MEDS2001 Report: Deep Brain Stimulation for Parkinson's Symptoms
VerifiedAdded on 2023/04/17
|7
|1275
|140
Report
AI Summary
This report explores the effectiveness of deep brain stimulation (DBS) in reducing the symptoms of Parkinson's disease (PD), a degenerative disorder affecting the central nervous system. It highlights the prevalence and cost of PD in Australia, noting that DBS involves implanting electrodes to generate electrical impulses in specific brain areas. The report traces the history of PD and its symptoms, emphasizing the role of DBS in managing motor symptoms like tremors, rigidity, and gait alterations. It discusses DBS targets such as the globus pallidus pars interna (GPi) and subthalamic nucleus (STN), as well as technological advancements aimed at improving battery life and targeting. Recent research into the use of DBS for opioid addiction and depression management is also mentioned. The report concludes that DBS is effective in treating PD by reducing motor symptoms, but further research is needed to determine its impact on non-motor symptoms and cognitive difficulties. Desklib offers a range of solved assignments and past papers for students.

Running head: MEDS2001
SID:
Title: how effective is deep brain stimulation in reducing the symptoms of Parkinson’s
disease and how far has the research progressed?
Name of the University
Author Note
SID:
Title: how effective is deep brain stimulation in reducing the symptoms of Parkinson’s
disease and how far has the research progressed?
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
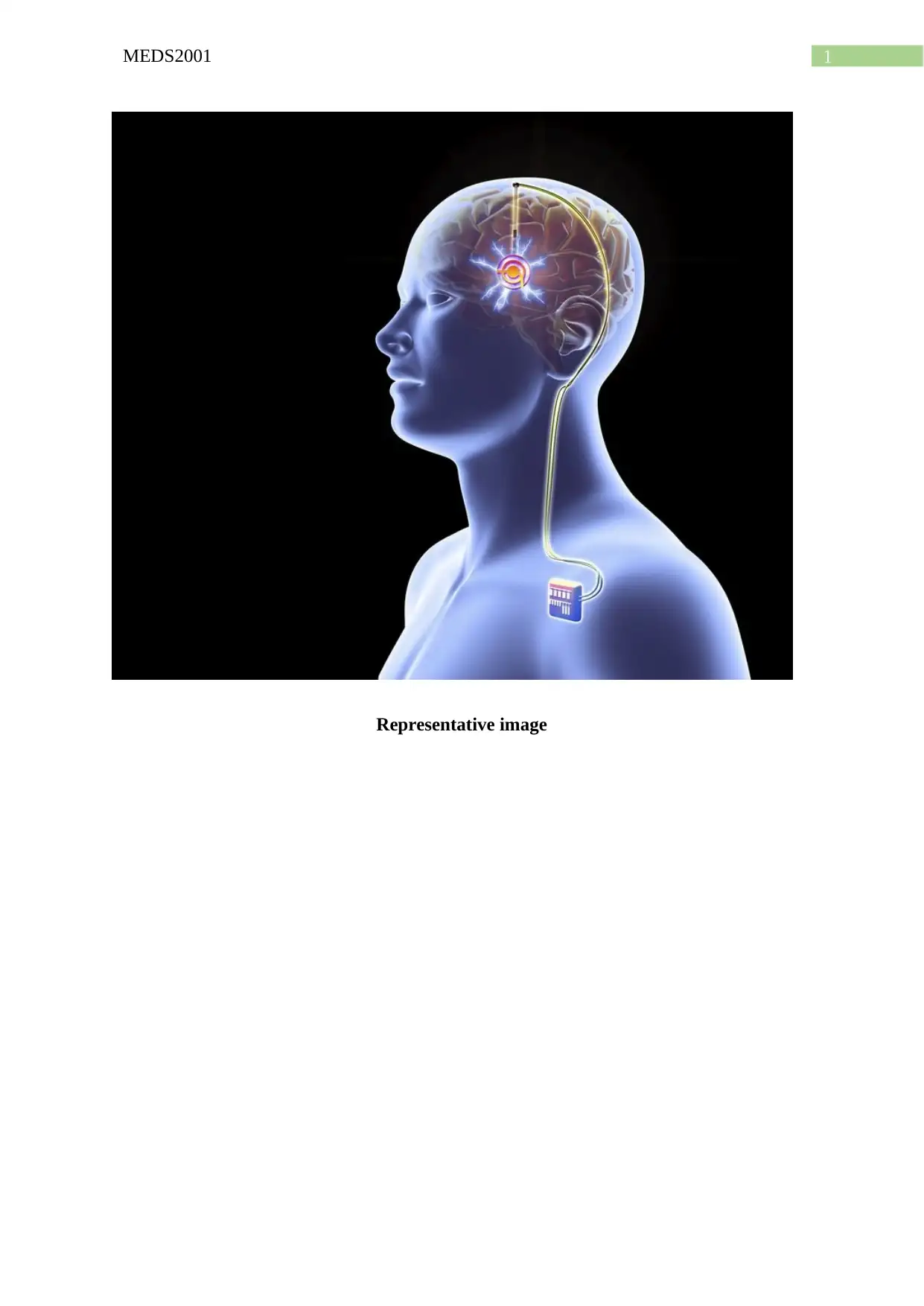
1MEDS2001
Representative image
Representative image

2MEDS2001
Parkinson’s disease (PD) refers to a degenerative disorder that affects the central
nervous system and creates an impact on motor functioning (Postuma et al. 2015). The
widespread prevalence of the disease can be associated with the fact that an estimated 340
Australians suffer from the degenerative disorder and 32 people get diagnosed with PD, every
day (Parkinson’s Queensland 2018). The mean age of onset of PD is roughly 60-65 years,
however, in rare cases it occurs among people aged between 20-50 years (Pringsheim et al.
2014). Recent reports also provide evidence for the fact that PD is the second most prevalent
neurological disorder in Australia and was associated with an estimated $10 billion cost in
2014 (Deloitte Access Economics 2015). Although there are several treatment modalities for
management of PD, deep brain stimulation is one procedure that has gained prominence in
recent years and involves the implantation of electrodes within specific areas of the brain,
thereby generating electrical impulses, and affecting the release of neurotransmitters. This
assessment will determine the impacts of deep brain stimulation on reducing the symptoms of
PD.
According to Berg et al. (2014) history of PD can be traced back to 1817, when the
book An Essay on the Shaking Palsy was published by James Parkinson. Great enhancement
in knowledge about the symptoms and treatment of the condition was made during the 20th
century, prior to which the condition was referred to as paralysis agitans (Sagna et al. 2014).
Some of the common signs and symptoms of the condition comprise of tremors, muscle
rigidity, and alterations in patterns of gait and speech (Sveinbjornsdottir 2016). Movement
difficulties that are found associated with PD are commonly referred to as Parkinsonism,
which in turn is characterized by progressive decrease in speed and several repetitive actions
like voluntary finger-tapping. Non-motor signs and symptoms of the condition comprise of
neuropsychiatric problems that encompass mood, behaviour, though alterations, and
cognition (Pfeiffer 2016). Some other symptoms encompass autonomic dysfunction and
Parkinson’s disease (PD) refers to a degenerative disorder that affects the central
nervous system and creates an impact on motor functioning (Postuma et al. 2015). The
widespread prevalence of the disease can be associated with the fact that an estimated 340
Australians suffer from the degenerative disorder and 32 people get diagnosed with PD, every
day (Parkinson’s Queensland 2018). The mean age of onset of PD is roughly 60-65 years,
however, in rare cases it occurs among people aged between 20-50 years (Pringsheim et al.
2014). Recent reports also provide evidence for the fact that PD is the second most prevalent
neurological disorder in Australia and was associated with an estimated $10 billion cost in
2014 (Deloitte Access Economics 2015). Although there are several treatment modalities for
management of PD, deep brain stimulation is one procedure that has gained prominence in
recent years and involves the implantation of electrodes within specific areas of the brain,
thereby generating electrical impulses, and affecting the release of neurotransmitters. This
assessment will determine the impacts of deep brain stimulation on reducing the symptoms of
PD.
According to Berg et al. (2014) history of PD can be traced back to 1817, when the
book An Essay on the Shaking Palsy was published by James Parkinson. Great enhancement
in knowledge about the symptoms and treatment of the condition was made during the 20th
century, prior to which the condition was referred to as paralysis agitans (Sagna et al. 2014).
Some of the common signs and symptoms of the condition comprise of tremors, muscle
rigidity, and alterations in patterns of gait and speech (Sveinbjornsdottir 2016). Movement
difficulties that are found associated with PD are commonly referred to as Parkinsonism,
which in turn is characterized by progressive decrease in speed and several repetitive actions
like voluntary finger-tapping. Non-motor signs and symptoms of the condition comprise of
neuropsychiatric problems that encompass mood, behaviour, though alterations, and
cognition (Pfeiffer 2016). Some other symptoms encompass autonomic dysfunction and
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

3MEDS2001
altered sensory perceptions. Taking into consideration the fact that the most commonly
reported complaint involves slow and coarse tremor of the hands during rest, which typically
disappears while showing voluntary movement of the arms that are affected, the major
objective of all treatment modalities focus on reducing the severity of such tremor. Although
there is no particular cure for PD management, physical treatment, surgery and medications
prove effective in providing relief and lowering the severity of the symptoms. DBS is the
most commonly implemented surgical procedure and is administered to patients who have
been diagnosed with the condition for more than four years, in addition to the presence of
dyskinesia, and a noteworthy “off time” (Shake It Up Australia 2018).
The efficacy of DBS in PD were explained by Anderson, Beecher and Ba (2017) who
suggested that some of the major targets of DBS were the globus pallidus pars interna (GPi),
subthalamic nucleus (STN), and the ventral intermediate nucleus (VIM) located in the
thalamus. They also elaborated on the fact that VIM-DBS helps in effectively suppressing
tremors, while bringing about CMPf stimulation. Results from another study suggested that
implementation of acute therapeutic DBS helps in bringing about a reversible reduction in
phase-amplitude interactions, over a period of that is comparable to reduction in motor signs
and symptoms of Parkinsonism (De Hemptinne et al. 2015). Herrington, Cheng and Eskandar
(2015) opined that most common form of DBS encompasses the usage of a four-contact
electrode that is stereotactically implanted and connected to an implantable pulse generator
(IPG), with the help of a subcutaneous wire. The efficacy of VIM thalamus DBS helps in
providing relief from essential tremor over seconds. Furthermore, less profound axial
symptoms often get delayed for days or hours, upon subjecting the patient to STN DBS. In
addition, it has been found that DBS plays an important role in inhibiting the target neuronal
networks, while activating efferent axons.
altered sensory perceptions. Taking into consideration the fact that the most commonly
reported complaint involves slow and coarse tremor of the hands during rest, which typically
disappears while showing voluntary movement of the arms that are affected, the major
objective of all treatment modalities focus on reducing the severity of such tremor. Although
there is no particular cure for PD management, physical treatment, surgery and medications
prove effective in providing relief and lowering the severity of the symptoms. DBS is the
most commonly implemented surgical procedure and is administered to patients who have
been diagnosed with the condition for more than four years, in addition to the presence of
dyskinesia, and a noteworthy “off time” (Shake It Up Australia 2018).
The efficacy of DBS in PD were explained by Anderson, Beecher and Ba (2017) who
suggested that some of the major targets of DBS were the globus pallidus pars interna (GPi),
subthalamic nucleus (STN), and the ventral intermediate nucleus (VIM) located in the
thalamus. They also elaborated on the fact that VIM-DBS helps in effectively suppressing
tremors, while bringing about CMPf stimulation. Results from another study suggested that
implementation of acute therapeutic DBS helps in bringing about a reversible reduction in
phase-amplitude interactions, over a period of that is comparable to reduction in motor signs
and symptoms of Parkinsonism (De Hemptinne et al. 2015). Herrington, Cheng and Eskandar
(2015) opined that most common form of DBS encompasses the usage of a four-contact
electrode that is stereotactically implanted and connected to an implantable pulse generator
(IPG), with the help of a subcutaneous wire. The efficacy of VIM thalamus DBS helps in
providing relief from essential tremor over seconds. Furthermore, less profound axial
symptoms often get delayed for days or hours, upon subjecting the patient to STN DBS. In
addition, it has been found that DBS plays an important role in inhibiting the target neuronal
networks, while activating efferent axons.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4MEDS2001
Besides suppression of pathological rhythms or imposing new rhythms it also
upregulates several neurotransmitter systems such as, GABA and dopamine, thus reducing
PD symptoms (Udupa and Chen 2015). Recent developments in this domain include
researching for technological advances that help in increasing the battery life of the
electrodes, in addition to facilitating specific targeting with multiple contacts. Research has
also progressed towards determining the role of DBS in management of opioid addiction by
accumbal stimulation (Kuhn et al. 2014). Investigators have also tried to determine the
effects of this treatment modality in management of depression and found a significant
improvement in the signs of depression among patients, upon using the Hamilton Depression
Rating Scale (Morishita et al. 2014).
To conclude, DBS refers to a neurosurgical procedure that comprises of the placement
of a neurostimulator for sending electrical impulses to particular targets in the brain, through
electrodes. An analysis of the aforementioned articles suggest that DBS works effective in the
treatment of PD by reducing specific motor symptoms such as, slowness, stiffness, and
tremor. Further research should be done to determine whether the procedure of DBS has any
impact on other PD signs such as, non-motor symptoms, cognitive difficulties, or freezing
during walking.
Besides suppression of pathological rhythms or imposing new rhythms it also
upregulates several neurotransmitter systems such as, GABA and dopamine, thus reducing
PD symptoms (Udupa and Chen 2015). Recent developments in this domain include
researching for technological advances that help in increasing the battery life of the
electrodes, in addition to facilitating specific targeting with multiple contacts. Research has
also progressed towards determining the role of DBS in management of opioid addiction by
accumbal stimulation (Kuhn et al. 2014). Investigators have also tried to determine the
effects of this treatment modality in management of depression and found a significant
improvement in the signs of depression among patients, upon using the Hamilton Depression
Rating Scale (Morishita et al. 2014).
To conclude, DBS refers to a neurosurgical procedure that comprises of the placement
of a neurostimulator for sending electrical impulses to particular targets in the brain, through
electrodes. An analysis of the aforementioned articles suggest that DBS works effective in the
treatment of PD by reducing specific motor symptoms such as, slowness, stiffness, and
tremor. Further research should be done to determine whether the procedure of DBS has any
impact on other PD signs such as, non-motor symptoms, cognitive difficulties, or freezing
during walking.

5MEDS2001
References
Anderson, D., Beecher, G. and Ba, F., 2017. Deep Brain Stimulation in Parkinson’s Disease:
New and Emerging Targets for Refractory Motor and Nonmotor Symptoms. Parkinson’s
Disease, 2017.
Berg, D., Postuma, R.B., Bloem, B., Chan, P., Dubois, B., Gasser, T., Goetz, C.G., Halliday,
G.M., Hardy, J., Lang, A.E. and Litvan, I., 2014. Time to redefine PD? Introductory
statement of the MDS Task Force on the definition of Parkinson's disease. Movement
Disorders, 29(4), pp.454-462.
De Hemptinne, C., Swann, N.C., Ostrem, J.L., Ryapolova-Webb, E.S., San Luciano, M.,
Galifianakis, N.B. and Starr, P.A., 2015. Therapeutic deep brain stimulation reduces cortical
phase-amplitude coupling in Parkinson's disease. Nature neuroscience, 18(5), p.779.
Deloitte Access Economics., 2015. Living with Parkinson's Disease – update. [online]
Available at: https://shakeitup.org.au/wp-content/uploads/2012/01/AER-2011-Living-with-
PD-FINAL.pdf [Accessed 26 Feb. 2019].
Herrington, T.M., Cheng, J.J. and Eskandar, E.N., 2015. Mechanisms of deep brain
stimulation. Journal of neurophysiology, 115(1), pp.19-38.
Kuhn, J., Möller, M., Treppmann, J.F., Bartsch, C., Lenartz, D., Gründler, T.O., Maarouf, M.,
Brosig, A., Barnikol, U.B., Klosterkötter, J. and Sturm, V., 2014. Deep brain stimulation of
the nucleus accumbens and its usefulness in severe opioid addiction. Molecular
psychiatry, 19(2), p.145.
Morishita, T., Fayad, S.M., Higuchi, M.A., Nestor, K.A. and Foote, K.D., 2014. Deep brain
stimulation for treatment-resistant depression: systematic review of clinical
outcomes. Neurotherapeutics, 11(3), pp.475-484.
References
Anderson, D., Beecher, G. and Ba, F., 2017. Deep Brain Stimulation in Parkinson’s Disease:
New and Emerging Targets for Refractory Motor and Nonmotor Symptoms. Parkinson’s
Disease, 2017.
Berg, D., Postuma, R.B., Bloem, B., Chan, P., Dubois, B., Gasser, T., Goetz, C.G., Halliday,
G.M., Hardy, J., Lang, A.E. and Litvan, I., 2014. Time to redefine PD? Introductory
statement of the MDS Task Force on the definition of Parkinson's disease. Movement
Disorders, 29(4), pp.454-462.
De Hemptinne, C., Swann, N.C., Ostrem, J.L., Ryapolova-Webb, E.S., San Luciano, M.,
Galifianakis, N.B. and Starr, P.A., 2015. Therapeutic deep brain stimulation reduces cortical
phase-amplitude coupling in Parkinson's disease. Nature neuroscience, 18(5), p.779.
Deloitte Access Economics., 2015. Living with Parkinson's Disease – update. [online]
Available at: https://shakeitup.org.au/wp-content/uploads/2012/01/AER-2011-Living-with-
PD-FINAL.pdf [Accessed 26 Feb. 2019].
Herrington, T.M., Cheng, J.J. and Eskandar, E.N., 2015. Mechanisms of deep brain
stimulation. Journal of neurophysiology, 115(1), pp.19-38.
Kuhn, J., Möller, M., Treppmann, J.F., Bartsch, C., Lenartz, D., Gründler, T.O., Maarouf, M.,
Brosig, A., Barnikol, U.B., Klosterkötter, J. and Sturm, V., 2014. Deep brain stimulation of
the nucleus accumbens and its usefulness in severe opioid addiction. Molecular
psychiatry, 19(2), p.145.
Morishita, T., Fayad, S.M., Higuchi, M.A., Nestor, K.A. and Foote, K.D., 2014. Deep brain
stimulation for treatment-resistant depression: systematic review of clinical
outcomes. Neurotherapeutics, 11(3), pp.475-484.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6MEDS2001
Parkinson’s Queensland., 2018. STATISTICS. [online] Available at: http://www.parkinsons-
qld.org.au/pqi-research/statistics/ [Accessed 26 Feb. 2019]
Pfeiffer, R.F., 2016. Non-motor symptoms in Parkinson's disease. Parkinsonism & related
disorders, 22, pp.S119-S122.
Postuma, R.B., Berg, D., Stern, M., Poewe, W., Olanow, C.W., Oertel, W., Obeso, J., Marek,
K., Litvan, I., Lang, A.E. and Halliday, G., 2015. MDS clinical diagnostic criteria for
Parkinson's disease. Movement Disorders, 30(12), pp.1591-1601.
Pringsheim, T., Jette, N., Frolkis, A. and Steeves, T.D., 2014. The prevalence of Parkinson's
disease: A systematic review and meta‐analysis. Movement disorders, 29(13), pp.1583-1590.
Sagna, A., Gallo, J.J. and Pontone, G.M., 2014. Systematic review of factors associated with
depression and anxiety disorders among older adults with Parkinson's disease. Parkinsonism
& related disorders, 20(7), pp.708-715.
Shake It Up Australia., 2018. DEEP BRAIN STIMULATION – DBS. [online] Available at:
https://shakeitup.org.au/understanding-parkinsons/dbs-deep-brain-stimulation/ [Accessed 26
Feb. 2019]
Sveinbjornsdottir, S., 2016. The clinical symptoms of Parkinson's disease. Journal of
neurochemistry, 139, pp.318-324.
Udupa, K. and Chen, R., 2015. The mechanisms of action of deep brain stimulation and ideas
for the future development. Progress in neurobiology, 133, pp.27-49.
Parkinson’s Queensland., 2018. STATISTICS. [online] Available at: http://www.parkinsons-
qld.org.au/pqi-research/statistics/ [Accessed 26 Feb. 2019]
Pfeiffer, R.F., 2016. Non-motor symptoms in Parkinson's disease. Parkinsonism & related
disorders, 22, pp.S119-S122.
Postuma, R.B., Berg, D., Stern, M., Poewe, W., Olanow, C.W., Oertel, W., Obeso, J., Marek,
K., Litvan, I., Lang, A.E. and Halliday, G., 2015. MDS clinical diagnostic criteria for
Parkinson's disease. Movement Disorders, 30(12), pp.1591-1601.
Pringsheim, T., Jette, N., Frolkis, A. and Steeves, T.D., 2014. The prevalence of Parkinson's
disease: A systematic review and meta‐analysis. Movement disorders, 29(13), pp.1583-1590.
Sagna, A., Gallo, J.J. and Pontone, G.M., 2014. Systematic review of factors associated with
depression and anxiety disorders among older adults with Parkinson's disease. Parkinsonism
& related disorders, 20(7), pp.708-715.
Shake It Up Australia., 2018. DEEP BRAIN STIMULATION – DBS. [online] Available at:
https://shakeitup.org.au/understanding-parkinsons/dbs-deep-brain-stimulation/ [Accessed 26
Feb. 2019]
Sveinbjornsdottir, S., 2016. The clinical symptoms of Parkinson's disease. Journal of
neurochemistry, 139, pp.318-324.
Udupa, K. and Chen, R., 2015. The mechanisms of action of deep brain stimulation and ideas
for the future development. Progress in neurobiology, 133, pp.27-49.
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





