Chapter 1: Metallic Glass - Manufacturing and Characteristics
VerifiedAdded on 2022/09/12
|17
|4785
|22
Report
AI Summary
This report provides a comprehensive overview of metallic glass, beginning with a comparison of amorphous and crystalline solids, highlighting the advantages and disadvantages of materials like plastic and steel, and introducing metallic glass as a potential solution to overcome these limitations. The report delves into the definition of metallic glass, its structural characteristics, and its categorization into metal-metalloid and metal-metal alloys. It then explores various preparation methods, including melt quench technique, chill block melt spinning, and planar flow casting, with detailed descriptions and schematic representations of each process. The report also discusses the properties of metallic glass, such as its high viscosity, resistance to plastic deformation, corrosion resistance, and lower thermal conductivity compared to crystalline metals. Furthermore, the report examines the glass-forming capability of metallic alloys, emphasizing the importance of rapid cooling, alloy composition, and atomic radii differences in achieving the amorphous structure. Finally, the report discusses potential applications of metallic glass in various fields, including electronics, medical devices, and sports equipment, highlighting the advantages and limitations of the material.

CHAPTER 1 1
METALLIC GLASS
By Name
Course
Instructor
Institution
Location
Date
METALLIC GLASS
By Name
Course
Instructor
Institution
Location
Date
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

CHAPTER 1 2
CHAPTER 1
Solids can be categorized as either amorphous solids or crystalline solids depending on their
internal structures. Diamonds and metals are examples of crystalline solids and their particles are
arranged uniformly, resulting into the formation of crystal lattice extending in every direction.
For the case of amorphous solids like gel, plastic, and glass, the particles are not organized in a
specific lattice configuration.
Majority of things surrounding us in our lives daily are either made of plastic (amorphous solids)
or steel (crystalline solids). All of these materials have their specific benefits and shortcomings
(Sen and Prasenjit, 2020). From an assessment of everybody, it is clear that steel cannot be used
in making toys of babies and plastic cannot be used in making surgical instruments.
Plastics are very valuable materials since they can be formed easily into different shapes. Being
cheaper makes them a perfect selection for functionalities that do not need a strong structure for
support. Additionally, plastics are much lighter compared to steel and have relatively better
resistance to corrosion. These characteristics and various others make plastic materials perfect
for numerous industrial applications globally. The major disadvantage of plastic materials is that
they easily bend and hence cannot be applied for force-intensive functionalities (Hentschel et al.,
2012). On the other hand, the popularity of steel is compromised between its cost and strength. It
is generally known that steel is the strongest metal, however, its cost-effectiveness has resulted
into steel being one of the most preferred selections for machineries and equipment in
construction. Nevertheless, it is difficult to freeze steel into various intricate shapes hence
limiting its potential application.
CHAPTER 1
Solids can be categorized as either amorphous solids or crystalline solids depending on their
internal structures. Diamonds and metals are examples of crystalline solids and their particles are
arranged uniformly, resulting into the formation of crystal lattice extending in every direction.
For the case of amorphous solids like gel, plastic, and glass, the particles are not organized in a
specific lattice configuration.
Majority of things surrounding us in our lives daily are either made of plastic (amorphous solids)
or steel (crystalline solids). All of these materials have their specific benefits and shortcomings
(Sen and Prasenjit, 2020). From an assessment of everybody, it is clear that steel cannot be used
in making toys of babies and plastic cannot be used in making surgical instruments.
Plastics are very valuable materials since they can be formed easily into different shapes. Being
cheaper makes them a perfect selection for functionalities that do not need a strong structure for
support. Additionally, plastics are much lighter compared to steel and have relatively better
resistance to corrosion. These characteristics and various others make plastic materials perfect
for numerous industrial applications globally. The major disadvantage of plastic materials is that
they easily bend and hence cannot be applied for force-intensive functionalities (Hentschel et al.,
2012). On the other hand, the popularity of steel is compromised between its cost and strength. It
is generally known that steel is the strongest metal, however, its cost-effectiveness has resulted
into steel being one of the most preferred selections for machineries and equipment in
construction. Nevertheless, it is difficult to freeze steel into various intricate shapes hence
limiting its potential application.

CHAPTER 1 3
The shortcomings of both steel and plastic materials have compelled us to come up with an
alternative material that can resolve the challenges highlighted above. The determination to
invent a new material is based on a type of material that is as adaptable as plastic and as strong as
steel. This has led to the introduction of metallic glass (Song, 2011).
The term “glass” is generally related to the familiar transparent glass such as drinking glass or
window glass which is made from silicate and composed of silicon dioxide. However, the term
‘glass’ can be used scientifically to refer to all amorphous solids that depict glass transitions near
melting points (Dasgupta et al., 2012). However, the ordinary glass is non-metallic hence does
not show any metallic behavior such as high durability or electrical conductivity. Therefore,
metallic glasses are solid like metallic materials with disordered atomic-scale structure. Majority
of metals are crystalline when in solid-state, which insinuates that they have a structure similar to
glass. However, amorphous metals have perfect conductivity of electrical energy unlike ordinary
glasses which are generally electrical insulators.
Metallic glasses are categorized broadly as metal metalloid and metal-metal alloys. The metal-
metal alloy category entails various metal combinations like Vanadium and Hafnium or niobium
and nickel (Denga et al., 2011).
The metal-metalloid category on the other hand is normally developed through metal integration
with metalloids like germanium, polonium, and silicon. Nevertheless, such interactions may be
quaternary, ternary or binary depending on the outcome of the product desired. Metallic glass is
suitable for solving the major challenges observed in the steel and plastic applications since this
material shows both traits of adaptability and strength (Koppensteiner and Schranz, 2010).
The shortcomings of both steel and plastic materials have compelled us to come up with an
alternative material that can resolve the challenges highlighted above. The determination to
invent a new material is based on a type of material that is as adaptable as plastic and as strong as
steel. This has led to the introduction of metallic glass (Song, 2011).
The term “glass” is generally related to the familiar transparent glass such as drinking glass or
window glass which is made from silicate and composed of silicon dioxide. However, the term
‘glass’ can be used scientifically to refer to all amorphous solids that depict glass transitions near
melting points (Dasgupta et al., 2012). However, the ordinary glass is non-metallic hence does
not show any metallic behavior such as high durability or electrical conductivity. Therefore,
metallic glasses are solid like metallic materials with disordered atomic-scale structure. Majority
of metals are crystalline when in solid-state, which insinuates that they have a structure similar to
glass. However, amorphous metals have perfect conductivity of electrical energy unlike ordinary
glasses which are generally electrical insulators.
Metallic glasses are categorized broadly as metal metalloid and metal-metal alloys. The metal-
metal alloy category entails various metal combinations like Vanadium and Hafnium or niobium
and nickel (Denga et al., 2011).
The metal-metalloid category on the other hand is normally developed through metal integration
with metalloids like germanium, polonium, and silicon. Nevertheless, such interactions may be
quaternary, ternary or binary depending on the outcome of the product desired. Metallic glass is
suitable for solving the major challenges observed in the steel and plastic applications since this
material shows both traits of adaptability and strength (Koppensteiner and Schranz, 2010).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

CHAPTER 1 4
METALLIC GLASS PREPARATION
There are various methods of producing amorphous metals, namely mechanical alloying, ion
irradiation, solid-state reaction, physical vapor deposition, and extreme rapid cooling.
Previously, small amorphous metal batches have been generated through various methods of fast
cooling. For example, the ribbons of amorphous metals are generally manufactured through the
process of melt spinning which involves sputtering molten metals onto a metal disk spinning.
The instant cooling involving cooling at a rate of a million drees per second, is very fast for the
formation of crystal and the material is ‘locked’ in a glassy state (Haibo et al., 2014). Recently,
various alloys with low rates of critical cooling sufficient to permit amorphous structure
formation is thick layers of about 1mm have been generated. Some of the significant techniques
of producing amorphous metallic alloy are discussed below:
MELT QUENCH TECHNIQUE
The method of melt quench is one of the first approaches to be applied in the preparation of
glasses by the glass manufacturing industries. This approach is very flexible for production of
numerous composition of glasses for specific system like phosphates, borate, silicates, and non-
oxides (Yugeswaran and Kobayashi, 2014). This techniques enables for the co-doping of various
categories of active ions.
Description
This technique entails the estimation of the input materials also referred to as starting materials
by the use of weighing balance after batch calculation. The next process of melting of glass batch
at a temperature of 80oC in the platinum crucible. The melted glass batch is then cast at room
temperature into steel mold. The process of meting is performed using an electric furnace. The
METALLIC GLASS PREPARATION
There are various methods of producing amorphous metals, namely mechanical alloying, ion
irradiation, solid-state reaction, physical vapor deposition, and extreme rapid cooling.
Previously, small amorphous metal batches have been generated through various methods of fast
cooling. For example, the ribbons of amorphous metals are generally manufactured through the
process of melt spinning which involves sputtering molten metals onto a metal disk spinning.
The instant cooling involving cooling at a rate of a million drees per second, is very fast for the
formation of crystal and the material is ‘locked’ in a glassy state (Haibo et al., 2014). Recently,
various alloys with low rates of critical cooling sufficient to permit amorphous structure
formation is thick layers of about 1mm have been generated. Some of the significant techniques
of producing amorphous metallic alloy are discussed below:
MELT QUENCH TECHNIQUE
The method of melt quench is one of the first approaches to be applied in the preparation of
glasses by the glass manufacturing industries. This approach is very flexible for production of
numerous composition of glasses for specific system like phosphates, borate, silicates, and non-
oxides (Yugeswaran and Kobayashi, 2014). This techniques enables for the co-doping of various
categories of active ions.
Description
This technique entails the estimation of the input materials also referred to as starting materials
by the use of weighing balance after batch calculation. The next process of melting of glass batch
at a temperature of 80oC in the platinum crucible. The melted glass batch is then cast at room
temperature into steel mold. The process of meting is performed using an electric furnace. The
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
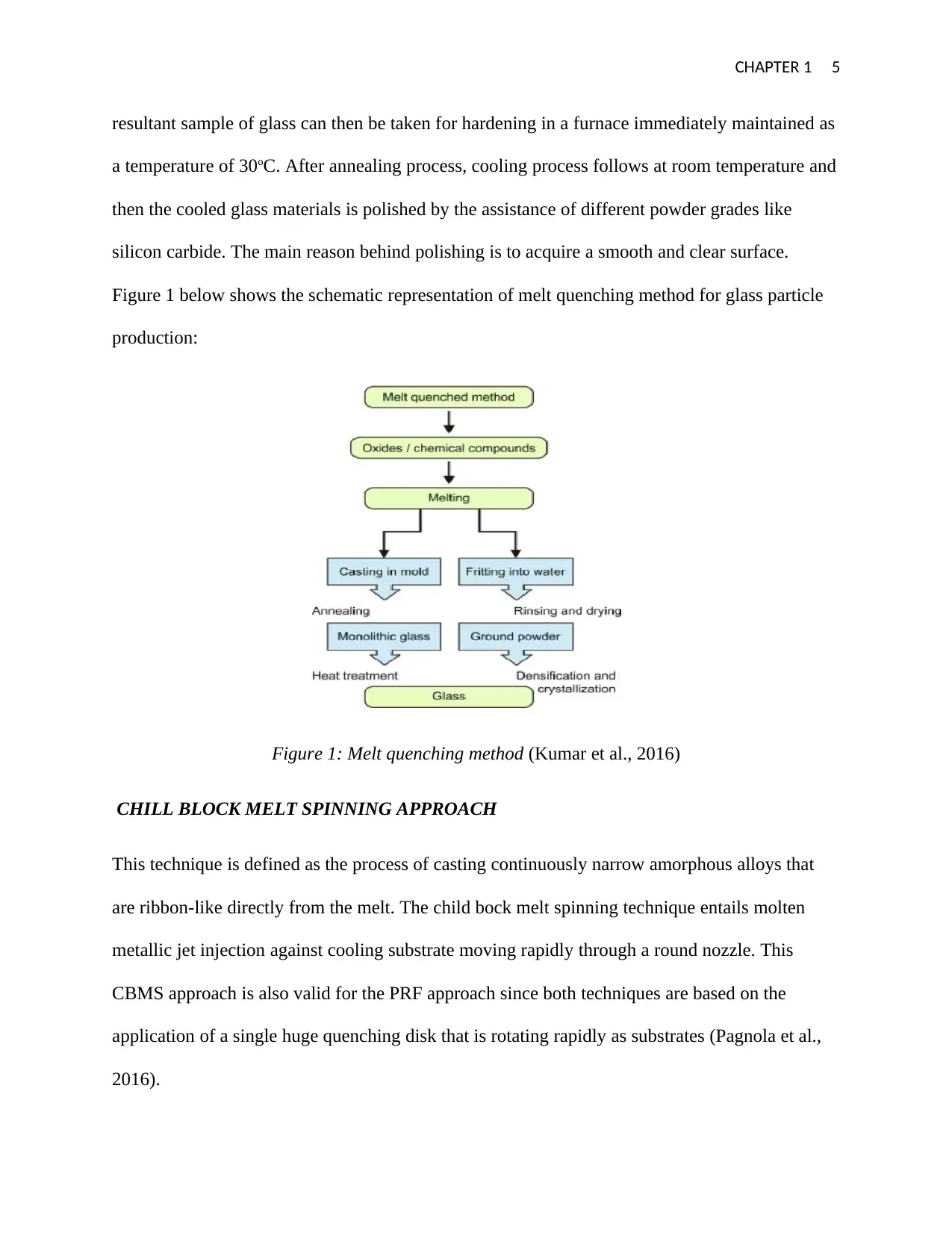
CHAPTER 1 5
resultant sample of glass can then be taken for hardening in a furnace immediately maintained as
a temperature of 30oC. After annealing process, cooling process follows at room temperature and
then the cooled glass materials is polished by the assistance of different powder grades like
silicon carbide. The main reason behind polishing is to acquire a smooth and clear surface.
Figure 1 below shows the schematic representation of melt quenching method for glass particle
production:
Figure 1: Melt quenching method (Kumar et al., 2016)
CHILL BLOCK MELT SPINNING APPROACH
This technique is defined as the process of casting continuously narrow amorphous alloys that
are ribbon-like directly from the melt. The child bock melt spinning technique entails molten
metallic jet injection against cooling substrate moving rapidly through a round nozzle. This
CBMS approach is also valid for the PRF approach since both techniques are based on the
application of a single huge quenching disk that is rotating rapidly as substrates (Pagnola et al.,
2016).
resultant sample of glass can then be taken for hardening in a furnace immediately maintained as
a temperature of 30oC. After annealing process, cooling process follows at room temperature and
then the cooled glass materials is polished by the assistance of different powder grades like
silicon carbide. The main reason behind polishing is to acquire a smooth and clear surface.
Figure 1 below shows the schematic representation of melt quenching method for glass particle
production:
Figure 1: Melt quenching method (Kumar et al., 2016)
CHILL BLOCK MELT SPINNING APPROACH
This technique is defined as the process of casting continuously narrow amorphous alloys that
are ribbon-like directly from the melt. The child bock melt spinning technique entails molten
metallic jet injection against cooling substrate moving rapidly through a round nozzle. This
CBMS approach is also valid for the PRF approach since both techniques are based on the
application of a single huge quenching disk that is rotating rapidly as substrates (Pagnola et al.,
2016).
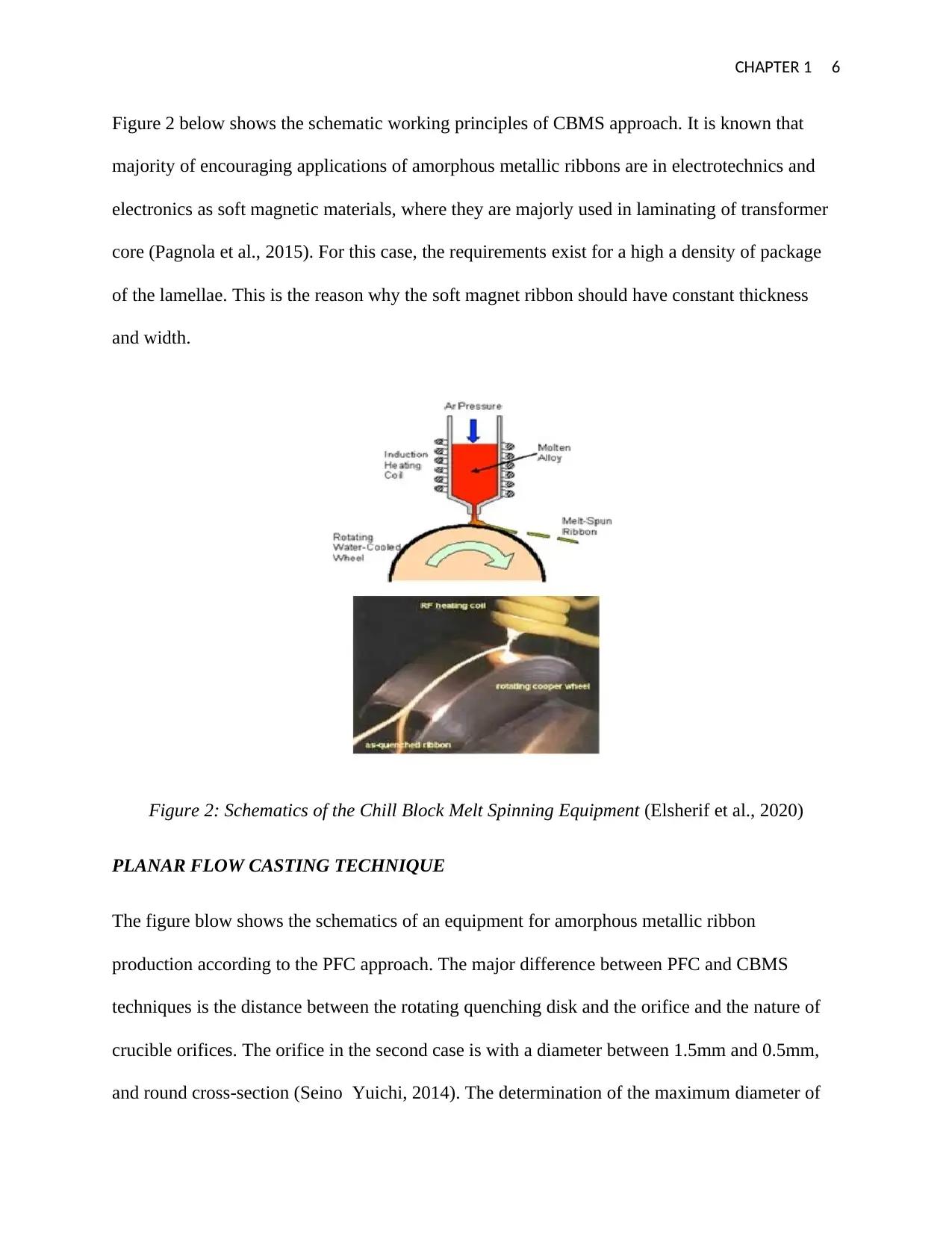
CHAPTER 1 6
Figure 2 below shows the schematic working principles of CBMS approach. It is known that
majority of encouraging applications of amorphous metallic ribbons are in electrotechnics and
electronics as soft magnetic materials, where they are majorly used in laminating of transformer
core (Pagnola et al., 2015). For this case, the requirements exist for a high a density of package
of the lamellae. This is the reason why the soft magnet ribbon should have constant thickness
and width.
Figure 2: Schematics of the Chill Block Melt Spinning Equipment (Elsherif et al., 2020)
PLANAR FLOW CASTING TECHNIQUE
The figure blow shows the schematics of an equipment for amorphous metallic ribbon
production according to the PFC approach. The major difference between PFC and CBMS
techniques is the distance between the rotating quenching disk and the orifice and the nature of
crucible orifices. The orifice in the second case is with a diameter between 1.5mm and 0.5mm,
and round cross-section (Seino Yuichi, 2014). The determination of the maximum diameter of
Figure 2 below shows the schematic working principles of CBMS approach. It is known that
majority of encouraging applications of amorphous metallic ribbons are in electrotechnics and
electronics as soft magnetic materials, where they are majorly used in laminating of transformer
core (Pagnola et al., 2015). For this case, the requirements exist for a high a density of package
of the lamellae. This is the reason why the soft magnet ribbon should have constant thickness
and width.
Figure 2: Schematics of the Chill Block Melt Spinning Equipment (Elsherif et al., 2020)
PLANAR FLOW CASTING TECHNIQUE
The figure blow shows the schematics of an equipment for amorphous metallic ribbon
production according to the PFC approach. The major difference between PFC and CBMS
techniques is the distance between the rotating quenching disk and the orifice and the nature of
crucible orifices. The orifice in the second case is with a diameter between 1.5mm and 0.5mm,
and round cross-section (Seino Yuichi, 2014). The determination of the maximum diameter of
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

CHAPTER 1 7
1.5mm is done according to the surface tension requirement to be able to successfully sustain the
weight of its melt hence causing an instant flow of melt through the orifice before any ejection
pressure is applied.
Figure 3: Schematics of Melt spinning equipment according to the PFC approach (Fan et al.,
2011)
Figure 4: (i) Different capacities of crucible for high flexibility, (ii) Internal view with rotating
cooling wheel and small crucible, and (iii) Entire view of facility of MS melt spinning with
periphery at Fraunhofer IFAM Dresden (Zhong and Liang, 2011).
1.5mm is done according to the surface tension requirement to be able to successfully sustain the
weight of its melt hence causing an instant flow of melt through the orifice before any ejection
pressure is applied.
Figure 3: Schematics of Melt spinning equipment according to the PFC approach (Fan et al.,
2011)
Figure 4: (i) Different capacities of crucible for high flexibility, (ii) Internal view with rotating
cooling wheel and small crucible, and (iii) Entire view of facility of MS melt spinning with
periphery at Fraunhofer IFAM Dresden (Zhong and Liang, 2011).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

CHAPTER 1 8
PROPERTIES
Amorphous metals are generally alloys and not pure metals. The alloys possess atoms of
noticeably different sizes, resulting in low free volumes in molten state hence have higher
viscosity compared to other alloys and metals. The high viscosity prevents the movement of the
atoms resulting in the formation of an ordered lattice. The structure of the material also results in
resistance to plastic deformation and low shrinkage during the process of cooling (Gunderov and
Astanin, 2020). Since the grain boundaries which is the weak section of crystalline material is
absent, then the resistance to corrosion and wear is improved. The amorphous metals,
specifically glasses, are also less brittle and much tougher compared to ceramics and oxide
glasses.
The amorphous materials have a lower thermal conductivity compared to crystalline metals.
Since the amorphous structure formation depends on fast cooling, the maximum attainable
amorphous structure thickness is limited (Bradley, 2013).
The alloy should be made of three or many components to attain the amorphous structure
formation during slower cooling, resulting into complex units of crystal with lower formation
chances and higher energy potential. The atomic radii of the components have to be different
substantially by over 12% to attain lower free volume and high packing density (Varouti, 2013).
The component combination should have negative mixing heat, prolonging the duration the
molten metals stay in supercooled states and preventing crystal nucleation.
The phosphorous, silicon, boron alloys and also other glass formers with magnetic metals such as
nickel, cobalt, and iron, have high electrical resistance, low coercivity, and susceptible to high
magnetism. Generally, the metallic glass conductivity is of the similar low order to low eddy
PROPERTIES
Amorphous metals are generally alloys and not pure metals. The alloys possess atoms of
noticeably different sizes, resulting in low free volumes in molten state hence have higher
viscosity compared to other alloys and metals. The high viscosity prevents the movement of the
atoms resulting in the formation of an ordered lattice. The structure of the material also results in
resistance to plastic deformation and low shrinkage during the process of cooling (Gunderov and
Astanin, 2020). Since the grain boundaries which is the weak section of crystalline material is
absent, then the resistance to corrosion and wear is improved. The amorphous metals,
specifically glasses, are also less brittle and much tougher compared to ceramics and oxide
glasses.
The amorphous materials have a lower thermal conductivity compared to crystalline metals.
Since the amorphous structure formation depends on fast cooling, the maximum attainable
amorphous structure thickness is limited (Bradley, 2013).
The alloy should be made of three or many components to attain the amorphous structure
formation during slower cooling, resulting into complex units of crystal with lower formation
chances and higher energy potential. The atomic radii of the components have to be different
substantially by over 12% to attain lower free volume and high packing density (Varouti, 2013).
The component combination should have negative mixing heat, prolonging the duration the
molten metals stay in supercooled states and preventing crystal nucleation.
The phosphorous, silicon, boron alloys and also other glass formers with magnetic metals such as
nickel, cobalt, and iron, have high electrical resistance, low coercivity, and susceptible to high
magnetism. Generally, the metallic glass conductivity is of the similar low order to low eddy

CHAPTER 1 9
current losses when exposed to magnetic fields alternating. This property is significant for
making magnetic cores of transformers (Aizawa, 2013). The low coercivity also promotes low
losses.
Amorphous metals also have higher limits of elastic strain and tensile yield strength compared to
polycrystalline metal alloy, however, they have lower fatigue and ductility strengths. The
amorphous alloys have various properties that potentially significant. Particularly, these alloys
are stronger compared to crystalline alloys of identical chemical compositions, and then can
withstand larger elastic (reversible) deformation compared to crystalline alloys (Shukla et al.,
2013). Amorphous metals acquires its strength directly from its non-crystalline structure, which
does not possess any dislocation defects which may reduce the crystalline alloy strength.
A single amorphous metal referred to as Vitreloy has a tensile strength that is double the strength
of titanium of high grade. Nevertheless, any metallic glass is not ductile at room temperature and
tend to suddenly fail when under tension hence limiting the applicability of the material to
applications that have critical reliability since there is no evidence of the impending failure
(Herzer, 2013). Consequently, there is substantial curiosity in metal matrix composite production
which consist of a metallic glass matrix having dendritic fibers or particles of a crystalline metals
that is ductile.
Possibly the most important characteristic of bulk amorphous alloys is that they are real glasses,
meaning that they flow and soften after heating. This promotes easy processing, such as through
injection molding similarly to polymers. Therefore, there have been commercialization of
amorphous alloys for application in electronic equipment, medical devices, and sports equipment
(Beloshenko and Voznyak, 2013). The deposition of thin film of amorphous metal can be
performed through the technique of oxygen fuel of high velocity as a coating for protection.
current losses when exposed to magnetic fields alternating. This property is significant for
making magnetic cores of transformers (Aizawa, 2013). The low coercivity also promotes low
losses.
Amorphous metals also have higher limits of elastic strain and tensile yield strength compared to
polycrystalline metal alloy, however, they have lower fatigue and ductility strengths. The
amorphous alloys have various properties that potentially significant. Particularly, these alloys
are stronger compared to crystalline alloys of identical chemical compositions, and then can
withstand larger elastic (reversible) deformation compared to crystalline alloys (Shukla et al.,
2013). Amorphous metals acquires its strength directly from its non-crystalline structure, which
does not possess any dislocation defects which may reduce the crystalline alloy strength.
A single amorphous metal referred to as Vitreloy has a tensile strength that is double the strength
of titanium of high grade. Nevertheless, any metallic glass is not ductile at room temperature and
tend to suddenly fail when under tension hence limiting the applicability of the material to
applications that have critical reliability since there is no evidence of the impending failure
(Herzer, 2013). Consequently, there is substantial curiosity in metal matrix composite production
which consist of a metallic glass matrix having dendritic fibers or particles of a crystalline metals
that is ductile.
Possibly the most important characteristic of bulk amorphous alloys is that they are real glasses,
meaning that they flow and soften after heating. This promotes easy processing, such as through
injection molding similarly to polymers. Therefore, there have been commercialization of
amorphous alloys for application in electronic equipment, medical devices, and sports equipment
(Beloshenko and Voznyak, 2013). The deposition of thin film of amorphous metal can be
performed through the technique of oxygen fuel of high velocity as a coating for protection.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

CHAPTER 1 10
GLASS FORMING CAPABILITY
The transition of glass is the principle feature of amorphous metallic alloy acquired from the belt
through spontaneous solidification. The molten metallic alloys have a very low viscosity of
2x10-3 Pa s. This is the reason why it is extreme rates of cooling of approximately 160 K/s is
required to prevent their crystallization and to acquire them in a vitreous state. During the
solidification of the glass forming melt in amorphous state, the viscosity becomes higher
resulting in prompt atom reduction (Nabiałek, 2016). This can occur in various ranges of
temperature. The perfect glass formers have high viscosity even at higher temperature more than
the melting points. However, there are other suitable glass forming substances, which at which at
which temperatures have moderately low viscosity.
For these substances, the condition that the crystal and nucleation growth is reduced during the
rapid cooling. This reduction can be as a result of the complicated nature of crystal structure and
it is impossible to be noticed. By considering this consideration, despite the perfect capability of
glass forming correlates with high values of melt viscosity at high temperatures, it is clear that it
is only essential condition for vitrification but not significant (Jiaojiao et al., 2015). Any metallic
glass obeys this general behavior. The process of vitrification can be performed in various
molten metallic alloys by the use of very high rates of quenching in the form of thin ribbons
without metastable or stable crystalline phases formation (Petkov et al., 2014). Lately, there have
been discovery of various metallic glasses, which can be acquired by low or moderate rates of
melt cooling, compared to the rates of cooling required for the traditional silicate glasses.
It is recognized generally that their viscosity of the melt rapidly develops with many magnitude
orders until attaining the temperature of glass transition of approximately Tm. However, there
GLASS FORMING CAPABILITY
The transition of glass is the principle feature of amorphous metallic alloy acquired from the belt
through spontaneous solidification. The molten metallic alloys have a very low viscosity of
2x10-3 Pa s. This is the reason why it is extreme rates of cooling of approximately 160 K/s is
required to prevent their crystallization and to acquire them in a vitreous state. During the
solidification of the glass forming melt in amorphous state, the viscosity becomes higher
resulting in prompt atom reduction (Nabiałek, 2016). This can occur in various ranges of
temperature. The perfect glass formers have high viscosity even at higher temperature more than
the melting points. However, there are other suitable glass forming substances, which at which at
which temperatures have moderately low viscosity.
For these substances, the condition that the crystal and nucleation growth is reduced during the
rapid cooling. This reduction can be as a result of the complicated nature of crystal structure and
it is impossible to be noticed. By considering this consideration, despite the perfect capability of
glass forming correlates with high values of melt viscosity at high temperatures, it is clear that it
is only essential condition for vitrification but not significant (Jiaojiao et al., 2015). Any metallic
glass obeys this general behavior. The process of vitrification can be performed in various
molten metallic alloys by the use of very high rates of quenching in the form of thin ribbons
without metastable or stable crystalline phases formation (Petkov et al., 2014). Lately, there have
been discovery of various metallic glasses, which can be acquired by low or moderate rates of
melt cooling, compared to the rates of cooling required for the traditional silicate glasses.
It is recognized generally that their viscosity of the melt rapidly develops with many magnitude
orders until attaining the temperature of glass transition of approximately Tm. However, there
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

CHAPTER 1 11
are metallic alloys which vitrify at temperature of about Tm. The major reason behind these
differences is the behavior of metallic melts during glass forming is not still understood well.
APPLICATIONS
The combination of unique and excellent properties of applications of metallic glasses are the
general features of these materials. Having this in mind and also the scheme of technological
production of the metallic glasses, associated with perfect energy savings, are the reasons for the
great interest in their practical applications (Bradley, 2012). The low losses of magnetization is
important for its application in transformers of high efficiency like amorphous metal
transformers at higher frequency transformers and line frequency. The amorphous steels are very
brittle materials which make it impossible to punch into laminations of motor (Wang and Wang,
2014). The theft control passive ID tags are example of electronic article surveillance which
normally applies metallic glasses due to their properties in magnetism.
An amorphous metal shows exclusive softening behavior of above their glass transition and this
softening behavior has been explored extensively for thermoplastic forming of metallic glasses.
This low softening temperatures enables for the development of simple approaches of making
nanoparticle composites such as BMGs and carbon nanotubes (Axinte, 2012). Research shows
that it is easy pattern metallic glasses on exceedingly small scale lengths ranging from 10nm to
many millimeters. The issue of nanoimprint lithography can be solved where costly nano-molds
made from silicon easily break. It is easy to fabricate nano-molds made from metallic glasses and
also more durable compared to silicon molds (Plummer and Todd, 2012). The superior
mechanical, thermal, and electronic properties of BMGs compared to polymers make them a
suitable option for nanocomposite development for electronic applications like devices of field
electron emission.
are metallic alloys which vitrify at temperature of about Tm. The major reason behind these
differences is the behavior of metallic melts during glass forming is not still understood well.
APPLICATIONS
The combination of unique and excellent properties of applications of metallic glasses are the
general features of these materials. Having this in mind and also the scheme of technological
production of the metallic glasses, associated with perfect energy savings, are the reasons for the
great interest in their practical applications (Bradley, 2012). The low losses of magnetization is
important for its application in transformers of high efficiency like amorphous metal
transformers at higher frequency transformers and line frequency. The amorphous steels are very
brittle materials which make it impossible to punch into laminations of motor (Wang and Wang,
2014). The theft control passive ID tags are example of electronic article surveillance which
normally applies metallic glasses due to their properties in magnetism.
An amorphous metal shows exclusive softening behavior of above their glass transition and this
softening behavior has been explored extensively for thermoplastic forming of metallic glasses.
This low softening temperatures enables for the development of simple approaches of making
nanoparticle composites such as BMGs and carbon nanotubes (Axinte, 2012). Research shows
that it is easy pattern metallic glasses on exceedingly small scale lengths ranging from 10nm to
many millimeters. The issue of nanoimprint lithography can be solved where costly nano-molds
made from silicon easily break. It is easy to fabricate nano-molds made from metallic glasses and
also more durable compared to silicon molds (Plummer and Todd, 2012). The superior
mechanical, thermal, and electronic properties of BMGs compared to polymers make them a
suitable option for nanocomposite development for electronic applications like devices of field
electron emission.

CHAPTER 1 12
A special type of noncarcinogenic material, Ti40Cu36Pd14Zr10, is approximately three times
stronger compared to titanium and its elastic modulus matches that of bones. It does not produce
abrasion powder and has a high resistance to wear. During solidification, the alloy does not
experience shrinkage (Qiao and Peker, 2012). A structure of the surface can be produced through
biological attachment by modification of the surface using laser pulses, hence enabling proper
combination with the bones.
Some of the applications of metallic glasses
Bio-medical industries
Metallic glasses may also be utilized as prosthetic material for human body implantation. The
metallic glasses are also suitable for making surgical and cutting instruments due to the high
resistance to corrosion (Kaushik et al., 2014).
Nuclear Reactor Engineering
The phosphorous and chromium based metallic lasses have high resistance to corrosion making
them suitable for application in internal surface of the reactor vessels. The irradiation effect does
not affect the magnetic properties of the metallic glasses hence making these materials
significant in preparation containers for magnets for fusion reactors and nuclear waste disposal
(Wang and Wong, 2012).
Electrical and Electronics
Since metallic glasses have high electrical resistance, they are used in the manufacture of
magnetic resistance sensors, computer memories, and accurate standard resistance.
Other Applications:
A special type of noncarcinogenic material, Ti40Cu36Pd14Zr10, is approximately three times
stronger compared to titanium and its elastic modulus matches that of bones. It does not produce
abrasion powder and has a high resistance to wear. During solidification, the alloy does not
experience shrinkage (Qiao and Peker, 2012). A structure of the surface can be produced through
biological attachment by modification of the surface using laser pulses, hence enabling proper
combination with the bones.
Some of the applications of metallic glasses
Bio-medical industries
Metallic glasses may also be utilized as prosthetic material for human body implantation. The
metallic glasses are also suitable for making surgical and cutting instruments due to the high
resistance to corrosion (Kaushik et al., 2014).
Nuclear Reactor Engineering
The phosphorous and chromium based metallic lasses have high resistance to corrosion making
them suitable for application in internal surface of the reactor vessels. The irradiation effect does
not affect the magnetic properties of the metallic glasses hence making these materials
significant in preparation containers for magnets for fusion reactors and nuclear waste disposal
(Wang and Wong, 2012).
Electrical and Electronics
Since metallic glasses have high electrical resistance, they are used in the manufacture of
magnetic resistance sensors, computer memories, and accurate standard resistance.
Other Applications:
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 17
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.