides, it offers Dual ion
funnel, IonBooster Source, CaptiveSpray nanoBooster (Roth et al., 2018).
Advantage and disadvantage:
Advantage:
• Time to flight mass spectrometry uses an approach of analyzing a broad molecular weight
range of signals associated with polar and non-polar compounds in a single sample.
MASS SPECTROMETRY
by means of time to flight digital converter. Microchannel detector is used as a detection system
in Bruker's Time to flight mass spectrometer. A Microchannel plate is a planner component used
for detection of the single particles such as an electron, ions, neutrons as well as low intensity
implementing radiation such as ultraviolet radiation and x-ray (Clark et al., 2018). It is closely
related to the electron multiplier as it intensifies both single and double by the multiplication of
electrons via secondary emission. However, because of the presence of the different slots, it can
provide spatial resolution. It acts an amplifier which turns the single particle into the cloud of
electrons. Therefore, the unique feature of Bruker's time to flight mass spectrometry that it gives
detailed and dynamic resolutions.
The recent technical advancement in technology:
Although the advantage of TOF mass spectrometry is the poor resolution compared to
others, The recent technical advancement Bruker’ s offer 2000- full sensitivity resolution for
gaining the finer details of the particles which higher indicated that it has the ability of the
separating two narrow spectral peaks (Li et al., 2016). It has a low picogram sensitivity which
means it can address the smallest differences in peak which can further change the reading of the
instrument. bruker’ s time to flight mass spectrometry offers advanced qualitative and
quantitative multitarget screening for forensic and doping control. Besides, it offers Dual ion
funnel, IonBooster Source, CaptiveSpray nanoBooster (Roth et al., 2018).
Advantage and disadvantage:
Advantage:
• Time to flight mass spectrometry uses an approach of analyzing a broad molecular weight
range of signals associated with polar and non-polar compounds in a single sample.

9
MASS SPECTROMETRY
• The mechanism of time to flight mass spectrometry increases mass accuracy and mass
resolution (Cai, Xu & Wu, 2015).
• Time to flight mass spectrometry increase dynamic range when over broad molecular weight
range.
• The incorporation of ion mobility offers the additional stages of separation of structurally
distinct isobaric compounds. The ion mobility is for the metabolites and an isobaric compound
which cannot be separated by chromatographic separation (Roth et al., 2018).
• It has the ability to record entire data at a time.
• Remarkably simple spectra which can be interpreted easily.
Disadvantages:
• The compound used for the creation of ion and detection has to be volatile in nature. Because
of volatile issues in the technology liquid chromatography-mass spectrometry is gaining
popularity.
• Another potential disadvantage is that it costs 2 to 3 times higher than others.
• Instruments drift that can be as high as 5-10% per hour (Roth et al., 2018).
• Interference effects may occur in this case.
Conclusion:
Thus, it can be concluded that in the field of research, the contribution of the mass
spectrometry is revolutionary. The hundreds of the researchers and laboratories around the globe
use mass spectrometry to investigate the phenomenon on the molecular level, especially for the
MASS SPECTROMETRY
• The mechanism of time to flight mass spectrometry increases mass accuracy and mass
resolution (Cai, Xu & Wu, 2015).
• Time to flight mass spectrometry increase dynamic range when over broad molecular weight
range.
• The incorporation of ion mobility offers the additional stages of separation of structurally
distinct isobaric compounds. The ion mobility is for the metabolites and an isobaric compound
which cannot be separated by chromatographic separation (Roth et al., 2018).
• It has the ability to record entire data at a time.
• Remarkably simple spectra which can be interpreted easily.
Disadvantages:
• The compound used for the creation of ion and detection has to be volatile in nature. Because
of volatile issues in the technology liquid chromatography-mass spectrometry is gaining
popularity.
• Another potential disadvantage is that it costs 2 to 3 times higher than others.
• Instruments drift that can be as high as 5-10% per hour (Roth et al., 2018).
• Interference effects may occur in this case.
Conclusion:
Thus, it can be concluded that in the field of research, the contribution of the mass
spectrometry is revolutionary. The hundreds of the researchers and laboratories around the globe
use mass spectrometry to investigate the phenomenon on the molecular level, especially for the

10
MASS SPECTROMETRY
drug discovery, quality control, and food safety protocol. Considering, time to flight mass
spectrometry, the key principle of ion separation is that the mass analyzer separate ions based on
the mass to charge ratio. The mass range is decided by an m/z ratio., m stands for mass and Z
stands for the charge of ions. In Bruker's time to flight mass spectrometry, a mass range is
39.0229 to 148 kDa, even can detect 70 to 900 m/z. Bruker’ s mass spectrometry uses
electrospray as well as MALDI as ion sources to generate ions. Microchannel detector is used as
a detection system in Bruker's Time to flight mass spectrometer. The advantages of TOF are it
increases dynamic range in case of broad molecular weight range and it has the ability to record
entire data at a time. The disadvantages of it are that it costs are 2 to 3 times higher than others
and interference may be observed.
MASS SPECTROMETRY
drug discovery, quality control, and food safety protocol. Considering, time to flight mass
spectrometry, the key principle of ion separation is that the mass analyzer separate ions based on
the mass to charge ratio. The mass range is decided by an m/z ratio., m stands for mass and Z
stands for the charge of ions. In Bruker's time to flight mass spectrometry, a mass range is
39.0229 to 148 kDa, even can detect 70 to 900 m/z. Bruker’ s mass spectrometry uses
electrospray as well as MALDI as ion sources to generate ions. Microchannel detector is used as
a detection system in Bruker's Time to flight mass spectrometer. The advantages of TOF are it
increases dynamic range in case of broad molecular weight range and it has the ability to record
entire data at a time. The disadvantages of it are that it costs are 2 to 3 times higher than others
and interference may be observed.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

11
MASS SPECTROMETRY
References:
Angeletti, S. (2017). Matrix assisted laser desorption time of flight mass spectrometry (MALDI-
TOF MS) in clinical microbiology. Journal of microbiological methods, 138, 20-29.
Bruker.com. (2019). Magnetic Resonance | Spectrometry | Technologies. Retrieved from
https://www.bruker.com/products/mr.html?
gclid=EAIaIQobChMI98vm_qP04AIVRpWPCh3yjAJdEAAYASAAEgLq-vD_BwE
Buckwalter, S. P., Olson, S. L., Connelly, B. J., Lucas, B. C., Rodning, A. A., Walchak, R. C., ...
& Wengenack, N. L. (2016). Evaluation of matrix-assisted laser desorption ionization−
time of flight mass spectrometry for identification of Mycobacterium species, Nocardia
species, and other aerobic Actinomycetes. Journal of clinical microbiology, 54(2), 376-
384.
Cai, T., Xu, X. Y., & Wu, Z. J. (2015). Analysis of diarylmethylamine compounds using
electrospray mass spectrometry: formation mechanisms of radical ions and dehydro
cations. Analyst, 140(23), 7864-7867.
Clark, C. M., Costa, M. S., Sanchez, L. M., & Murphy, B. T. (2018). Coupling MALDI-TOF
mass spectrometry protein and specialized metabolite analyses to rapidly discriminate
bacterial function. Proceedings of the National Academy of Sciences, 115(19), 4981-
4986.
Doern, C. D., & Butler-Wu, S. M. (2016). Emerging and future applications of matrix-assisted
laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry in the
clinical microbiology laboratory: a report of the association for molecular pathology. The
Journal of Molecular Diagnostics, 18(6), 789-802.
MASS SPECTROMETRY
References:
Angeletti, S. (2017). Matrix assisted laser desorption time of flight mass spectrometry (MALDI-
TOF MS) in clinical microbiology. Journal of microbiological methods, 138, 20-29.
Bruker.com. (2019). Magnetic Resonance | Spectrometry | Technologies. Retrieved from
https://www.bruker.com/products/mr.html?
gclid=EAIaIQobChMI98vm_qP04AIVRpWPCh3yjAJdEAAYASAAEgLq-vD_BwE
Buckwalter, S. P., Olson, S. L., Connelly, B. J., Lucas, B. C., Rodning, A. A., Walchak, R. C., ...
& Wengenack, N. L. (2016). Evaluation of matrix-assisted laser desorption ionization−
time of flight mass spectrometry for identification of Mycobacterium species, Nocardia
species, and other aerobic Actinomycetes. Journal of clinical microbiology, 54(2), 376-
384.
Cai, T., Xu, X. Y., & Wu, Z. J. (2015). Analysis of diarylmethylamine compounds using
electrospray mass spectrometry: formation mechanisms of radical ions and dehydro
cations. Analyst, 140(23), 7864-7867.
Clark, C. M., Costa, M. S., Sanchez, L. M., & Murphy, B. T. (2018). Coupling MALDI-TOF
mass spectrometry protein and specialized metabolite analyses to rapidly discriminate
bacterial function. Proceedings of the National Academy of Sciences, 115(19), 4981-
4986.
Doern, C. D., & Butler-Wu, S. M. (2016). Emerging and future applications of matrix-assisted
laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry in the
clinical microbiology laboratory: a report of the association for molecular pathology. The
Journal of Molecular Diagnostics, 18(6), 789-802.

12
MASS SPECTROMETRY
Gouriet, F., Ghiab, F., Couderc, C., Bittar, F., Dupont, H. T., Flaudrops, C., ... & Fenollar, F.
(2016). Evaluation of a new extraction protocol for yeast identification by mass
spectrometry. Journal of microbiological methods, 129, 61-65.
Hagiwara, Y., Sieverling, L., Hanif, F., Anton, J., Dickinson, E. R., Bui, T. T., ... & Nikolova, P.
V. (2016). Consequences of point mutations in melanoma-associated antigen 4 (MAGE-
A4) protein: Insights from structural and biophysical studies. Scientific reports, 6, 25182.
Idelevich, E. A., Sparbier, K., Kostrzewa, M., & Becker, K. (2018). Rapid detection of antibiotic
resistance by MALDI-TOF mass spectrometry using a novel direct-on-target
microdroplet growth assay. Clinical Microbiology and Infection, 24(7), 738-743.
Kathuria, S., Singh, P. K., Sharma, C., Prakash, A., Masih, A., Kumar, A., ... & Chowdhary, A.
(2015). Multidrug-resistant Candida auris misidentified as Candida haemulonii:
characterization by matrix-assisted laser desorption ionization–time of flight mass
spectrometry and DNA sequencing and its antifungal susceptibility profile variability by
Vitek 2, CLSI broth microdilution, and Etest method. Journal of clinical
microbiology, 53(6), 1823-1830.
Li, H., Nguyen, H. H., Loo, R. R. O., Campuzano, I. D., & Loo, J. A. (2018). An integrated
native mass spectrometry and top-down proteomics method that connects sequence to
structure and function of macromolecular complexes. Nature chemistry, 10(2), 139.
Ouyang, X., Leonards, P. E., Tousova, Z., Slobodnik, J., de Boer, J., & Lamoree, M. H. (2016).
Rapid screening of acetylcholinesterase inhibitors by effect-directed analysis using LC×
LC fractionation, a high throughput in vitro assay, and parallel identification by time of
flight mass spectrometry. Analytical chemistry, 88(4), 2353-2360.
MASS SPECTROMETRY
Gouriet, F., Ghiab, F., Couderc, C., Bittar, F., Dupont, H. T., Flaudrops, C., ... & Fenollar, F.
(2016). Evaluation of a new extraction protocol for yeast identification by mass
spectrometry. Journal of microbiological methods, 129, 61-65.
Hagiwara, Y., Sieverling, L., Hanif, F., Anton, J., Dickinson, E. R., Bui, T. T., ... & Nikolova, P.
V. (2016). Consequences of point mutations in melanoma-associated antigen 4 (MAGE-
A4) protein: Insights from structural and biophysical studies. Scientific reports, 6, 25182.
Idelevich, E. A., Sparbier, K., Kostrzewa, M., & Becker, K. (2018). Rapid detection of antibiotic
resistance by MALDI-TOF mass spectrometry using a novel direct-on-target
microdroplet growth assay. Clinical Microbiology and Infection, 24(7), 738-743.
Kathuria, S., Singh, P. K., Sharma, C., Prakash, A., Masih, A., Kumar, A., ... & Chowdhary, A.
(2015). Multidrug-resistant Candida auris misidentified as Candida haemulonii:
characterization by matrix-assisted laser desorption ionization–time of flight mass
spectrometry and DNA sequencing and its antifungal susceptibility profile variability by
Vitek 2, CLSI broth microdilution, and Etest method. Journal of clinical
microbiology, 53(6), 1823-1830.
Li, H., Nguyen, H. H., Loo, R. R. O., Campuzano, I. D., & Loo, J. A. (2018). An integrated
native mass spectrometry and top-down proteomics method that connects sequence to
structure and function of macromolecular complexes. Nature chemistry, 10(2), 139.
Ouyang, X., Leonards, P. E., Tousova, Z., Slobodnik, J., de Boer, J., & Lamoree, M. H. (2016).
Rapid screening of acetylcholinesterase inhibitors by effect-directed analysis using LC×
LC fractionation, a high throughput in vitro assay, and parallel identification by time of
flight mass spectrometry. Analytical chemistry, 88(4), 2353-2360.

13
MASS SPECTROMETRY
Oviaño, M., & Bou, G. (2018). Matrix-assisted laser desorption ionization–time of flight mass
spectrometry for the rapid detection of antimicrobial resistance mechanisms and
beyond. Clinical microbiology reviews, 32(1), e00037-18.
Perrenoud, A. G. G., Guillarme, D., Boccard, J., Veuthey, J. L., Barron, D., & Moco, S. (2016).
Ultra-high performance supercritical fluid chromatography coupled with quadrupole-
time-of-flight mass spectrometry as a performing tool for bioactive analysis. Journal of
Chromatography A, 1450, 101-111.
Roth, S. P., Burk, J., Schiller, J., & Nimptsch, A. (2018). De novo synthesis of
glycosaminoglycans by equine multipotent mesenchymal stromal cells in vitro–Studied
by stable isotopic labeling and matrix-assisted laser desorption ionization mass
spectrometry. Journal of Carbohydrate Chemistry, 37(2), 69-80.
Sanguinetti, M., & Posteraro, B. (2017). Identification of molds by matrix-assisted laser
desorption ionization–time of flight mass spectrometry. Journal of clinical
microbiology, 55(2), 369-379.
Stockwell, C. E., Veres, P. R., Williams, J., & Yokelson, R. J. (2015). Characterization of
biomass burning emissions from cooking fires, peat, crop residue, and other fuels with
high-resolution proton-transfer-reaction time-of-flight mass spectrometry. Atmospheric
Chemistry and Physics, 15(2), 845-865.
MASS SPECTROMETRY
Oviaño, M., & Bou, G. (2018). Matrix-assisted laser desorption ionization–time of flight mass
spectrometry for the rapid detection of antimicrobial resistance mechanisms and
beyond. Clinical microbiology reviews, 32(1), e00037-18.
Perrenoud, A. G. G., Guillarme, D., Boccard, J., Veuthey, J. L., Barron, D., & Moco, S. (2016).
Ultra-high performance supercritical fluid chromatography coupled with quadrupole-
time-of-flight mass spectrometry as a performing tool for bioactive analysis. Journal of
Chromatography A, 1450, 101-111.
Roth, S. P., Burk, J., Schiller, J., & Nimptsch, A. (2018). De novo synthesis of
glycosaminoglycans by equine multipotent mesenchymal stromal cells in vitro–Studied
by stable isotopic labeling and matrix-assisted laser desorption ionization mass
spectrometry. Journal of Carbohydrate Chemistry, 37(2), 69-80.
Sanguinetti, M., & Posteraro, B. (2017). Identification of molds by matrix-assisted laser
desorption ionization–time of flight mass spectrometry. Journal of clinical
microbiology, 55(2), 369-379.
Stockwell, C. E., Veres, P. R., Williams, J., & Yokelson, R. J. (2015). Characterization of
biomass burning emissions from cooking fires, peat, crop residue, and other fuels with
high-resolution proton-transfer-reaction time-of-flight mass spectrometry. Atmospheric
Chemistry and Physics, 15(2), 845-865.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

14
MASS SPECTROMETRY
MASS SPECTROMETRY
1 out of 14
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.



