Production of Omega 3 Fatty Acids Using E. coli: A Detailed Analysis
VerifiedAdded on 2023/04/25
|16
|4303
|138
Report
AI Summary
This report explores the production of Omega 3 fatty acids using E. coli with lignocellulosic hydrolysate as a feedstock. It delves into the chemical characteristics of Omega 3 fatty acids and lignocellulose, highlighting the importance of accurate compositional analysis for optimal yield. The report details the metabolic engineering pathway in E. coli, including the enzymes involved in fatty acid synthesis and process modification strategies to enhance production. Enzyme engineering, specifically focusing on enzymes like acetyl-CoA carboxylase and mutant acyl-CoA thioesterase I (TesA), is discussed to illustrate their impact on Omega 3 fatty acid quantity. The report also examines methods for immobilizing E. coli cells to improve fatty acid production, enhancing cost-effectiveness and stability in industrial applications. The conclusion emphasizes the potential of engineered enzymes and immobilized cells in optimizing Omega 3 fatty acid production.

PRODUCTION OF OMEGA 3 FATTY ACIDS USING E.COLI AS YOUR
ORGANISM AND LIGNOCELLULOSIC HYDROLYSATE AS YOUR FEEDSTOCK
ORGANISM AND LIGNOCELLULOSIC HYDROLYSATE AS YOUR FEEDSTOCK
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Introduction
Omega 3 fatty acids are a polyunsaturated type of fat, which typically means that the
chemical structure of their carbon atoms has more double bonds. Most of the natural Omega
3 bulk is found in fish, but some does exist in plants such as walnuts, hemp, and flaxseed
(Demirbas, 2009, p.89). Although the latter is quite different, when it gets into the body, it
gets converted into the type found within fish. On the other hand, lignocellulose hydrolysate
facilitates an abundant renewable resource during generation of polymers, chemicals, and
biofuels. The feedstock includes residues from pulp mills, biorefineries, forestry, and
agriculture. Due to the need for a consistent source of energy, there has been a significant
focus of researches on the biosynthesis of microbial fatty acids with the aim of producing
Omega 3 fatty acids and other substitute derivatives of diesel.
Chemical characteristics
As an organism that is industrially significant and one that has the best result of fatty
acid biosynthesis, Escherichia coli has been regularly chosen as a producer for these studies
and several conclusions have been made in the field of E.coli bio-fuels or omega three fatty
acid biosynthesis. Omega 3 fatty acids are regarded as conditionally essential because
usually, their synthesized quantity is insufficient to meet human needs. When it comes to the
lignocellulosichydrolysate feedstock, it is ideally a non-edible plant material that is primarily
composed of hemicellulose and polysaccharides cellulose(Huffer, 2012, p.30). Accurate
compositional analysis of this feedstock and a good measurement of its biomass are of prime
significance as it is directly proportional to the omega-3 fatty acids yield.
Omega-3 fatty acids are one of the most researched nutrients as they have a high energy
density which is valuable for storage (Walther and Farese, 2012). ALA is the precursor of
two essential bio-active long-chain polyunsaturated fatty acids (LC-PUFA), which are the
docosahexaenoic acid (DHA- C22:6, omega-3) and eicosapentaenoic acid (EPA- C20:5,
omega-3). ALA is converted to DHA and EPA through the occurrence of desaturation
reactions and enzymatic elongation, where Δ-5 desaturase, Δ-6 desaturase,andelongase
enzymes are involved. Linolenic acid (ALA- C18:3, omega-3) is not produced in human
body synthesis and thus has to be consumed orally in the diet (Abedi and Sahari, 2014).
Omega 3 fatty acids are a polyunsaturated type of fat, which typically means that the
chemical structure of their carbon atoms has more double bonds. Most of the natural Omega
3 bulk is found in fish, but some does exist in plants such as walnuts, hemp, and flaxseed
(Demirbas, 2009, p.89). Although the latter is quite different, when it gets into the body, it
gets converted into the type found within fish. On the other hand, lignocellulose hydrolysate
facilitates an abundant renewable resource during generation of polymers, chemicals, and
biofuels. The feedstock includes residues from pulp mills, biorefineries, forestry, and
agriculture. Due to the need for a consistent source of energy, there has been a significant
focus of researches on the biosynthesis of microbial fatty acids with the aim of producing
Omega 3 fatty acids and other substitute derivatives of diesel.
Chemical characteristics
As an organism that is industrially significant and one that has the best result of fatty
acid biosynthesis, Escherichia coli has been regularly chosen as a producer for these studies
and several conclusions have been made in the field of E.coli bio-fuels or omega three fatty
acid biosynthesis. Omega 3 fatty acids are regarded as conditionally essential because
usually, their synthesized quantity is insufficient to meet human needs. When it comes to the
lignocellulosichydrolysate feedstock, it is ideally a non-edible plant material that is primarily
composed of hemicellulose and polysaccharides cellulose(Huffer, 2012, p.30). Accurate
compositional analysis of this feedstock and a good measurement of its biomass are of prime
significance as it is directly proportional to the omega-3 fatty acids yield.
Omega-3 fatty acids are one of the most researched nutrients as they have a high energy
density which is valuable for storage (Walther and Farese, 2012). ALA is the precursor of
two essential bio-active long-chain polyunsaturated fatty acids (LC-PUFA), which are the
docosahexaenoic acid (DHA- C22:6, omega-3) and eicosapentaenoic acid (EPA- C20:5,
omega-3). ALA is converted to DHA and EPA through the occurrence of desaturation
reactions and enzymatic elongation, where Δ-5 desaturase, Δ-6 desaturase,andelongase
enzymes are involved. Linolenic acid (ALA- C18:3, omega-3) is not produced in human
body synthesis and thus has to be consumed orally in the diet (Abedi and Sahari, 2014).

METABOLIC ENGINEERING
Pathway of fatty acid production in E. coli:
The first stage of the fatty acid synthesis is the formation of malonyl-CoA which is formed by
carboxylation of acetyl-CoA. This process cost one ATP. Then, ACP or acyl carrier protein
exchanged the Coenzyme A in the malonyl-CoA and became malonyl-ACP. The action of
this ACP or acyl carrier protein is to prevent this growing fatty acid chain from being
degraded or used in anabolic reactions. Condensation of malonyl-ACP with acetyl-CoA starts
the first steps of the cycle of fatty acid synthesis. The by- products of this steps are free
coenzyme A, hydrogencarbonate, and acetoacetyl-ACP. Acetoacetyl-ACP then reduced to 3-
hydroxybutyryl-ACP which is then converted to 2-butenoyl-ACP by dehydration. 2-butenoyl-
ACP further reduced to butyryl-ACP. The next step of this cycles begins with butyryl-ACP
and its condensation with malonyl-ACP. The synthesis of the fatty acids continues until it
reaches a certain length. After reaching that required chain length, membrane synthesis
performed using the acyl-ACP. Reduction equivalent required by these two reduction step are
derived from NADPH or nicotinamide adenine dinucleotide (Schweizer and Hofmann 2004).
Pathway of fatty acid production in E. coli:
The first stage of the fatty acid synthesis is the formation of malonyl-CoA which is formed by
carboxylation of acetyl-CoA. This process cost one ATP. Then, ACP or acyl carrier protein
exchanged the Coenzyme A in the malonyl-CoA and became malonyl-ACP. The action of
this ACP or acyl carrier protein is to prevent this growing fatty acid chain from being
degraded or used in anabolic reactions. Condensation of malonyl-ACP with acetyl-CoA starts
the first steps of the cycle of fatty acid synthesis. The by- products of this steps are free
coenzyme A, hydrogencarbonate, and acetoacetyl-ACP. Acetoacetyl-ACP then reduced to 3-
hydroxybutyryl-ACP which is then converted to 2-butenoyl-ACP by dehydration. 2-butenoyl-
ACP further reduced to butyryl-ACP. The next step of this cycles begins with butyryl-ACP
and its condensation with malonyl-ACP. The synthesis of the fatty acids continues until it
reaches a certain length. After reaching that required chain length, membrane synthesis
performed using the acyl-ACP. Reduction equivalent required by these two reduction step are
derived from NADPH or nicotinamide adenine dinucleotide (Schweizer and Hofmann 2004).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
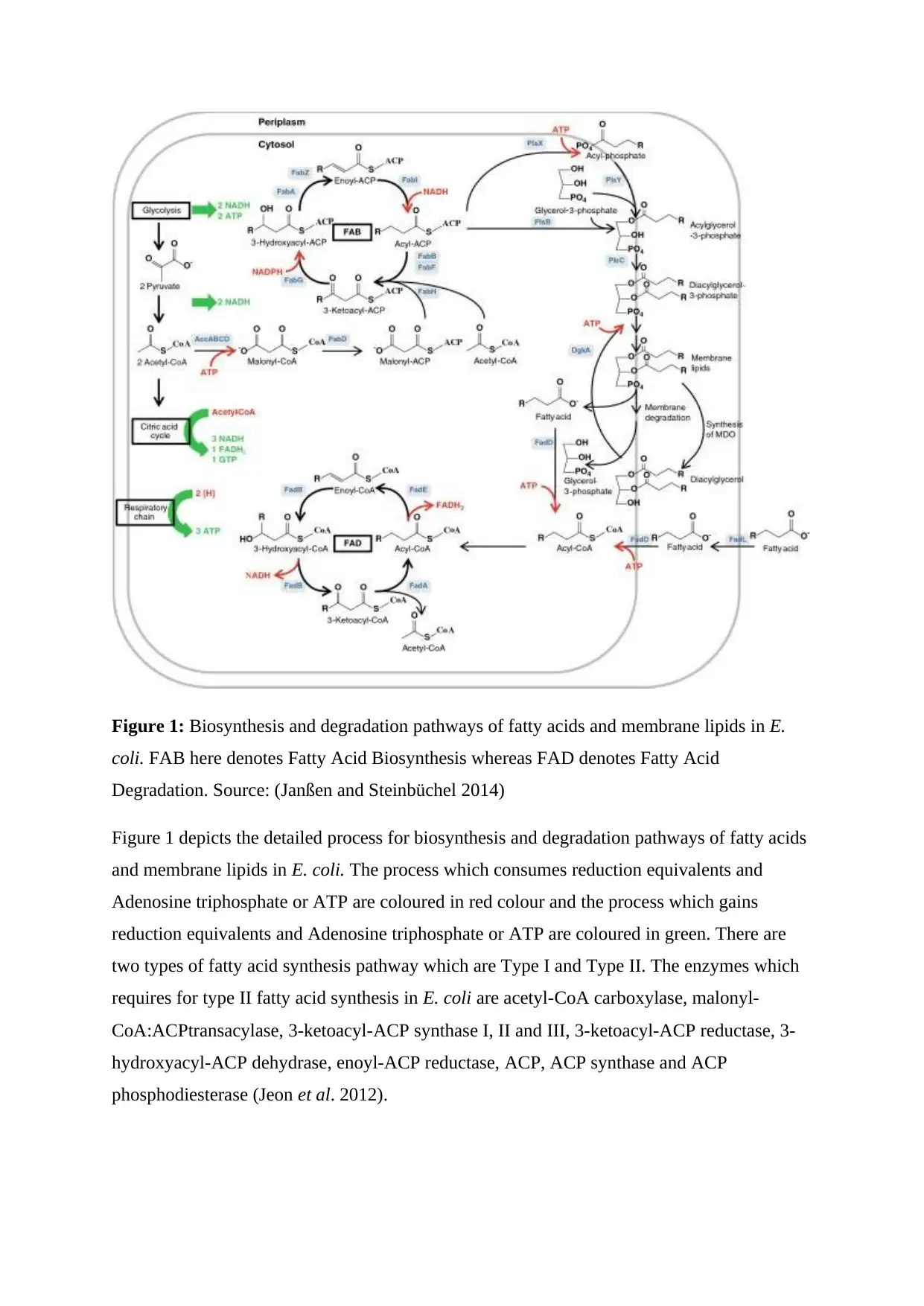
Figure 1: Biosynthesis and degradation pathways of fatty acids and membrane lipids in E.
coli. FAB here denotes Fatty Acid Biosynthesis whereas FAD denotes Fatty Acid
Degradation. Source: (Janßen and Steinbüchel 2014)
Figure 1 depicts the detailed process for biosynthesis and degradation pathways of fatty acids
and membrane lipids in E. coli. The process which consumes reduction equivalents and
Adenosine triphosphate or ATP are coloured in red colour and the process which gains
reduction equivalents and Adenosine triphosphate or ATP are coloured in green. There are
two types of fatty acid synthesis pathway which are Type I and Type II. The enzymes which
requires for type II fatty acid synthesis in E. coli are acetyl-CoA carboxylase, malonyl-
CoA:ACPtransacylase, 3-ketoacyl-ACP synthase I, II and III, 3-ketoacyl-ACP reductase, 3-
hydroxyacyl-ACP dehydrase, enoyl-ACP reductase, ACP, ACP synthase and ACP
phosphodiesterase (Jeon et al. 2012).
coli. FAB here denotes Fatty Acid Biosynthesis whereas FAD denotes Fatty Acid
Degradation. Source: (Janßen and Steinbüchel 2014)
Figure 1 depicts the detailed process for biosynthesis and degradation pathways of fatty acids
and membrane lipids in E. coli. The process which consumes reduction equivalents and
Adenosine triphosphate or ATP are coloured in red colour and the process which gains
reduction equivalents and Adenosine triphosphate or ATP are coloured in green. There are
two types of fatty acid synthesis pathway which are Type I and Type II. The enzymes which
requires for type II fatty acid synthesis in E. coli are acetyl-CoA carboxylase, malonyl-
CoA:ACPtransacylase, 3-ketoacyl-ACP synthase I, II and III, 3-ketoacyl-ACP reductase, 3-
hydroxyacyl-ACP dehydrase, enoyl-ACP reductase, ACP, ACP synthase and ACP
phosphodiesterase (Jeon et al. 2012).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Process modification of omega -3- fatty acids synthesis in E. coli:
Four different modification directions can be taken to improve the fatty acid synthesis in E.
coli. These modification directions are namely a) Increase of export rates, b) Regulation of
membrane saturation, c) Addressing the regulatory and metabolic bottlenecks, and d)
Structural, genetic and biochemistry studies. From the fore mentioned methods, identification
of metabolic bottlenecks and optimal cultivation strategy design might be the best possible
option to generate maximum yield for fatty acid synthesis. The reason behind the selection of
this method is that this particular direction has the maximum chance to succeed in regard to
other methods. Lennenet al. (2011) have modified the actions of ΔfadD with BTE
thioesterase and have achieved a total theoretical yield of less than 40 per cent. They have
used K-12 MG1655 base strain for their experiment. Another study has modified the actions
of ΔfadL with TesA′ thioesterase in the base strain of BL21 (DE3). The total theoretical yield
in that study was reported at around 12 per cent (Liu et al. 2012).
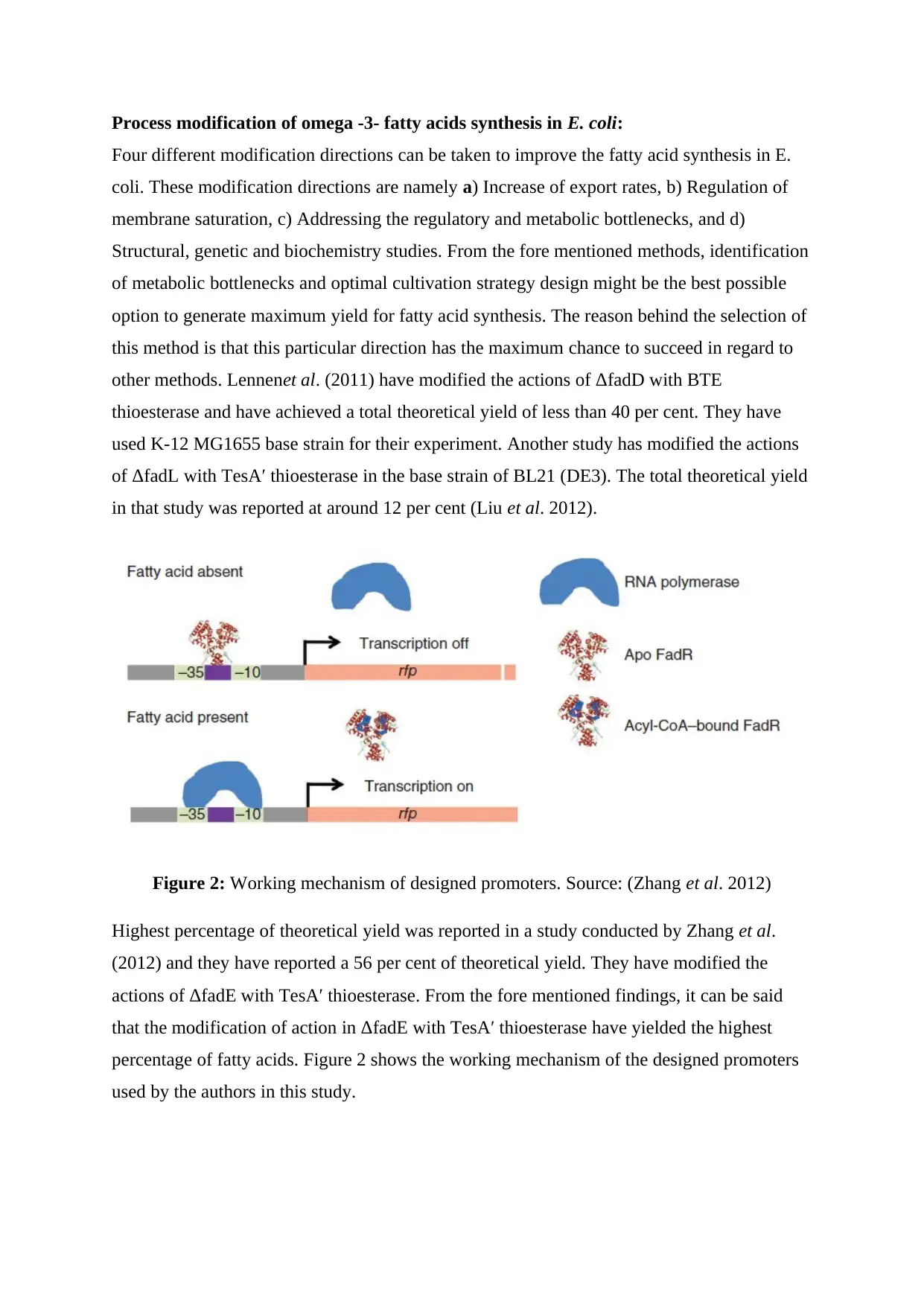
Figure 2: Working mechanism of designed promoters. Source: (Zhang et al. 2012)
Highest percentage of theoretical yield was reported in a study conducted by Zhang et al.
(2012) and they have reported a 56 per cent of theoretical yield. They have modified the
actions of ΔfadE with TesA′ thioesterase. From the fore mentioned findings, it can be said
that the modification of action in ΔfadE with TesA′ thioesterase have yielded the highest
percentage of fatty acids. Figure 2 shows the working mechanism of the designed promoters
used by the authors in this study.
Four different modification directions can be taken to improve the fatty acid synthesis in E.
coli. These modification directions are namely a) Increase of export rates, b) Regulation of
membrane saturation, c) Addressing the regulatory and metabolic bottlenecks, and d)
Structural, genetic and biochemistry studies. From the fore mentioned methods, identification
of metabolic bottlenecks and optimal cultivation strategy design might be the best possible
option to generate maximum yield for fatty acid synthesis. The reason behind the selection of
this method is that this particular direction has the maximum chance to succeed in regard to
other methods. Lennenet al. (2011) have modified the actions of ΔfadD with BTE
thioesterase and have achieved a total theoretical yield of less than 40 per cent. They have
used K-12 MG1655 base strain for their experiment. Another study has modified the actions
of ΔfadL with TesA′ thioesterase in the base strain of BL21 (DE3). The total theoretical yield
in that study was reported at around 12 per cent (Liu et al. 2012).
Figure 2: Working mechanism of designed promoters. Source: (Zhang et al. 2012)
Highest percentage of theoretical yield was reported in a study conducted by Zhang et al.
(2012) and they have reported a 56 per cent of theoretical yield. They have modified the
actions of ΔfadE with TesA′ thioesterase. From the fore mentioned findings, it can be said
that the modification of action in ΔfadE with TesA′ thioesterase have yielded the highest
percentage of fatty acids. Figure 2 shows the working mechanism of the designed promoters
used by the authors in this study.

ENZYME ENGINEERING
Impact of each enzyme on the production of omega 3 fatty acids
Acetyl-CoA carboxylase
This is the first enzyme and is the one responsible for increasing the length of the chain and
facilitates transfer ofacetyl-Coa to de-novo fatty acid where biosynthesis occur. Two
subcomplexes are produced by purifying four subunits which are in unstable complex.They
are the biotin carboxylase-biotin carboxyl carrier protein comprising of AccC and AccB, the
other one is the carboxyl transferase which consists AccA and AccD. This reaction can occur
in two half-reactions with the carboxylation of biotin under activation of ATP in presence of
Mg2+-ions, secondly there is transfer of the carboxyl group to acetyl-CoA amounting to
malonyl-CoA (Selwood & Jaffe, 2012).
The subunits are supposed to be synthesized in equimolar quantities therefore there should be
strict regulation of transcription of accABCD.Carboxylation of acetyl-Coa requires energy
because it is driven by ATP cleavage. AccB and AccC which constitute mRNA have their
transcription being more so that it can be auto regulated by the N-terminal domain of
AccB.Studies show that acyl-ACP with chain lengths of C6 to C20 inhibit the enzyme
activity of the E. coli acetyl-CoA carboxylase (Desbois & Smith, 2010).
Malonyl-CoA: acyl carrier proteins (ACPs) transacylase
It catalyses the exchange of malonyl-moiety to ACP which guides it towards unsaturated fat
neogenesis and unsaturated fat chain extension. Studies demonstrates that cancellation of
gene fabD (gene of ACP transacylase) is dangerous and its overexpression in E coli
encourages the alteration of structure of the unsaturated fats. The shifted extents of
palmitoleic acid and cis-vaccenic acid is brought about by malonyl-ACP pool adding to
movement of the 3-ketoacyl-ACP synthase II (FabF), an enzyme in charge of tie stretching of
C16:1 to C18:1. Measure of free unsaturated fat is expanded by about 11% when there is
overexpression of the E. coli fabD, together with tesA contrasted with when there is
overexpression of thioesterase quality alone (Amiri-Jami & Griffiths, 2011) .The fab D
quality interpreted in E. coli prompts a somewhat adjusted unsaturated fat piece. The extent
of palmithetoleic acid reductions though the extent of cis-vaccenic acid increments.
Impact of each enzyme on the production of omega 3 fatty acids
Acetyl-CoA carboxylase
This is the first enzyme and is the one responsible for increasing the length of the chain and
facilitates transfer ofacetyl-Coa to de-novo fatty acid where biosynthesis occur. Two
subcomplexes are produced by purifying four subunits which are in unstable complex.They
are the biotin carboxylase-biotin carboxyl carrier protein comprising of AccC and AccB, the
other one is the carboxyl transferase which consists AccA and AccD. This reaction can occur
in two half-reactions with the carboxylation of biotin under activation of ATP in presence of
Mg2+-ions, secondly there is transfer of the carboxyl group to acetyl-CoA amounting to
malonyl-CoA (Selwood & Jaffe, 2012).
The subunits are supposed to be synthesized in equimolar quantities therefore there should be
strict regulation of transcription of accABCD.Carboxylation of acetyl-Coa requires energy
because it is driven by ATP cleavage. AccB and AccC which constitute mRNA have their
transcription being more so that it can be auto regulated by the N-terminal domain of
AccB.Studies show that acyl-ACP with chain lengths of C6 to C20 inhibit the enzyme
activity of the E. coli acetyl-CoA carboxylase (Desbois & Smith, 2010).
Malonyl-CoA: acyl carrier proteins (ACPs) transacylase
It catalyses the exchange of malonyl-moiety to ACP which guides it towards unsaturated fat
neogenesis and unsaturated fat chain extension. Studies demonstrates that cancellation of
gene fabD (gene of ACP transacylase) is dangerous and its overexpression in E coli
encourages the alteration of structure of the unsaturated fats. The shifted extents of
palmitoleic acid and cis-vaccenic acid is brought about by malonyl-ACP pool adding to
movement of the 3-ketoacyl-ACP synthase II (FabF), an enzyme in charge of tie stretching of
C16:1 to C18:1. Measure of free unsaturated fat is expanded by about 11% when there is
overexpression of the E. coli fabD, together with tesA contrasted with when there is
overexpression of thioesterase quality alone (Amiri-Jami & Griffiths, 2011) .The fab D
quality interpreted in E. coli prompts a somewhat adjusted unsaturated fat piece. The extent
of palmithetoleic acid reductions though the extent of cis-vaccenic acid increments.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

FabB, FabF and FabH: 3-ketoacyl-ACP synthase I, II and III
This enzyme through buildup of fatty acyl-ACP with malonyl-ACP catalyzes the
development of 3-ketoacyl-ACP. FabH start the main cycle of chain prolongation amid
unsaturated fat biosynthesis while FabF and FabB play out the ensuing lengthening steps
(Nikaido & Takatsuka, 2009).
FabA and FabZ: 3-hydroxyacyl-ACP dehydrase
3-hydroxyacyl-ACP dehydrase perform dehydration of 3-hyroxyacyl-ACP (Kosa &
Ragauskas, 2011). Trans-2-decenoyl-ACP is isomerized by FabA into cis-3-decenoyl-ACP
making the primary response towards amalgamation of unsaturated fats. FabA catalyzes the
dehydration of saturated 3-hydroxyacyl-ACPs with various chain lengths and its
overexpression builds the measure of immersed unsaturated fats. It can likewise get
dehydrate 3-hydroxydecanoyl-CoA with a movement of 11% in contrast with 3-
hydroxydecanoyl-ACP. FabZ performs drying out of 3-hydroxymyristoyl-ACP (Walther &
Farese, 2012).
FabI: enoyl-ACP reductase
This enzyme encoded by FabI catalyzes the decrease of 2-enoyl-ACP to fatty acyl-ACP to the
detriment of NADPH + H+ or NADH + H+. Palmitoyl-ACP hinder the enoyl-ACP reductace
at focus which is around 50 times high. Overexpression of the fabIqene does not result in any
development imperfection and does not build the cell lipid, palmitic acid or stearic acid
substance (Wymann & Schneiter, 2008).
ACP, ACP synthase and ACP phosphodiesterase
A phosphopantethein group is appended to a serine of the deciphered apo-ACP by the activity
of the ACP synthase (AcpS) in order to get the physiologically dynamic frame. The
physiological job of ACP is to separate unsaturated fat biosynthesis where all intermediates
are bound to ACP from unsaturated fat catabolism. In E. coli, ACP speaks to 0.25% of every
single soluble protein. Overexpression of acpS prompts end of cell development which is
because of solid hindrance of the glycerol-3-phosphate acyltransferase. Heterologous
articulation of acpP offers some potential, as it has been demonstrated that the outflow of
acpP from Azospirillumbrasilense modifies the E. coli unsaturated fat profile and the
substance of C18:1 is expanded to 2-overlay at 30°C (Walther & Farese, 2012).
This enzyme through buildup of fatty acyl-ACP with malonyl-ACP catalyzes the
development of 3-ketoacyl-ACP. FabH start the main cycle of chain prolongation amid
unsaturated fat biosynthesis while FabF and FabB play out the ensuing lengthening steps
(Nikaido & Takatsuka, 2009).
FabA and FabZ: 3-hydroxyacyl-ACP dehydrase
3-hydroxyacyl-ACP dehydrase perform dehydration of 3-hyroxyacyl-ACP (Kosa &
Ragauskas, 2011). Trans-2-decenoyl-ACP is isomerized by FabA into cis-3-decenoyl-ACP
making the primary response towards amalgamation of unsaturated fats. FabA catalyzes the
dehydration of saturated 3-hydroxyacyl-ACPs with various chain lengths and its
overexpression builds the measure of immersed unsaturated fats. It can likewise get
dehydrate 3-hydroxydecanoyl-CoA with a movement of 11% in contrast with 3-
hydroxydecanoyl-ACP. FabZ performs drying out of 3-hydroxymyristoyl-ACP (Walther &
Farese, 2012).
FabI: enoyl-ACP reductase
This enzyme encoded by FabI catalyzes the decrease of 2-enoyl-ACP to fatty acyl-ACP to the
detriment of NADPH + H+ or NADH + H+. Palmitoyl-ACP hinder the enoyl-ACP reductace
at focus which is around 50 times high. Overexpression of the fabIqene does not result in any
development imperfection and does not build the cell lipid, palmitic acid or stearic acid
substance (Wymann & Schneiter, 2008).
ACP, ACP synthase and ACP phosphodiesterase
A phosphopantethein group is appended to a serine of the deciphered apo-ACP by the activity
of the ACP synthase (AcpS) in order to get the physiologically dynamic frame. The
physiological job of ACP is to separate unsaturated fat biosynthesis where all intermediates
are bound to ACP from unsaturated fat catabolism. In E. coli, ACP speaks to 0.25% of every
single soluble protein. Overexpression of acpS prompts end of cell development which is
because of solid hindrance of the glycerol-3-phosphate acyltransferase. Heterologous
articulation of acpP offers some potential, as it has been demonstrated that the outflow of
acpP from Azospirillumbrasilense modifies the E. coli unsaturated fat profile and the
substance of C18:1 is expanded to 2-overlay at 30°C (Walther & Farese, 2012).
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Impact of engineered enzyme on the quantity of omega 3 fatty acids
The enzyme that I will design is mutant acyl-CoA thioesterase I (TesA) in light because it has
high action in E coli (Wymann & Schneiter, 2008). This was concentrated by developing a
detecting framework with a combination protein of antibiotic medication obstruction and red
fluorescent protein under the control of FadR responsive promoter which selects suitable
mutants. TesA mutant that produces double the quantity of FFAs was separated from an
error-prone PCR mutant collection of E.coliTesA. The kinetic analysis showed that
replacement of Arg64 with Cys64 in the enzyme causes roughly double increase in catalytic
activity.
In order to increase the rate of production of omega 3 fatty acids it is more preferable to
increase the ratio of every component including fatty acid synthase and TesA which enhances
optimal production. Engineering the catalytic activity of TesA is also necessary than just
increasing the enzyme expression in order to enhance omega 3 fatty acids. (Kwang, et al.,
2016)
IMMOBILISATION
Methods used to immobilize E. coli cells
Omega 3 fatty acids include eicosapentaenoic acid (EPA), docosahexaenoic acid
(DHA) and Alpha-linolenic acid (ALA) found in plant sources and fishes. The fatty acids
have several health benefits such as they lower triglyceride levels in the blood (Drachuk et al.
2017). Immobilization of E.coli is an engineering technique that improves the production of
fatty acids. Also, the process confines cells in inert support for either functional reuse or
stability. The activity makes cells cost-effective and efficient for the use in industries (Jha
2014).The immobilized cells are more efficient and stable in function. Furthermore, they are
more preferred than mobilized cells because of their specificity in releasing yield in pure
form. Moreover, they have a natural environment with the availability of cofactors (Gregory
& Mello 2005).
The conventional methods of immobilizing cells include entrapment, adsorption,
crosslinking and covalent binding. Adsorption is the physical binding of cells in inert
support. It consists of both inorganic support materials such as silica gel or alumina and
The enzyme that I will design is mutant acyl-CoA thioesterase I (TesA) in light because it has
high action in E coli (Wymann & Schneiter, 2008). This was concentrated by developing a
detecting framework with a combination protein of antibiotic medication obstruction and red
fluorescent protein under the control of FadR responsive promoter which selects suitable
mutants. TesA mutant that produces double the quantity of FFAs was separated from an
error-prone PCR mutant collection of E.coliTesA. The kinetic analysis showed that
replacement of Arg64 with Cys64 in the enzyme causes roughly double increase in catalytic
activity.
In order to increase the rate of production of omega 3 fatty acids it is more preferable to
increase the ratio of every component including fatty acid synthase and TesA which enhances
optimal production. Engineering the catalytic activity of TesA is also necessary than just
increasing the enzyme expression in order to enhance omega 3 fatty acids. (Kwang, et al.,
2016)
IMMOBILISATION
Methods used to immobilize E. coli cells
Omega 3 fatty acids include eicosapentaenoic acid (EPA), docosahexaenoic acid
(DHA) and Alpha-linolenic acid (ALA) found in plant sources and fishes. The fatty acids
have several health benefits such as they lower triglyceride levels in the blood (Drachuk et al.
2017). Immobilization of E.coli is an engineering technique that improves the production of
fatty acids. Also, the process confines cells in inert support for either functional reuse or
stability. The activity makes cells cost-effective and efficient for the use in industries (Jha
2014).The immobilized cells are more efficient and stable in function. Furthermore, they are
more preferred than mobilized cells because of their specificity in releasing yield in pure
form. Moreover, they have a natural environment with the availability of cofactors (Gregory
& Mello 2005).
The conventional methods of immobilizing cells include entrapment, adsorption,
crosslinking and covalent binding. Adsorption is the physical binding of cells in inert
support. It consists of both inorganic support materials such as silica gel or alumina and

organic support materials such as DEAE – Sephadex or carboxymethyl cellulose. The cells
can be removed merely by temperature changes, ionic strength and changes in ph(Jha 2014).
Cells can be immobilized by entrapment in a gel matrix or a polymer. The pores of
the gel allow for the retention of cells. Also, the cell is not subjected to structural distortions
and strong binding forces. Matrices used for the physical entrapment of cells include
collagen, polyacrylamide gel and silicon (Nomanbay & Hussain 2015).
Microencapsulation involves the formation of spherical particles in a suspension or a
liquid in a semi-permeable membrane. The membrane can be lipoidal, polymeric, or
lipoprotein based. It is the recent method used in the immobilization of E. coli cells
(Nomanbay & Hussain 2015).
E. coli cells can also be immobilized through covalent binding, which is the formation
of covalent bonds between cells. Furthermore, the technique is widely used. However, it is
involved with the loss of cell activity. The conventional methods of covalent binding include
cyanogen bromide and the treatment of support materials using HCl (Jha 2014).
Lastly, cross-linking is used in the immobilization of cells. Polyfunctional reagents
are used to create cross-links between cells. The reagents react to form bridges which can be
used to hold cell molecules. Several reagents used in cross-linking include glutaraldehyde
and diazobenzidine (Amarjeet 2013).
The method chosen for E.coli cells
The preferred method for immobilization of cells is cross-linking. It is the most
convenient because the cross-links formed between the cells and glutaraldehyde are
irreversible. Furthermore, the method can withstand high temperatures and extreme ph
conditions (Steinbuchel & Jan 2014).
The engineering constraints governing cross-linking
The polyfunctional reagent can lead to the denaturation of enzymes. Furthermore,
some the biological activity of some cells can be lost. Also, it is expensive since it involves
the use of sophisticated equipment (Jha, 2014).
The size of particles containing immobilized cells.
can be removed merely by temperature changes, ionic strength and changes in ph(Jha 2014).
Cells can be immobilized by entrapment in a gel matrix or a polymer. The pores of
the gel allow for the retention of cells. Also, the cell is not subjected to structural distortions
and strong binding forces. Matrices used for the physical entrapment of cells include
collagen, polyacrylamide gel and silicon (Nomanbay & Hussain 2015).
Microencapsulation involves the formation of spherical particles in a suspension or a
liquid in a semi-permeable membrane. The membrane can be lipoidal, polymeric, or
lipoprotein based. It is the recent method used in the immobilization of E. coli cells
(Nomanbay & Hussain 2015).
E. coli cells can also be immobilized through covalent binding, which is the formation
of covalent bonds between cells. Furthermore, the technique is widely used. However, it is
involved with the loss of cell activity. The conventional methods of covalent binding include
cyanogen bromide and the treatment of support materials using HCl (Jha 2014).
Lastly, cross-linking is used in the immobilization of cells. Polyfunctional reagents
are used to create cross-links between cells. The reagents react to form bridges which can be
used to hold cell molecules. Several reagents used in cross-linking include glutaraldehyde
and diazobenzidine (Amarjeet 2013).
The method chosen for E.coli cells
The preferred method for immobilization of cells is cross-linking. It is the most
convenient because the cross-links formed between the cells and glutaraldehyde are
irreversible. Furthermore, the method can withstand high temperatures and extreme ph
conditions (Steinbuchel & Jan 2014).
The engineering constraints governing cross-linking
The polyfunctional reagent can lead to the denaturation of enzymes. Furthermore,
some the biological activity of some cells can be lost. Also, it is expensive since it involves
the use of sophisticated equipment (Jha, 2014).
The size of particles containing immobilized cells.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Flick's law is used to determine the diffusion coefficient.Flick's first law is used to
determine the radius of particles used in immobilization. First, some unknowns can be
derived to apply the law.
Deo
Daq
= (1−φp )
( 1+ φp )2
3
(Westrin and Axelsson 1991).
The diffusion coefficient of gel divided by diffusion coefficient diffusion in water is 0.62.
This depended on gel beads using oxygen solute. The equation was rearranged to determine
the polymer volume fraction of parts of the cell not occupied with cells. This was derived as
0.032 (Westrin and Axelsson 1991).
0.62= ( 1−0.032 )
( 1+0.032 )2
3
Deo= ( 1−φp )
(1+ φp )2
3
= (1−0.032 )
( 1+0.032 )2
3
× Daq=0.62 ×2.10 ×10−9=1.54 × 10−9 m2 s−1
Daq was found as the diffusion coefficient of oxygen to water: 2.10 x 10-9 m2/s (Cussler 2008)
The following equation was then used to calculate De:
De
Deo
=1−∅ c
(Westrin and Axelsson 1991)
∅ c, cell volume fraction was assumed as 0.05
De
1.54 ×10−9 m2 s−1 =1−0.05
De=1.5 ×10−9
determine the radius of particles used in immobilization. First, some unknowns can be
derived to apply the law.
Deo
Daq
= (1−φp )
( 1+ φp )2
3
(Westrin and Axelsson 1991).
The diffusion coefficient of gel divided by diffusion coefficient diffusion in water is 0.62.
This depended on gel beads using oxygen solute. The equation was rearranged to determine
the polymer volume fraction of parts of the cell not occupied with cells. This was derived as
0.032 (Westrin and Axelsson 1991).
0.62= ( 1−0.032 )
( 1+0.032 )2
3
Deo= ( 1−φp )
(1+ φp )2
3
= (1−0.032 )
( 1+0.032 )2
3
× Daq=0.62 ×2.10 ×10−9=1.54 × 10−9 m2 s−1
Daq was found as the diffusion coefficient of oxygen to water: 2.10 x 10-9 m2/s (Cussler 2008)
The following equation was then used to calculate De:
De
Deo
=1−∅ c
(Westrin and Axelsson 1991)
∅ c, cell volume fraction was assumed as 0.05
De
1.54 ×10−9 m2 s−1 =1−0.05
De=1.5 ×10−9
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
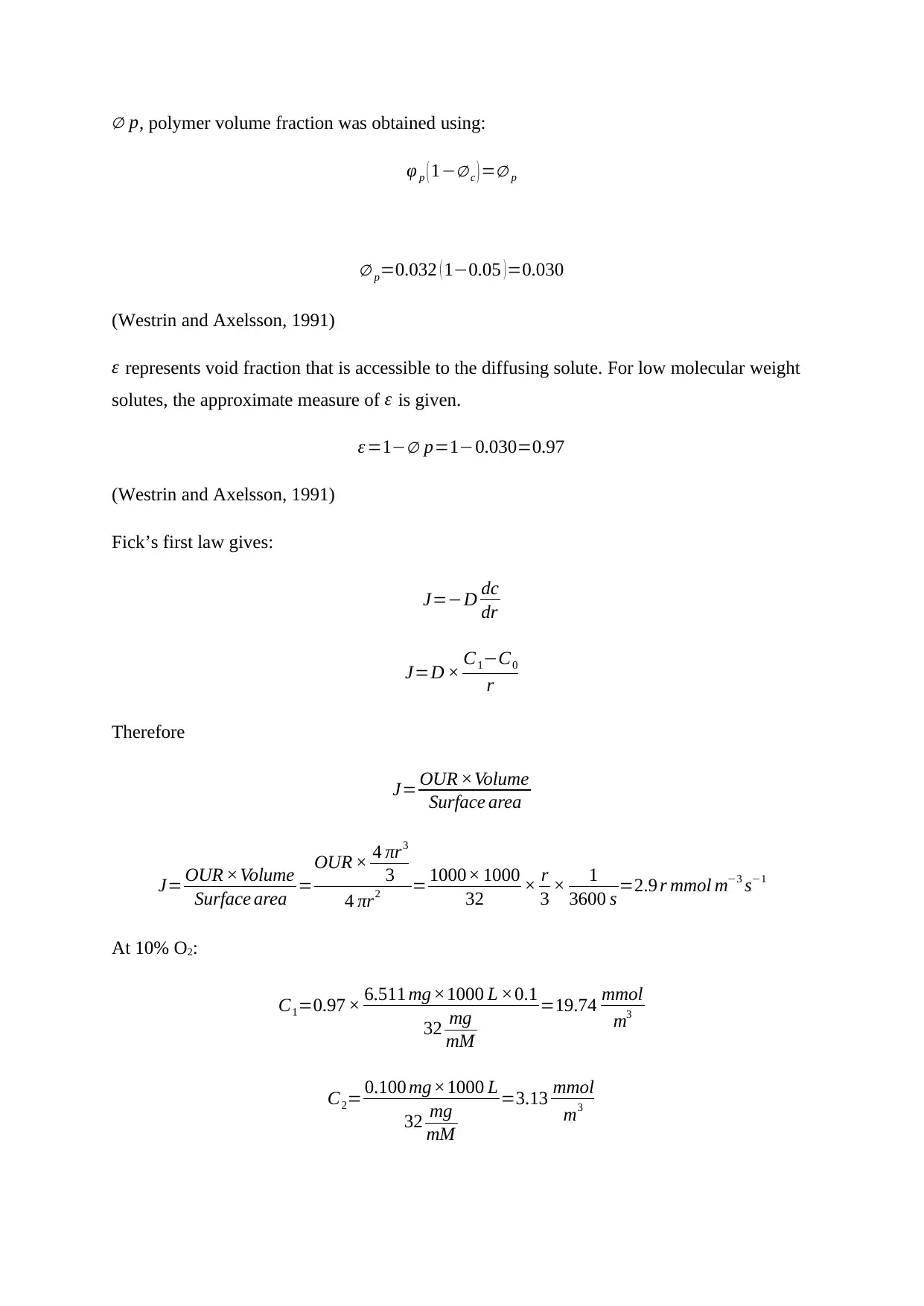
∅ p, polymer volume fraction was obtained using:
φ p ( 1−∅ c ) =∅ p
∅ p=0.032 ( 1−0.05 )=0.030
(Westrin and Axelsson, 1991)
ε represents void fraction that is accessible to the diffusing solute. For low molecular weight
solutes, the approximate measure of ε is given.
ε =1−∅ p=1−0.030=0.97
(Westrin and Axelsson, 1991)
Fick’s first law gives:
J=−D dc
dr
J=D × C1−C0
r
Therefore
J= OUR ×Volume
Surface area
J= OUR ×Volume
Surface area =
OUR × 4 πr3
3
4 πr2 = 1000× 1000
32 × r
3 × 1
3600 s =2.9 r mmol m−3 s−1
At 10% O2:
C1=0.97 × 6.511 mg ×1000 L ×0.1
32 mg
mM
=19.74 mmol
m3
C2= 0.100 mg×1000 L
32 mg
mM
=3.13 mmol
m3
φ p ( 1−∅ c ) =∅ p
∅ p=0.032 ( 1−0.05 )=0.030
(Westrin and Axelsson, 1991)
ε represents void fraction that is accessible to the diffusing solute. For low molecular weight
solutes, the approximate measure of ε is given.
ε =1−∅ p=1−0.030=0.97
(Westrin and Axelsson, 1991)
Fick’s first law gives:
J=−D dc
dr
J=D × C1−C0
r
Therefore
J= OUR ×Volume
Surface area
J= OUR ×Volume
Surface area =
OUR × 4 πr3
3
4 πr2 = 1000× 1000
32 × r
3 × 1
3600 s =2.9 r mmol m−3 s−1
At 10% O2:
C1=0.97 × 6.511 mg ×1000 L ×0.1
32 mg
mM
=19.74 mmol
m3
C2= 0.100 mg×1000 L
32 mg
mM
=3.13 mmol
m3
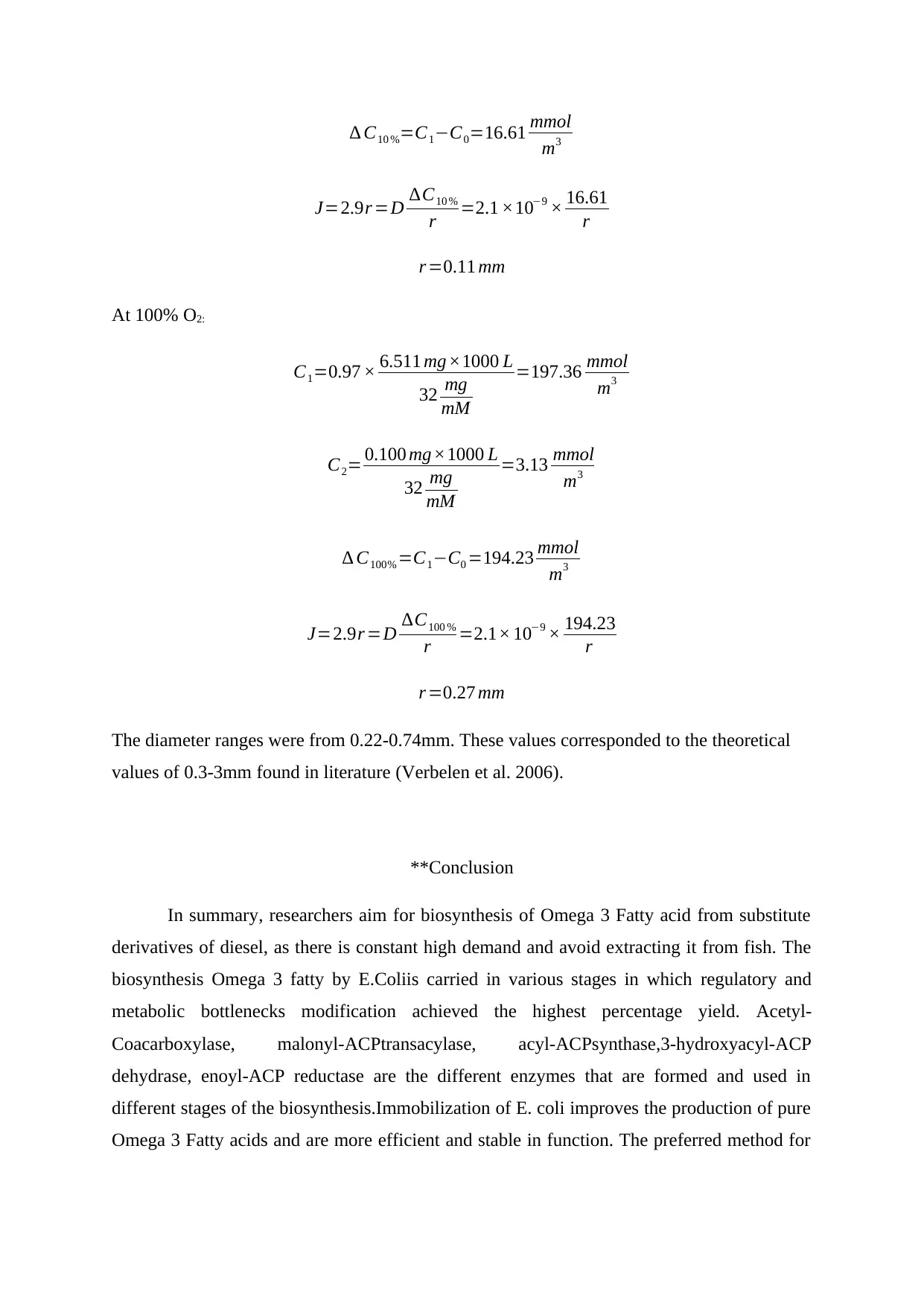
∆ C10 %=C1−C0=16.61 mmol
m3
J=2.9r =D ∆C10 %
r =2.1 ×10−9 × 16.61
r
r =0.11 mm
At 100% O2:
C1=0.97 × 6.511 mg×1000 L
32 mg
mM
=197.36 mmol
m3
C2= 0.100 mg×1000 L
32 mg
mM
=3.13 mmol
m3
∆ C100% =C1−C0 =194.23 mmol
m3
J=2.9r =D ∆C100 %
r =2.1× 10−9 × 194.23
r
r =0.27 mm
The diameter ranges were from 0.22-0.74mm. These values corresponded to the theoretical
values of 0.3-3mm found in literature (Verbelen et al. 2006).
**Conclusion
In summary, researchers aim for biosynthesis of Omega 3 Fatty acid from substitute
derivatives of diesel, as there is constant high demand and avoid extracting it from fish. The
biosynthesis Omega 3 fatty by E.Coliis carried in various stages in which regulatory and
metabolic bottlenecks modification achieved the highest percentage yield. Acetyl-
Coacarboxylase, malonyl-ACPtransacylase, acyl-ACPsynthase,3-hydroxyacyl-ACP
dehydrase, enoyl-ACP reductase are the different enzymes that are formed and used in
different stages of the biosynthesis.Immobilization of E. coli improves the production of pure
Omega 3 Fatty acids and are more efficient and stable in function. The preferred method for
m3
J=2.9r =D ∆C10 %
r =2.1 ×10−9 × 16.61
r
r =0.11 mm
At 100% O2:
C1=0.97 × 6.511 mg×1000 L
32 mg
mM
=197.36 mmol
m3
C2= 0.100 mg×1000 L
32 mg
mM
=3.13 mmol
m3
∆ C100% =C1−C0 =194.23 mmol
m3
J=2.9r =D ∆C100 %
r =2.1× 10−9 × 194.23
r
r =0.27 mm
The diameter ranges were from 0.22-0.74mm. These values corresponded to the theoretical
values of 0.3-3mm found in literature (Verbelen et al. 2006).
**Conclusion
In summary, researchers aim for biosynthesis of Omega 3 Fatty acid from substitute
derivatives of diesel, as there is constant high demand and avoid extracting it from fish. The
biosynthesis Omega 3 fatty by E.Coliis carried in various stages in which regulatory and
metabolic bottlenecks modification achieved the highest percentage yield. Acetyl-
Coacarboxylase, malonyl-ACPtransacylase, acyl-ACPsynthase,3-hydroxyacyl-ACP
dehydrase, enoyl-ACP reductase are the different enzymes that are formed and used in
different stages of the biosynthesis.Immobilization of E. coli improves the production of pure
Omega 3 Fatty acids and are more efficient and stable in function. The preferred method for
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 16
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.




