Medical Devices Report: Pacemaker, Materials, and Clinical Efficacy
VerifiedAdded on 2020/03/16
|14
|3305
|77
Report
AI Summary
This report provides a comprehensive overview of medical devices, with a specific focus on pacemakers and their role in treating cardiac arrhythmias. It begins with an introduction to pacemakers, their function, and the materials used in their construction, including biocompatible materials like titanium and silicone. The report then delves into the design and mechanism of action of pacemakers, explaining how they monitor and control heartbeats. A significant portion of the report is dedicated to the clinical safety and efficacy of pacemakers, drawing on multiple studies that demonstrate their effectiveness and safety in various contexts, including use with MRI scanners and in patients with bradycardia. The report also discusses the evolution of pacemaker technology, including the emergence of leadless pacemakers, highlighting their advantages and clinical outcomes. The studies analyzed cover implantation techniques, adverse events, and long-term follow-up data, providing a detailed understanding of the benefits and potential risks associated with pacemaker use. The conclusion emphasizes the importance of pacemakers in modern healthcare and their contribution to improving the quality of life for patients with heart conditions.

Running head: MEDICAL DEVICES
Medical Devices and Diagbostics
Name of the student:
Name of the university:
Author note:
Medical Devices and Diagbostics
Name of the student:
Name of the university:
Author note:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1MEDICAL DEVICES
Abstract
This assignment deals with the study of the medical implants and their use in the field of
healthcare and treatment. A medical implant is a type of device that can be used as a
replacement of a biological structure. These implants are generally manmade as compared to
the transplant because it is a biomedical tissue. The surfaces of the implants that are in contact
with the body are generally made up of biomedical material such as titanium or silicone
depending upon its needs. Some of the implants contains electronics, for example, pacemaker.
Some are bioactive implants. In this assignment there is a detailed description about two
different types of medical implants, its material, design, clinical efficacy and safety. From this
paper you can get a clear idea of the use of medical implants.
Abstract
This assignment deals with the study of the medical implants and their use in the field of
healthcare and treatment. A medical implant is a type of device that can be used as a
replacement of a biological structure. These implants are generally manmade as compared to
the transplant because it is a biomedical tissue. The surfaces of the implants that are in contact
with the body are generally made up of biomedical material such as titanium or silicone
depending upon its needs. Some of the implants contains electronics, for example, pacemaker.
Some are bioactive implants. In this assignment there is a detailed description about two
different types of medical implants, its material, design, clinical efficacy and safety. From this
paper you can get a clear idea of the use of medical implants.

2MEDICAL DEVICES
Pacemaker
Introduction
A pacemaker is a device which is very small in size and is placed in the chest or
abdomen. It is help to control the abnormality of the heart beats (as shown in fig. 1). This
device works by using low energy electric for prompting the heart to beat at normal rates.
Pacemakers are generally used for treating arrhythmias. Arrhythmia is type of dysfunction of
the heart where a person suffers from the problems with the rate of the rhythm of heartbeat.
In case of arrhythmia, the heartbeat may become too fast or too slow or with an irregular
rhythm (Foster et al. 2017). Having a pacemaker can extraordinarily enhance your personal
satisfaction and for a few people it can be life saving. Most pacemakers are extremely solid and
agreeable. They're littler than a normal matchbox and weigh around 20 to 50 grams.
Materials
The materials that are used for making the pacemakers must be nontoxic, sterilizable
and have the ability to function according to the conditions of the body. The parts of the
pacemaker include the casing, electronics and the leads. These parts are generally made up of
biocompatible materials. The casing is generally made up of titanium or metal alloy but these
are insulated with a polymer such as polyurethane. In this design the metal tip is only exposed
and the circuit is generally made up of semiconductors of silicon (Miller, Nazarian and Halperin
2016).
Design
Pacemaker
Introduction
A pacemaker is a device which is very small in size and is placed in the chest or
abdomen. It is help to control the abnormality of the heart beats (as shown in fig. 1). This
device works by using low energy electric for prompting the heart to beat at normal rates.
Pacemakers are generally used for treating arrhythmias. Arrhythmia is type of dysfunction of
the heart where a person suffers from the problems with the rate of the rhythm of heartbeat.
In case of arrhythmia, the heartbeat may become too fast or too slow or with an irregular
rhythm (Foster et al. 2017). Having a pacemaker can extraordinarily enhance your personal
satisfaction and for a few people it can be life saving. Most pacemakers are extremely solid and
agreeable. They're littler than a normal matchbox and weigh around 20 to 50 grams.
Materials
The materials that are used for making the pacemakers must be nontoxic, sterilizable
and have the ability to function according to the conditions of the body. The parts of the
pacemaker include the casing, electronics and the leads. These parts are generally made up of
biocompatible materials. The casing is generally made up of titanium or metal alloy but these
are insulated with a polymer such as polyurethane. In this design the metal tip is only exposed
and the circuit is generally made up of semiconductors of silicon (Miller, Nazarian and Halperin
2016).
Design
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3MEDICAL DEVICES
The pacemaker generally made up of battery, leads and the circuit which consist of
resistors, diodes, capacitors, and semiconductors. The battery is needed to store energy for the
stimulation of heart. It is also used to provide the sensors with power. The leads that are used
to build up the pacemaker are thin and insulated. For the single chambers pacemakers a single
lead is needed and for dual chambers pacemakers, two leads are needed. The modern day’s
pacemakers are a bigger improvement as compared to the earlier models, as the circuit models
have become much smaller (Weiss et al. 2013). These results in the consumption of less space
in the body; require less energy and are greatly reliable.
Mechanism of action
The pacemaker helps for monitoring and control the heartbeat. The electrodes present in the
pacemaker detect the heartbeat and sends the data through the wires to the computer. If the
heart beat is abnormal then the generator will send electric pulses to the heart. Then these
pulses travel through the wires and reach the heart. They can also adjust the heart rate so that
the heart can work in a better way (Brunner et al. 2014).
Clinical safety and efficacy
The modern pacemakers are safe to use. According to Khan et al. (2013), the pacemaker
can be used during electromagnetic bronchosopy. They had proved that pacemakers of 1.5-T or
3-T magnetic fields can be used in the MRI scanners. They selected some 24 patients with the
pacemakers and suffering from lung lesions. Then the electromagnetic bronchoscopy was done,
then pacing systems were interrogated and then ECG was recorded. It was seen that there were
no disturbances in the pacemaker device and those are working well. It is safe while going
The pacemaker generally made up of battery, leads and the circuit which consist of
resistors, diodes, capacitors, and semiconductors. The battery is needed to store energy for the
stimulation of heart. It is also used to provide the sensors with power. The leads that are used
to build up the pacemaker are thin and insulated. For the single chambers pacemakers a single
lead is needed and for dual chambers pacemakers, two leads are needed. The modern day’s
pacemakers are a bigger improvement as compared to the earlier models, as the circuit models
have become much smaller (Weiss et al. 2013). These results in the consumption of less space
in the body; require less energy and are greatly reliable.
Mechanism of action
The pacemaker helps for monitoring and control the heartbeat. The electrodes present in the
pacemaker detect the heartbeat and sends the data through the wires to the computer. If the
heart beat is abnormal then the generator will send electric pulses to the heart. Then these
pulses travel through the wires and reach the heart. They can also adjust the heart rate so that
the heart can work in a better way (Brunner et al. 2014).
Clinical safety and efficacy
The modern pacemakers are safe to use. According to Khan et al. (2013), the pacemaker
can be used during electromagnetic bronchosopy. They had proved that pacemakers of 1.5-T or
3-T magnetic fields can be used in the MRI scanners. They selected some 24 patients with the
pacemakers and suffering from lung lesions. Then the electromagnetic bronchoscopy was done,
then pacing systems were interrogated and then ECG was recorded. It was seen that there were
no disturbances in the pacemaker device and those are working well. It is safe while going
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4MEDICAL DEVICES
through the security detectors of malls, railway stations or airport. They will not damage the
pacemaker or ICD. Implantation of the pacemaker for heart is choice of treatment in case of
severe or symptomatic bradycardia. So it can be concluded that electromagnetic Navigation
Bronchoscopy is safe to perform in the patients having pacemakers. Generally now-a-days,
pacemaker implantation has been greatly evolved. Some highly sophisticated devices are now
available in the market which provides optimal support for the treatment of any type of
arrhythmia.
In the second study, Xiang et al. (2016) had done a randomized controlled trial at 14
centers in china for the safety and efficacy of a cardiac pacemaker. Cardiac arrhythmia is a
noteworthy clinical issue prompting significant grimness and mortality. As indicated by the
insights of American Heart Association, the occurrence of bradyarrhythmia was accounted for
to be 4%. It is assessed to influence 5.6– 12.0 million individuals in 2050 and will prompt more
than 400,000 yearly sudden heart deaths in the United States. Implantation a cardiovascular
pacemaker is the best path for treating patients with bradyarrhythmia. In the previous decades,
heart pacemakers have spared a huge number of patients experiencing cardiovascular
bradyarrhythmia and have enhanced the personal satisfaction of patients. They collected the
parameters of the pacemaker systems immediately after the implantation of the device. A 6-
month follow-up for the pacing rate was being recorded. Electrical properties, single-pole and
double-pole polarity conversion, magnet response, adverse events and rate response function
of the pacing system were analyzed. For measuring the primary qualitative result Chi-square
test, Wilcoxon signed-rank test and paired t-test were used (Weiss et al. 2013). The outcomes
were analyzed and compared for achieving the results of the distributed measurement data.
through the security detectors of malls, railway stations or airport. They will not damage the
pacemaker or ICD. Implantation of the pacemaker for heart is choice of treatment in case of
severe or symptomatic bradycardia. So it can be concluded that electromagnetic Navigation
Bronchoscopy is safe to perform in the patients having pacemakers. Generally now-a-days,
pacemaker implantation has been greatly evolved. Some highly sophisticated devices are now
available in the market which provides optimal support for the treatment of any type of
arrhythmia.
In the second study, Xiang et al. (2016) had done a randomized controlled trial at 14
centers in china for the safety and efficacy of a cardiac pacemaker. Cardiac arrhythmia is a
noteworthy clinical issue prompting significant grimness and mortality. As indicated by the
insights of American Heart Association, the occurrence of bradyarrhythmia was accounted for
to be 4%. It is assessed to influence 5.6– 12.0 million individuals in 2050 and will prompt more
than 400,000 yearly sudden heart deaths in the United States. Implantation a cardiovascular
pacemaker is the best path for treating patients with bradyarrhythmia. In the previous decades,
heart pacemakers have spared a huge number of patients experiencing cardiovascular
bradyarrhythmia and have enhanced the personal satisfaction of patients. They collected the
parameters of the pacemaker systems immediately after the implantation of the device. A 6-
month follow-up for the pacing rate was being recorded. Electrical properties, single-pole and
double-pole polarity conversion, magnet response, adverse events and rate response function
of the pacing system were analyzed. For measuring the primary qualitative result Chi-square
test, Wilcoxon signed-rank test and paired t-test were used (Weiss et al. 2013). The outcomes
were analyzed and compared for achieving the results of the distributed measurement data.

5MEDICAL DEVICES
Safety evaluations were directed by recording all-causes of deaths, pacemaker-related
antagonistic occasions and cardiovascular deaths within six months of implantation. In addition,
the clinical side effects, essential signs (circulatory strain and heart rate), and research center
parameters were checked. In the event that irregular changes were noticed, their relationship
with the pacemaker was examined. Research facility parameters included finish blood check,
liver capacity, renal capacity, and blood coagulating tests (Bailey et al. 2015).
In the third study of Reddy et al. (2015), it has been described that every year, around 1
million individuals everywhere throughout the world get standard transvenous cardiovascular
pacemakers with dynamic fixation prompts treat bradycardia and heart piece. Despite huge
mechanical types of progress as the introduction of the pacemakers before six decades,
antagonistic events related to pacemaker occurring in 1 out of 10 patients. The events are
consistently related to surgical pocket, the transvenous lead, or pulse generator. These leads
are weak and easily, breaks or insurance dissatisfaction and can in like manner causes polluting,
cardiovascular gap, venous obstruction, and tricuspid regurgitating forward. Pulse generators
are being connected with ailment, stash hematoma, and skin breaking down.
As of late a gadget is being produced that is totally autonomous, leadless cardiovascular
pacemaker with joined battery, equipment, and terminals. Through the femoral vein, the
catheter is inserted and the leadless heart pacemaker is non-surgically placed inside the right
ventricle. Wiping out the device takes and transvenous lead furthermore conceivably restricts
some whole deal bothers saw with customary pacemakers, for instance, tricuspid valvular
heaving forward and thrombo-embolism over a patent foramen ovale (Durrani et al. 2016).
Achievability of the leadless heart pacemaker in individuals was showed up in the LEADLESS
Safety evaluations were directed by recording all-causes of deaths, pacemaker-related
antagonistic occasions and cardiovascular deaths within six months of implantation. In addition,
the clinical side effects, essential signs (circulatory strain and heart rate), and research center
parameters were checked. In the event that irregular changes were noticed, their relationship
with the pacemaker was examined. Research facility parameters included finish blood check,
liver capacity, renal capacity, and blood coagulating tests (Bailey et al. 2015).
In the third study of Reddy et al. (2015), it has been described that every year, around 1
million individuals everywhere throughout the world get standard transvenous cardiovascular
pacemakers with dynamic fixation prompts treat bradycardia and heart piece. Despite huge
mechanical types of progress as the introduction of the pacemakers before six decades,
antagonistic events related to pacemaker occurring in 1 out of 10 patients. The events are
consistently related to surgical pocket, the transvenous lead, or pulse generator. These leads
are weak and easily, breaks or insurance dissatisfaction and can in like manner causes polluting,
cardiovascular gap, venous obstruction, and tricuspid regurgitating forward. Pulse generators
are being connected with ailment, stash hematoma, and skin breaking down.
As of late a gadget is being produced that is totally autonomous, leadless cardiovascular
pacemaker with joined battery, equipment, and terminals. Through the femoral vein, the
catheter is inserted and the leadless heart pacemaker is non-surgically placed inside the right
ventricle. Wiping out the device takes and transvenous lead furthermore conceivably restricts
some whole deal bothers saw with customary pacemakers, for instance, tricuspid valvular
heaving forward and thrombo-embolism over a patent foramen ovale (Durrani et al. 2016).
Achievability of the leadless heart pacemaker in individuals was showed up in the LEADLESS
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6MEDICAL DEVICES
trial. A nonrandomized trial was finished reviewing the clinical security and sufficiency of
nonsurgical implantation of the Nanostim leadless cardiovascular pacemaker in patients who
require interminable ventricular pacing.
The arranged examination, that was reported here, incorporates the essential
investigation of viability and wellbeing in the underlying 300 patients who were taken after for
a half year (the essential companion) and results for every one of the 526 patients who were
enlisted as of June 2015 (the aggregate partner).
A global directing board of trustees, with the investment of the support, was in charge of the
outline and lead of the investigation and the revealing of the discoveries. Observing and
accumulation of the information and introductory information examinations were performed
by the support in organization with the guiding council (Bailey, Gleva and Woodard 2015).
Patients were rejected on the off chance that they had mechanical tricuspid-valve prosthesis,
pneumonic blood vessel hypertension, prior endocardial pacing or defibrillation leads, or a
substandard vena cava channel or in the event that they had experienced cardiovascular or
fringe vascular surgery inside 30 days before enlistment. The essential result investigation was a
prespecified evaluation of the essential viability and wellbeing end focuses in the initial 300
patients who were taken after for a half year. The composite essential viability end point was
both a restoratively adequate pacing catch edge (≤2.0 V at 0.4 msec) and remedially worthy
detecting sufficiency (R wave ≥5.0 mV, or an esteem equivalent to or more noteworthy than the
incentive at implantation) through a half year. The essential wellbeing end point was flexibility
from gadget related genuine unfavorable occasions amid the underlying a half year after
implantation (Falk et al. 2017).
trial. A nonrandomized trial was finished reviewing the clinical security and sufficiency of
nonsurgical implantation of the Nanostim leadless cardiovascular pacemaker in patients who
require interminable ventricular pacing.
The arranged examination, that was reported here, incorporates the essential
investigation of viability and wellbeing in the underlying 300 patients who were taken after for
a half year (the essential companion) and results for every one of the 526 patients who were
enlisted as of June 2015 (the aggregate partner).
A global directing board of trustees, with the investment of the support, was in charge of the
outline and lead of the investigation and the revealing of the discoveries. Observing and
accumulation of the information and introductory information examinations were performed
by the support in organization with the guiding council (Bailey, Gleva and Woodard 2015).
Patients were rejected on the off chance that they had mechanical tricuspid-valve prosthesis,
pneumonic blood vessel hypertension, prior endocardial pacing or defibrillation leads, or a
substandard vena cava channel or in the event that they had experienced cardiovascular or
fringe vascular surgery inside 30 days before enlistment. The essential result investigation was a
prespecified evaluation of the essential viability and wellbeing end focuses in the initial 300
patients who were taken after for a half year. The composite essential viability end point was
both a restoratively adequate pacing catch edge (≤2.0 V at 0.4 msec) and remedially worthy
detecting sufficiency (R wave ≥5.0 mV, or an esteem equivalent to or more noteworthy than the
incentive at implantation) through a half year. The essential wellbeing end point was flexibility
from gadget related genuine unfavorable occasions amid the underlying a half year after
implantation (Falk et al. 2017).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7MEDICAL DEVICES
Every single antagonistic occasion were mediated by an autonomous clinical-occasions
board of trustees. A genuine antagonistic occasion was characterized as any untoward
restorative event that prompted passing or to a genuine disintegration in the soundness of a
patient that brought about hazardous ailment or damage, perpetual impedance of a body
structure or a body capacity, inpatient or delayed hospitalization, or a therapeutic or surgical
mediation to counteract perilous sickness or damage or changeless weakness to a body
structure or a body work. Genuine unfavorable occasions were named gadget related on the off
chance that they were considered by the clinical-occasions board to be owing to the
investigational gadget or methodology (Lee et al. 2014).
The cohort study was assessed for all non– gadget related genuine unfriendly occasions
amid a half year of development. Such occasions were thought to be random to the
investigational gadget or method. Since the LEADLESS II trial is progressing, auxiliary
examinations were performed on information from extra patients who were enlisted as of June
2015, joined with information from the initial 300 patients, who had broadened follow-up past
a half year (add up to companion) (Figure 2). Extra investigations in the aggregate associate
included assurance of all gadgets related and non–gadget related genuine antagonistic
occasions amid development and the impact of administrator encounter (Miller et al. 2015).
It has been evaluated that if 300 patients were taken after for a half year, the
examination would have 90% power, at a two-sided 5.0% importance level, to demonstrate
rates of security and viability that would be better than foreordained execution objectives for
wellbeing and adequacy. The execution objective for the essential viability end purpose of both
a restoratively adequate pacing catch limit and a remedially satisfactory detecting sufficiency
Every single antagonistic occasion were mediated by an autonomous clinical-occasions
board of trustees. A genuine antagonistic occasion was characterized as any untoward
restorative event that prompted passing or to a genuine disintegration in the soundness of a
patient that brought about hazardous ailment or damage, perpetual impedance of a body
structure or a body capacity, inpatient or delayed hospitalization, or a therapeutic or surgical
mediation to counteract perilous sickness or damage or changeless weakness to a body
structure or a body work. Genuine unfavorable occasions were named gadget related on the off
chance that they were considered by the clinical-occasions board to be owing to the
investigational gadget or methodology (Lee et al. 2014).
The cohort study was assessed for all non– gadget related genuine unfriendly occasions
amid a half year of development. Such occasions were thought to be random to the
investigational gadget or method. Since the LEADLESS II trial is progressing, auxiliary
examinations were performed on information from extra patients who were enlisted as of June
2015, joined with information from the initial 300 patients, who had broadened follow-up past
a half year (add up to companion) (Figure 2). Extra investigations in the aggregate associate
included assurance of all gadgets related and non–gadget related genuine antagonistic
occasions amid development and the impact of administrator encounter (Miller et al. 2015).
It has been evaluated that if 300 patients were taken after for a half year, the
examination would have 90% power, at a two-sided 5.0% importance level, to demonstrate
rates of security and viability that would be better than foreordained execution objectives for
wellbeing and adequacy. The execution objective for the essential viability end purpose of both
a restoratively adequate pacing catch limit and a remedially satisfactory detecting sufficiency

8MEDICAL DEVICES
through a half year was 85%, and the investigation was fueled under the supposition that the
rate of this end point would be 91.5% or higher. The execution objective for adequacy
depended on a continuous pacemaker contemplates that is supported by St. Jude Medical
(Phillips 2015). The execution objective for the essential wellbeing end purpose of flexibility
from gadget related genuine unfriendly occasions through a half year was 86%, and the
investigation was fueled under the presumption that the occasion free rate would be 92%. The
outcomes demonstrate that pacemaker implantation was effective in 504 of the 526 patients
(95.8%). Most patients (70.2%) did not require gadget repositioning after starting sending. The
term of healing facility remain from implantation to release was 1.1±1.7 days (run, 0 to 33)
(Ritter et al. 2015).
Conclusion
Thus from the above discussion, we can get an idea about pacemaker and the
importance of its use. A pacemaker is a device which is very small in size and is placed in the
chest or abdomen. The modern day’s pacemakers are a bigger improvement as compared to
the earlier models, as the circuit models have become much smaller. The pacemaker helps for
monitoring and control the heartbeat. Most pacemakers are extremely solid and agreeable.
They're a bit small than a normal matchbox and weigh around 20 to 50 grams. A pacemaker is
placed simply under your neckline bone and will have at least one leads which are set into your
heart through a vein.
through a half year was 85%, and the investigation was fueled under the supposition that the
rate of this end point would be 91.5% or higher. The execution objective for adequacy
depended on a continuous pacemaker contemplates that is supported by St. Jude Medical
(Phillips 2015). The execution objective for the essential wellbeing end purpose of flexibility
from gadget related genuine unfriendly occasions through a half year was 86%, and the
investigation was fueled under the presumption that the occasion free rate would be 92%. The
outcomes demonstrate that pacemaker implantation was effective in 504 of the 526 patients
(95.8%). Most patients (70.2%) did not require gadget repositioning after starting sending. The
term of healing facility remain from implantation to release was 1.1±1.7 days (run, 0 to 33)
(Ritter et al. 2015).
Conclusion
Thus from the above discussion, we can get an idea about pacemaker and the
importance of its use. A pacemaker is a device which is very small in size and is placed in the
chest or abdomen. The modern day’s pacemakers are a bigger improvement as compared to
the earlier models, as the circuit models have become much smaller. The pacemaker helps for
monitoring and control the heartbeat. Most pacemakers are extremely solid and agreeable.
They're a bit small than a normal matchbox and weigh around 20 to 50 grams. A pacemaker is
placed simply under your neckline bone and will have at least one leads which are set into your
heart through a vein.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
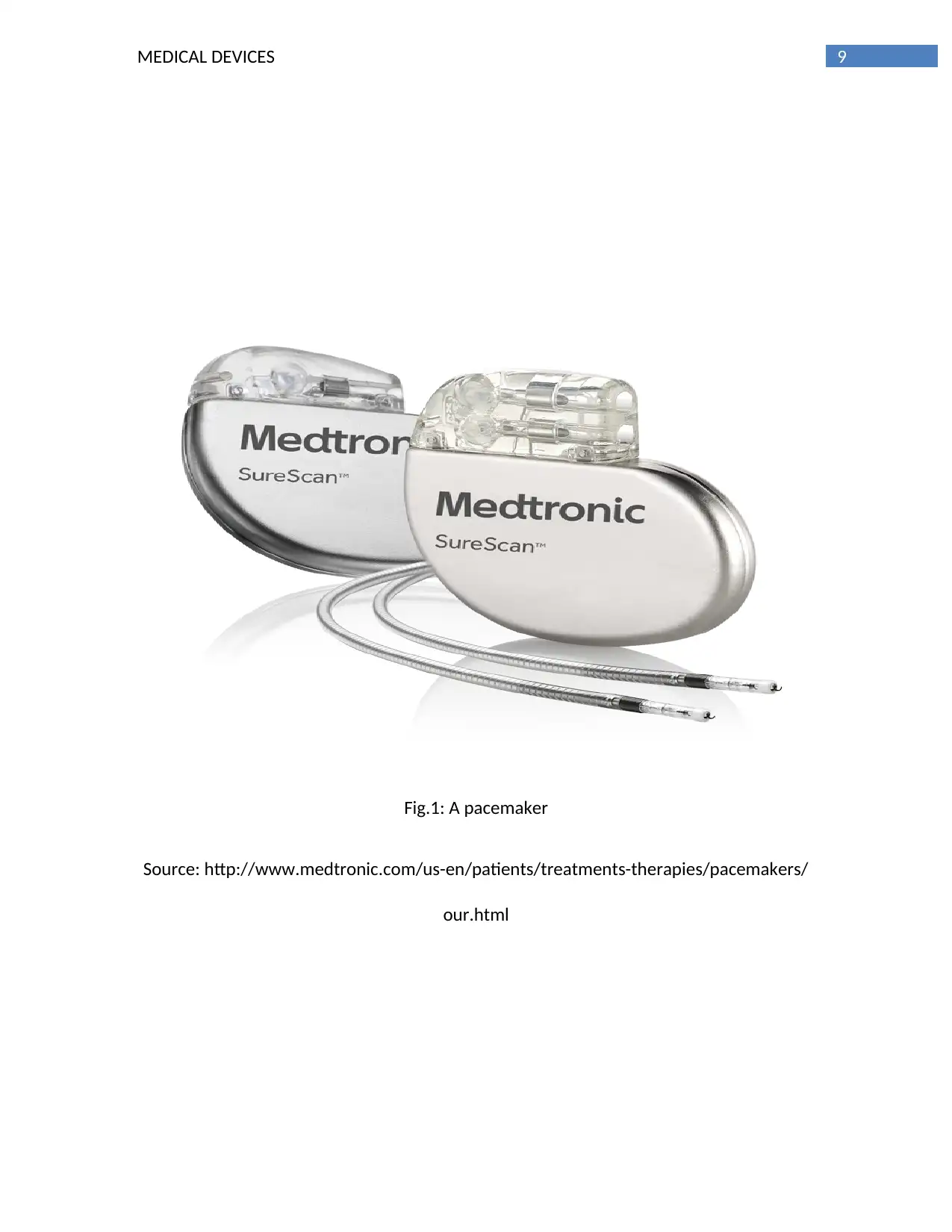
9MEDICAL DEVICES
Fig.1: A pacemaker
Source: http://www.medtronic.com/us-en/patients/treatments-therapies/pacemakers/
our.html
Fig.1: A pacemaker
Source: http://www.medtronic.com/us-en/patients/treatments-therapies/pacemakers/
our.html
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
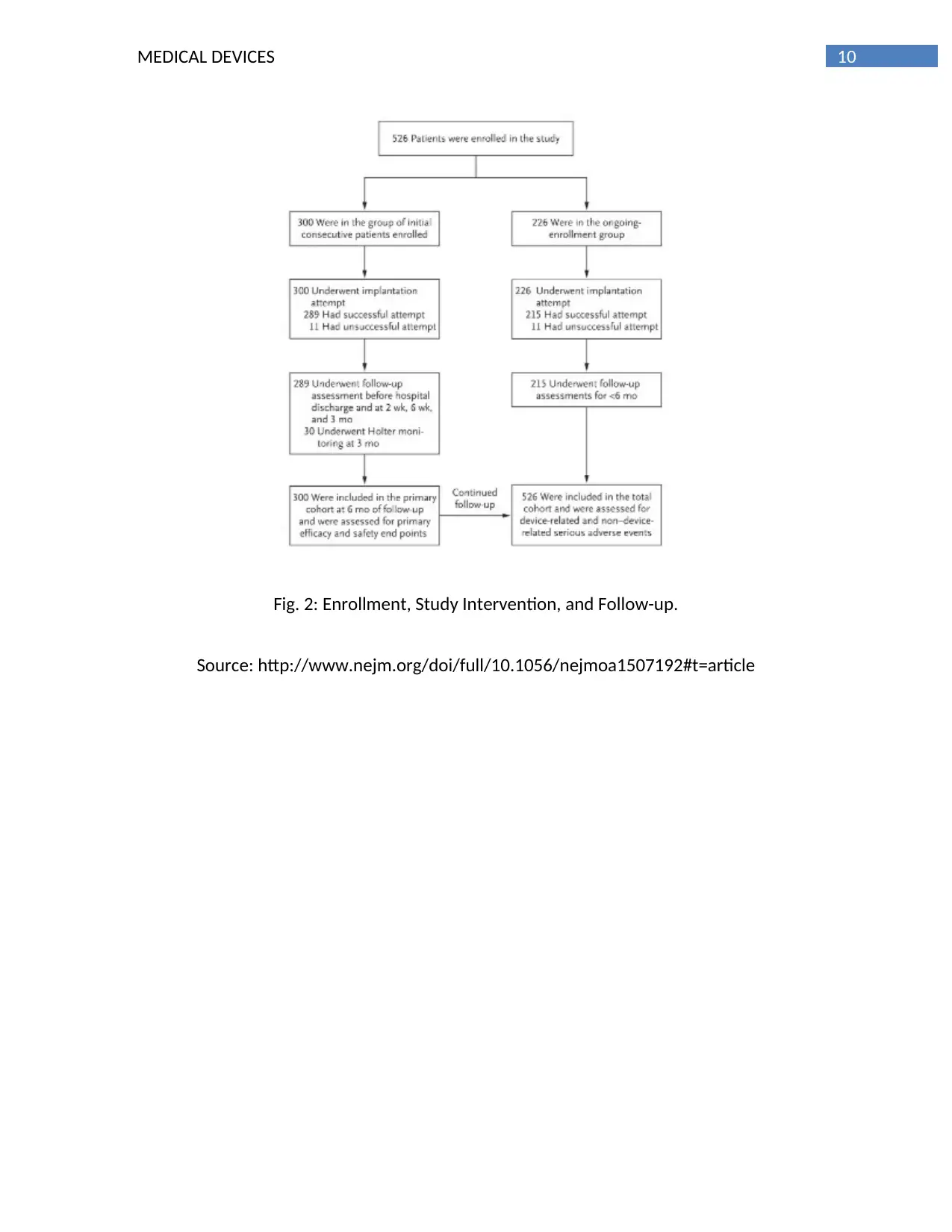
10MEDICAL DEVICES
Fig. 2: Enrollment, Study Intervention, and Follow-up.
Source: http://www.nejm.org/doi/full/10.1056/nejmoa1507192#t=article
Fig. 2: Enrollment, Study Intervention, and Follow-up.
Source: http://www.nejm.org/doi/full/10.1056/nejmoa1507192#t=article

11MEDICAL DEVICES
References
1. Foster, A.J., Tockman, B.A., Liu, L., Simms Jr, H.D. and Bustillos, A.M., Cardiac
Pacemakers, Inc., 2017. Implantable medical devices with separate fixation mechanism.
U.S. Patent 9,694,172.
2. Miller, J.D., Nazarian, S. and Halperin, H.R., 2016. Implantable electronic cardiac devices
and compatibility with magnetic resonance imaging. Journal of the American College of
Cardiology, 68(14), pp.1590-1598.
3. Weiss, R., Knight, B.P., Gold, M.R., Leon, A.R., Herre, J.M., Hood, M., Rashtian, M.,
Kremers, M., Crozier, I., Lee, K.L. and Smith, W., 2013. Safety and efficacy of a totally
subcutaneous implantable-cardioverter defibrillator. Circulation, 128(9), pp.944-953.
4. Brunner, M.P., Cronin, E.M., Duarte, V.E., Yu, C., Tarakji, K.G., Martin, D.O., Callahan, T.,
Cantillon, D.J., Niebauer, M.J., Saliba, W.I. and Kanj, M., 2014. Clinical predictors of
adverse patient outcomes in an experience of more than 5000 chronic endovascular
pacemaker and defibrillator lead extractions. Heart Rhythm, 11(5), pp.799-805.
5. Khan, A.Y., Berkowitz, D., Krimsky, W.S., Hogarth, D.K., Parks, C. and Bechara, R., 2013.
Safety of pacemakers and defibrillators in electromagnetic navigation bronchoscopy.
CHEST Journal, 143(1), pp.75-81.
6. Xiang, M.X., Wang, D.Q., Xu, J., Zhang, Z., Hu, J.X., Wang, D.M., Gu, X., Liu, H.P., Guo, T.,
Yang, X.J. and Ling, F., 2016. Evaluation of Safety and Efficacy of Qinming8631 DR
Implantable Cardiac Pacemaker in Chinese Patients: A Prospective, Multicenter,
Randomized Controlled Trial of the First Domestically Developed Pacemaker of China.
Chinese medical journal, 129(22), p.2659.
References
1. Foster, A.J., Tockman, B.A., Liu, L., Simms Jr, H.D. and Bustillos, A.M., Cardiac
Pacemakers, Inc., 2017. Implantable medical devices with separate fixation mechanism.
U.S. Patent 9,694,172.
2. Miller, J.D., Nazarian, S. and Halperin, H.R., 2016. Implantable electronic cardiac devices
and compatibility with magnetic resonance imaging. Journal of the American College of
Cardiology, 68(14), pp.1590-1598.
3. Weiss, R., Knight, B.P., Gold, M.R., Leon, A.R., Herre, J.M., Hood, M., Rashtian, M.,
Kremers, M., Crozier, I., Lee, K.L. and Smith, W., 2013. Safety and efficacy of a totally
subcutaneous implantable-cardioverter defibrillator. Circulation, 128(9), pp.944-953.
4. Brunner, M.P., Cronin, E.M., Duarte, V.E., Yu, C., Tarakji, K.G., Martin, D.O., Callahan, T.,
Cantillon, D.J., Niebauer, M.J., Saliba, W.I. and Kanj, M., 2014. Clinical predictors of
adverse patient outcomes in an experience of more than 5000 chronic endovascular
pacemaker and defibrillator lead extractions. Heart Rhythm, 11(5), pp.799-805.
5. Khan, A.Y., Berkowitz, D., Krimsky, W.S., Hogarth, D.K., Parks, C. and Bechara, R., 2013.
Safety of pacemakers and defibrillators in electromagnetic navigation bronchoscopy.
CHEST Journal, 143(1), pp.75-81.
6. Xiang, M.X., Wang, D.Q., Xu, J., Zhang, Z., Hu, J.X., Wang, D.M., Gu, X., Liu, H.P., Guo, T.,
Yang, X.J. and Ling, F., 2016. Evaluation of Safety and Efficacy of Qinming8631 DR
Implantable Cardiac Pacemaker in Chinese Patients: A Prospective, Multicenter,
Randomized Controlled Trial of the First Domestically Developed Pacemaker of China.
Chinese medical journal, 129(22), p.2659.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 14
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.