Physical and Chemical Agents for Microbial Growth Control Lab Report
VerifiedAdded on 2020/05/08
|11
|2486
|322
Practical Assignment
AI Summary
This lab report details experiments on controlling microbial growth using physical and chemical agents. Four experiments were conducted: the Kirby-Bauer test to determine antibiotic susceptibility of *B. subtilis* and *E. coli*; the determination of the minimum inhibitory concentration (MIC) of antimicrobial drugs; bleach tests to assess the effectiveness of sodium hypochlorite; and tests on the lethal effects of temperature on the two microorganisms. Results from the Kirby-Bauer test revealed varying susceptibilities to antibiotics, with *B. subtilis* being susceptible to ciprofloxacin, vancomycin, and polymyxin B, while *E. coli* showed resistance to several antibiotics. MIC tests showed that *B. subtilis* required lower antimicrobial concentrations for growth inhibition compared to *E. coli*. Bleach tests demonstrated the effectiveness of sodium hypochlorite in inhibiting microbial growth, with *E. coli* being more susceptible. Temperature experiments showed that *B. subtilis* could grow up to 100°C, while *E. coli* growth was limited to 60°C. The report provides an analysis of these findings and a discussion of the methods used to control microbial growth.

Physical and Chemical Agents for the Control of Microbial Growth
Name
Lab Section
Date of Study
Names of Lab Partners
Name
Lab Section
Date of Study
Names of Lab Partners
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Abstract
The report illustrates the concepts of microbial growth control using various physical and
chemical methods. Different terminologies relating to microbial controls have been defined to
enhance the understanding of the lab report findings. A total of four experiments have been
done on microbial growth inhibition and they include the Kirby-Bauer test, Bleaching test,
Minimum inhibitory concentration (MIC) determination and the lethal effects of temperature
on the microbial activities. The Carpenter-Cleland (2017) BIOL 2P98 Principles of
Microbiology 2017FW Lab Manual was used to conduct the four practical. The Kirby-Bauer
results has shown that B. Sabtilis were reported to be susceptible to ciprofloxacin,
vancomycin, polymyxin B and slightly susceptible to colistin. The organisms are
insusceptible to moxalactam and penicillin. E. coli species on the other hand are insusceptible
to moxalactam, vancomycin and penicillin and sensitive to ciprofloxacin, polymyxin B and
the colistin. The study has indicated that B. Subtilis require the lowest concentration of
antimicrobial to inhibit growth as compared to E. coli. The growth of B. subtilis can occure
up to 100 degrees Celsius however, its growth at 100 degrees Celsius is dramatically slowed.
E. coli species can only grow up to 60 degrees Celsius. The study outcome has also shown
that E. coli species has been shown to be more susceptible to bleaching agent as compared to
B. Subtilis species.
The report illustrates the concepts of microbial growth control using various physical and
chemical methods. Different terminologies relating to microbial controls have been defined to
enhance the understanding of the lab report findings. A total of four experiments have been
done on microbial growth inhibition and they include the Kirby-Bauer test, Bleaching test,
Minimum inhibitory concentration (MIC) determination and the lethal effects of temperature
on the microbial activities. The Carpenter-Cleland (2017) BIOL 2P98 Principles of
Microbiology 2017FW Lab Manual was used to conduct the four practical. The Kirby-Bauer
results has shown that B. Sabtilis were reported to be susceptible to ciprofloxacin,
vancomycin, polymyxin B and slightly susceptible to colistin. The organisms are
insusceptible to moxalactam and penicillin. E. coli species on the other hand are insusceptible
to moxalactam, vancomycin and penicillin and sensitive to ciprofloxacin, polymyxin B and
the colistin. The study has indicated that B. Subtilis require the lowest concentration of
antimicrobial to inhibit growth as compared to E. coli. The growth of B. subtilis can occure
up to 100 degrees Celsius however, its growth at 100 degrees Celsius is dramatically slowed.
E. coli species can only grow up to 60 degrees Celsius. The study outcome has also shown
that E. coli species has been shown to be more susceptible to bleaching agent as compared to
B. Subtilis species.

Introduction
The control of microbial growth is an essential procedure to prevent the transmission
of infections and diseases caused by the microbial agents. The microbial growth control is
essential as it stops the decomposition and spoilage of foods and other consumable products
and also preventing the unwanted microbial contaminations. The control of the microbial
growth can be achieved through the use of chemical agents and physical agents. The physical
agents and processes that can be used to control the microbial growth include osmotic
pressure, filtrations, use of either too high or too low temperatures, radiations or desiccation
(Carpenter-Cleland, 2017). The chemical methods commonly used to control the microbial
growth include use of antiseptics, disinfectants and chemotherapeutic antimicrobial
chemicals. The scientists and the healthcare professionals’ uses specific terminologies to
precisely refer to microbial control environment hence the need to be familiar with such
terms in order to fully understand and learn microbial growth control concepts (Kilbey,
2015). Some of these terms are defined and discussed below.
1. Antibiotic refers to a microbial derivative with the ability to kill susceptible
microorganisms on inhibit their growth and proliferation in specific environment
(Slonczewski, Foster, & Gillen, 2014).
2. Antimicrobial agents refer to any chemical compound that can kill or inhibit the
growth of the microorganisms. The agents may vary in terms of selective toxicity and
they can either be natural or chemically synthesized (Kilbey, 2015).
3. Antisepsis is the reduction in the number of microbes with potential pathogenesis on
living cells while antiseptic refers to the procedure or environment free of pathogenic
contaminants such as bacteria, virus or fungus (Kirchman, 2011).
4. Disinfection refers to elimination or reduction of the pathogenic microorganism in or
on a material surface so as to make it safe. Disinfection process can be achieved
The control of microbial growth is an essential procedure to prevent the transmission
of infections and diseases caused by the microbial agents. The microbial growth control is
essential as it stops the decomposition and spoilage of foods and other consumable products
and also preventing the unwanted microbial contaminations. The control of the microbial
growth can be achieved through the use of chemical agents and physical agents. The physical
agents and processes that can be used to control the microbial growth include osmotic
pressure, filtrations, use of either too high or too low temperatures, radiations or desiccation
(Carpenter-Cleland, 2017). The chemical methods commonly used to control the microbial
growth include use of antiseptics, disinfectants and chemotherapeutic antimicrobial
chemicals. The scientists and the healthcare professionals’ uses specific terminologies to
precisely refer to microbial control environment hence the need to be familiar with such
terms in order to fully understand and learn microbial growth control concepts (Kilbey,
2015). Some of these terms are defined and discussed below.
1. Antibiotic refers to a microbial derivative with the ability to kill susceptible
microorganisms on inhibit their growth and proliferation in specific environment
(Slonczewski, Foster, & Gillen, 2014).
2. Antimicrobial agents refer to any chemical compound that can kill or inhibit the
growth of the microorganisms. The agents may vary in terms of selective toxicity and
they can either be natural or chemically synthesized (Kilbey, 2015).
3. Antisepsis is the reduction in the number of microbes with potential pathogenesis on
living cells while antiseptic refers to the procedure or environment free of pathogenic
contaminants such as bacteria, virus or fungus (Kirchman, 2011).
4. Disinfection refers to elimination or reduction of the pathogenic microorganism in or
on a material surface so as to make it safe. Disinfection process can be achieved
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

through the use of antiseptic which is used externally on animal tissues, disinfectant
that is used on inanimate objects and not animal tissues and the sanitizer that is used
on food preparation equipments (Reis, Paula, Casarotti, & Penna, 2012).
5. Decontamination is the process of treating an objective or inanimate surface to
render it safe to handle while sterilization refers to the process of destroying all the
living microbes such as viruses, bacteria, fungi, protozoa among others. A sterile
object is one which is free of all forms of life (Santagati, Scillato, Patanè, Aiello, &
Stefani, 2012).
6. Chemotherapeutic antimicrobial chemicals refer to synthetic chemicals that can be
used therapeutically to kill or control the growth and proliferation of the microbes.
The agents with the ability to kill the microbes are termed cidal agents while those
that inhibit the growth and proliferations of the microorganisms are termed static
agents (Kirchman, 2011).
The purpose of the experiment was to investigate how the physical and chemical agents
and processes can be used to control the growth and proliferation of two specifically chosen
microbes. The two microorganisms that were used in the experiment included B. Subtilis and
the E.coli. The experiments to be conducted include the Kirby-Bauer test, the lethal effects of
the temperature, bleach test and determination of minimum inhibitory concentration (MIC) of
antibiotic drugs.
Materials and Methods
1. The Kirby-Bauer test
The test procedure was developed to help in determining the microbial susceptibility to
different antibiotics. Materials used included small forceps, sterile cotton swabs, Mueller-
Hinton agar plates and the paper disks impregnated with antibiotics (Slonczewski, Foster, &
that is used on inanimate objects and not animal tissues and the sanitizer that is used
on food preparation equipments (Reis, Paula, Casarotti, & Penna, 2012).
5. Decontamination is the process of treating an objective or inanimate surface to
render it safe to handle while sterilization refers to the process of destroying all the
living microbes such as viruses, bacteria, fungi, protozoa among others. A sterile
object is one which is free of all forms of life (Santagati, Scillato, Patanè, Aiello, &
Stefani, 2012).
6. Chemotherapeutic antimicrobial chemicals refer to synthetic chemicals that can be
used therapeutically to kill or control the growth and proliferation of the microbes.
The agents with the ability to kill the microbes are termed cidal agents while those
that inhibit the growth and proliferations of the microorganisms are termed static
agents (Kirchman, 2011).
The purpose of the experiment was to investigate how the physical and chemical agents
and processes can be used to control the growth and proliferation of two specifically chosen
microbes. The two microorganisms that were used in the experiment included B. Subtilis and
the E.coli. The experiments to be conducted include the Kirby-Bauer test, the lethal effects of
the temperature, bleach test and determination of minimum inhibitory concentration (MIC) of
antibiotic drugs.
Materials and Methods
1. The Kirby-Bauer test
The test procedure was developed to help in determining the microbial susceptibility to
different antibiotics. Materials used included small forceps, sterile cotton swabs, Mueller-
Hinton agar plates and the paper disks impregnated with antibiotics (Slonczewski, Foster, &
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Gillen, 2014). The antibiotics used included ciprofloxacin, moxalactam, Vancomycin,
Penicilin, Polymyxin B and the Colistin. Procedure 3 was followed without any alterations on
the methodology (Carpenter-Cleland, 2017). The diameters of microbes (E. coli and B.
Sabtilis) migration in the plate were measured for the different drugs used. The plates were
exposed in similar environmental conditions.
2. Determining the Minimum Inhibitory concentration of an Antimicrobial Drug
This is a complementary Kirby-Bauer disk test. It is a test used in microbial research
laboratories to determine the lowest concentration of the drug required to prevent the growth
of the microbial agent. It is usually a quantitative test as opposed to Kirby-Bauer disc
diffusion test which is a qualitative test. Procedure 5 in the practical manual guide will be
used to conduct the experiment (Carpenter-Cleland, 2017). The lowest concentration of the
drugs that inhibits the culture growth will be recorded.
3. Bleach Tests
The experiment will employ the use of commercially sold household bleaching items
such as sodium hypochlorite to determine their ability to disinfect surfaces through moderate
oxidizing activities (Reis, Paula, Casarotti, & Penna, 2012). The bleaching agents have the
ability to control the growth and proliferations of the microbes. The experiment deployed the
use of nutrient ager plates, sterile cotton swab, bleach solutions and filter discs culture and
10% bleach agent. The amount of time taken to clear the culture in the plates was recorded.
Procedure 6 in the practical manual guide will be used to conduct the experiment (Carpenter-
Cleland, 2017).
4. Lethal Effects of Temperature on Microbes
Penicilin, Polymyxin B and the Colistin. Procedure 3 was followed without any alterations on
the methodology (Carpenter-Cleland, 2017). The diameters of microbes (E. coli and B.
Sabtilis) migration in the plate were measured for the different drugs used. The plates were
exposed in similar environmental conditions.
2. Determining the Minimum Inhibitory concentration of an Antimicrobial Drug
This is a complementary Kirby-Bauer disk test. It is a test used in microbial research
laboratories to determine the lowest concentration of the drug required to prevent the growth
of the microbial agent. It is usually a quantitative test as opposed to Kirby-Bauer disc
diffusion test which is a qualitative test. Procedure 5 in the practical manual guide will be
used to conduct the experiment (Carpenter-Cleland, 2017). The lowest concentration of the
drugs that inhibits the culture growth will be recorded.
3. Bleach Tests
The experiment will employ the use of commercially sold household bleaching items
such as sodium hypochlorite to determine their ability to disinfect surfaces through moderate
oxidizing activities (Reis, Paula, Casarotti, & Penna, 2012). The bleaching agents have the
ability to control the growth and proliferations of the microbes. The experiment deployed the
use of nutrient ager plates, sterile cotton swab, bleach solutions and filter discs culture and
10% bleach agent. The amount of time taken to clear the culture in the plates was recorded.
Procedure 6 in the practical manual guide will be used to conduct the experiment (Carpenter-
Cleland, 2017).
4. Lethal Effects of Temperature on Microbes

The experiment is set to determine the thermal death time and thermal death point for the
two microorganisms under study. The material required in the experimented included water
baths, test-tube racks, thermometer, nutrient agar plate, inoculating loop and the 3 days
culture organisms (B. Subtilis). Procedure 7 in the practical manual was employed to carry
out the practical (Carpenter-Cleland, 2017). The amount of growth in the test-tubes at
different temperature was recorded.
Results
1. The Kirby-Bauer test
Drugs
Diameter of growth ( mm)
B. sabtilis E. coli
Ciprofloxacin 40 45
moxalactam No clear circle No visible circle
Vancomycin 30 No visible circle
Polymyxin B 20 20
Colistin 12 15
Penicilin No clear circle No visible circle
Table 1
2. Determining the Minimum Inhibitory concentration (MIC) of an Antimicrobial
Drug
Microorganism Concentration Used
B. subtilis 0.015
E. coli 8
Table 2
3. Bleach Tests
S. subtilis
Concentration of bleaching
agent
Time Taken in the Bleaching Agent
10minutes 30 minutes
0% No clear margin No clear margin
10% No clear margin No clear margin
20% No clear margin 5mm
30% No clear margin 5mm
35% 8mm 7mm
Table 3
E. coli
two microorganisms under study. The material required in the experimented included water
baths, test-tube racks, thermometer, nutrient agar plate, inoculating loop and the 3 days
culture organisms (B. Subtilis). Procedure 7 in the practical manual was employed to carry
out the practical (Carpenter-Cleland, 2017). The amount of growth in the test-tubes at
different temperature was recorded.
Results
1. The Kirby-Bauer test
Drugs
Diameter of growth ( mm)
B. sabtilis E. coli
Ciprofloxacin 40 45
moxalactam No clear circle No visible circle
Vancomycin 30 No visible circle
Polymyxin B 20 20
Colistin 12 15
Penicilin No clear circle No visible circle
Table 1
2. Determining the Minimum Inhibitory concentration (MIC) of an Antimicrobial
Drug
Microorganism Concentration Used
B. subtilis 0.015
E. coli 8
Table 2
3. Bleach Tests
S. subtilis
Concentration of bleaching
agent
Time Taken in the Bleaching Agent
10minutes 30 minutes
0% No clear margin No clear margin
10% No clear margin No clear margin
20% No clear margin 5mm
30% No clear margin 5mm
35% 8mm 7mm
Table 3
E. coli
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
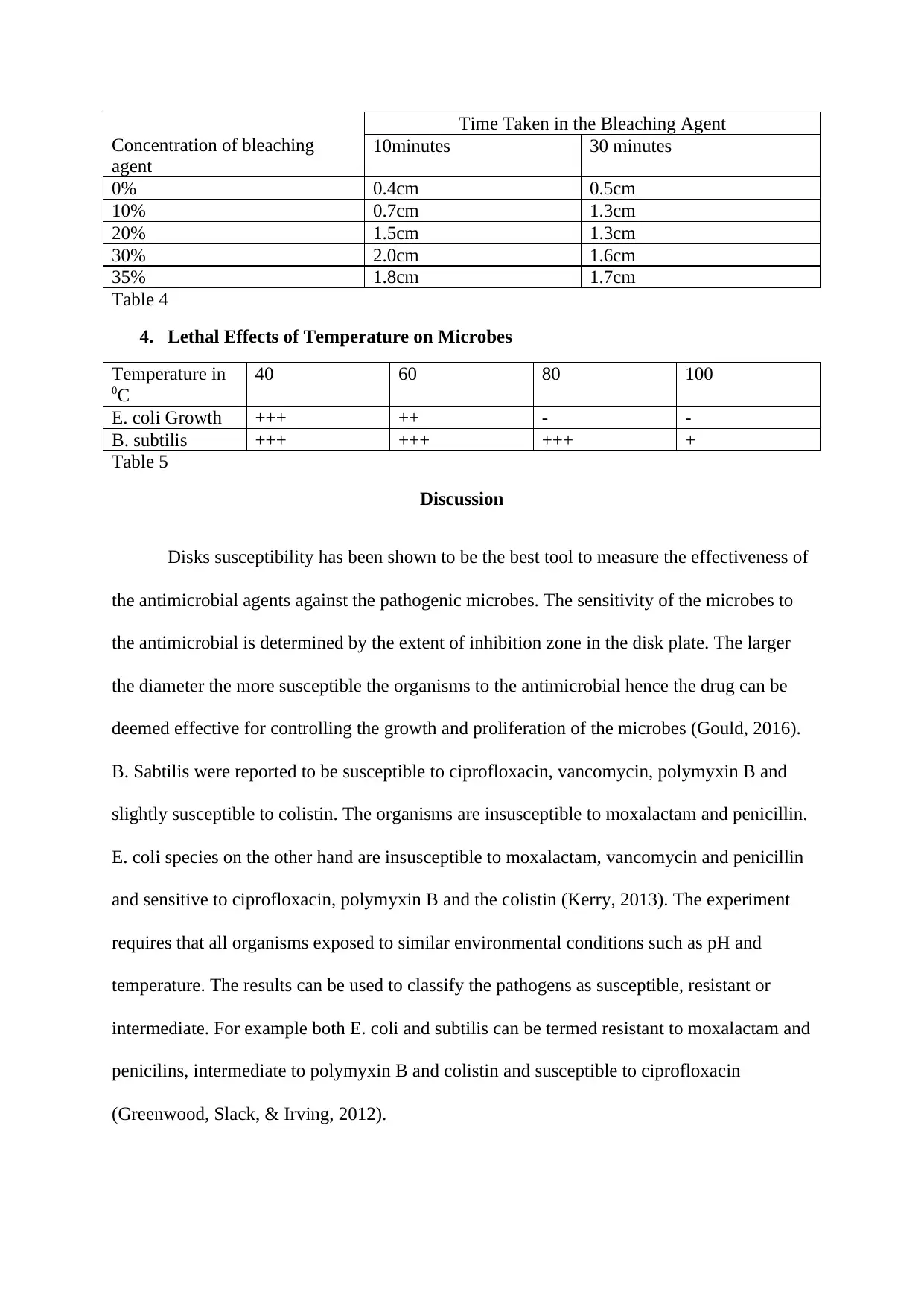
Concentration of bleaching
agent
Time Taken in the Bleaching Agent
10minutes 30 minutes
0% 0.4cm 0.5cm
10% 0.7cm 1.3cm
20% 1.5cm 1.3cm
30% 2.0cm 1.6cm
35% 1.8cm 1.7cm
Table 4
4. Lethal Effects of Temperature on Microbes
Temperature in
0C
40 60 80 100
E. coli Growth +++ ++ - -
B. subtilis +++ +++ +++ +
Table 5
Discussion
Disks susceptibility has been shown to be the best tool to measure the effectiveness of
the antimicrobial agents against the pathogenic microbes. The sensitivity of the microbes to
the antimicrobial is determined by the extent of inhibition zone in the disk plate. The larger
the diameter the more susceptible the organisms to the antimicrobial hence the drug can be
deemed effective for controlling the growth and proliferation of the microbes (Gould, 2016).
B. Sabtilis were reported to be susceptible to ciprofloxacin, vancomycin, polymyxin B and
slightly susceptible to colistin. The organisms are insusceptible to moxalactam and penicillin.
E. coli species on the other hand are insusceptible to moxalactam, vancomycin and penicillin
and sensitive to ciprofloxacin, polymyxin B and the colistin (Kerry, 2013). The experiment
requires that all organisms exposed to similar environmental conditions such as pH and
temperature. The results can be used to classify the pathogens as susceptible, resistant or
intermediate. For example both E. coli and subtilis can be termed resistant to moxalactam and
penicilins, intermediate to polymyxin B and colistin and susceptible to ciprofloxacin
(Greenwood, Slack, & Irving, 2012).
agent
Time Taken in the Bleaching Agent
10minutes 30 minutes
0% 0.4cm 0.5cm
10% 0.7cm 1.3cm
20% 1.5cm 1.3cm
30% 2.0cm 1.6cm
35% 1.8cm 1.7cm
Table 4
4. Lethal Effects of Temperature on Microbes
Temperature in
0C
40 60 80 100
E. coli Growth +++ ++ - -
B. subtilis +++ +++ +++ +
Table 5
Discussion
Disks susceptibility has been shown to be the best tool to measure the effectiveness of
the antimicrobial agents against the pathogenic microbes. The sensitivity of the microbes to
the antimicrobial is determined by the extent of inhibition zone in the disk plate. The larger
the diameter the more susceptible the organisms to the antimicrobial hence the drug can be
deemed effective for controlling the growth and proliferation of the microbes (Gould, 2016).
B. Sabtilis were reported to be susceptible to ciprofloxacin, vancomycin, polymyxin B and
slightly susceptible to colistin. The organisms are insusceptible to moxalactam and penicillin.
E. coli species on the other hand are insusceptible to moxalactam, vancomycin and penicillin
and sensitive to ciprofloxacin, polymyxin B and the colistin (Kerry, 2013). The experiment
requires that all organisms exposed to similar environmental conditions such as pH and
temperature. The results can be used to classify the pathogens as susceptible, resistant or
intermediate. For example both E. coli and subtilis can be termed resistant to moxalactam and
penicilins, intermediate to polymyxin B and colistin and susceptible to ciprofloxacin
(Greenwood, Slack, & Irving, 2012).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

The minimum inhibitory concentration (MIC) test is quantitative tests which seek to
determine the amount of the antimicrobials that can be used to control the growth and
proliferations of the microbes. The study has indicated that B. Subtilis require the lowest
concentration of antimicrobial to inhibit growth as compared to E. coli. As such, B. Subtilis
are more susceptible as compared to E. coli. The knowledge of minimum inhibitory
concentration can be used to determine the antimicrobial dosage regimen and predict the
toxicity (Greenwood, Slack, & Irving, 2012).
The bleaching agents act as disinfect of the surfaces through reduction process hence
depriving the microbe oxygen and air for respiration. The agents are used to target aerobic
microbes which cannot survive in absence of oxygen. The degree of growth inhibition is
greatly determined by the concentration of the bleaching agent and amount of time allowed
for the reduction process (Hauschild, 2015). The higher the concentration of the bleaching
agent, the higher the potential of inhibiting microbial growth as indicated in table 3 and table
4. E. coli species has been shown to be more susceptible to bleaching agent as compared to B.
Subtilis species. B. subtilis is only susceptible to bleaching agent used when exposed for
long time and at higher concentrations. On the other hand, E. coli is susceptible to the
bleaching agent at both low concentrations and almost immediately it is exposed (Florence,
2014).
The low temperature and extremely high temperatures affects the activities of the
microbial hence it can be used to control the growth and proliferations of the
microorganisms. Low temperatures inactivate the microbes hence inability to infect, spoil or
degrade substances while the high temperature denatures the enzymes in microbes hence
inactivating them (Hauschild, 2015). Therefore the growth of bacteria can be controlled by
regulating the temperatures. The practical results shown in table 6 have shown that the
growth of B. subtilis can occure up to 100 degrees Celsius however, its growth at 100 degrees
determine the amount of the antimicrobials that can be used to control the growth and
proliferations of the microbes. The study has indicated that B. Subtilis require the lowest
concentration of antimicrobial to inhibit growth as compared to E. coli. As such, B. Subtilis
are more susceptible as compared to E. coli. The knowledge of minimum inhibitory
concentration can be used to determine the antimicrobial dosage regimen and predict the
toxicity (Greenwood, Slack, & Irving, 2012).
The bleaching agents act as disinfect of the surfaces through reduction process hence
depriving the microbe oxygen and air for respiration. The agents are used to target aerobic
microbes which cannot survive in absence of oxygen. The degree of growth inhibition is
greatly determined by the concentration of the bleaching agent and amount of time allowed
for the reduction process (Hauschild, 2015). The higher the concentration of the bleaching
agent, the higher the potential of inhibiting microbial growth as indicated in table 3 and table
4. E. coli species has been shown to be more susceptible to bleaching agent as compared to B.
Subtilis species. B. subtilis is only susceptible to bleaching agent used when exposed for
long time and at higher concentrations. On the other hand, E. coli is susceptible to the
bleaching agent at both low concentrations and almost immediately it is exposed (Florence,
2014).
The low temperature and extremely high temperatures affects the activities of the
microbial hence it can be used to control the growth and proliferations of the
microorganisms. Low temperatures inactivate the microbes hence inability to infect, spoil or
degrade substances while the high temperature denatures the enzymes in microbes hence
inactivating them (Hauschild, 2015). Therefore the growth of bacteria can be controlled by
regulating the temperatures. The practical results shown in table 6 have shown that the
growth of B. subtilis can occure up to 100 degrees Celsius however, its growth at 100 degrees

Celsius is dramatically slowed. E. coli species can only grow up to 60 degrees Celsius hence
its growth can be minimized by increasing the temperatures beyond 60 degrees Celsius
(Clifford, 2015).
its growth can be minimized by increasing the temperatures beyond 60 degrees Celsius
(Clifford, 2015).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

References
Carpenter-Cleland, C. (2017). BIOL 2P98 Principles of Microbiology 2017FW Lab
Manual. St. Catharines: Brock University.
Clifford, D. (2015). Biocides – A Reasonable Alternative to Prevent and Control
Microorganisms? Frontiers in Antimicrobial Agents, 12(9), 208-233.
doi:10.2174/9781681081403115010011
Florence, K. (2014). Microbes and pathogens. Theoretical Approaches to Biological
Control, 13(3), 305-306. doi:10.1017/cbo9780511542077.022
Gould, G. W. (2016). Control of Microbial Growth through the Exclusion of Air.
Biodeterioration 7, 10(14), 529-534. doi:10.1007/978-94-009-1363-9_70
Greenwood, D., Slack, R. C., & Irving, W. L. (2012). Medical Microbiology: A Guide
to Microbial Infections: Pathogenesis, Immunity, Laboratory Diagnosis and
Control. With STUDENT CONSULT Online Access (3rd ed.). London: Elsevier
Health Sciences UK.
Hauschild, R. (2015). Safety and regulation of microbial pest control agents and
microbial plant growth promoters - introduction and overview. Beneficial
microorganisms in agriculture, food and the environment: safety assessment
and regulation, 9(5), 67-71. doi:10.1079/9781845938109.0067
Kerry, B. (2013). The use of microbial agents for the biological control of plant
parasitic nematodes. Exploitation of Microorganisms, 7(3), 81-104.
doi:10.1007/978-94-011-1532-2_4
Kilbey, B. J. (2015). Determinants of the Mutagenic Specificity of Chemical and
Physical Agents in Microorganisms. Radiation Research, 19(9), 966-975.
doi:10.1016/b978-0-12-523350-7.50094-8
Carpenter-Cleland, C. (2017). BIOL 2P98 Principles of Microbiology 2017FW Lab
Manual. St. Catharines: Brock University.
Clifford, D. (2015). Biocides – A Reasonable Alternative to Prevent and Control
Microorganisms? Frontiers in Antimicrobial Agents, 12(9), 208-233.
doi:10.2174/9781681081403115010011
Florence, K. (2014). Microbes and pathogens. Theoretical Approaches to Biological
Control, 13(3), 305-306. doi:10.1017/cbo9780511542077.022
Gould, G. W. (2016). Control of Microbial Growth through the Exclusion of Air.
Biodeterioration 7, 10(14), 529-534. doi:10.1007/978-94-009-1363-9_70
Greenwood, D., Slack, R. C., & Irving, W. L. (2012). Medical Microbiology: A Guide
to Microbial Infections: Pathogenesis, Immunity, Laboratory Diagnosis and
Control. With STUDENT CONSULT Online Access (3rd ed.). London: Elsevier
Health Sciences UK.
Hauschild, R. (2015). Safety and regulation of microbial pest control agents and
microbial plant growth promoters - introduction and overview. Beneficial
microorganisms in agriculture, food and the environment: safety assessment
and regulation, 9(5), 67-71. doi:10.1079/9781845938109.0067
Kerry, B. (2013). The use of microbial agents for the biological control of plant
parasitic nematodes. Exploitation of Microorganisms, 7(3), 81-104.
doi:10.1007/978-94-011-1532-2_4
Kilbey, B. J. (2015). Determinants of the Mutagenic Specificity of Chemical and
Physical Agents in Microorganisms. Radiation Research, 19(9), 966-975.
doi:10.1016/b978-0-12-523350-7.50094-8
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Kirchman, D. L. (2011). Physical-chemical environment of microbes. Processes in
Microbial Ecology, 11(2), 35-54.
doi:10.1093/acprof:oso/9780199586936.003.0003
Reis, J. A., Paula, A. T., Casarotti, S. N., & Penna, A. L. (2012). Lactic Acid Bacteria
Antimicrobial Compounds: Characteristics and Applications. Food Engineering
Reviews, 4(2), 124-140. doi:10.1007/s12393-012-9051-2
Santagati, M., Scillato, M., Patanè, F., Aiello, C., & Stefani, S. (2012). Bacteriocin-
producing oral streptococci and inhibition of respiratory pathogens. FEMS
Immunology & Medical Microbiology, 65(1), 23-31. doi:10.1111/j.1574-
695x.2012.00928.x
Slonczewski, J., Foster, J. W., & Gillen, K. M. (2014). Microbiology: An evolving
science. New york: W w norton.
Microbial Ecology, 11(2), 35-54.
doi:10.1093/acprof:oso/9780199586936.003.0003
Reis, J. A., Paula, A. T., Casarotti, S. N., & Penna, A. L. (2012). Lactic Acid Bacteria
Antimicrobial Compounds: Characteristics and Applications. Food Engineering
Reviews, 4(2), 124-140. doi:10.1007/s12393-012-9051-2
Santagati, M., Scillato, M., Patanè, F., Aiello, C., & Stefani, S. (2012). Bacteriocin-
producing oral streptococci and inhibition of respiratory pathogens. FEMS
Immunology & Medical Microbiology, 65(1), 23-31. doi:10.1111/j.1574-
695x.2012.00928.x
Slonczewski, J., Foster, J. W., & Gillen, K. M. (2014). Microbiology: An evolving
science. New york: W w norton.
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.