MAE 204 Thermodynamics Homework #8: Problem Solutions and Analysis
VerifiedAdded on 2022/10/04
|3
|456
|14
Homework Assignment
AI Summary
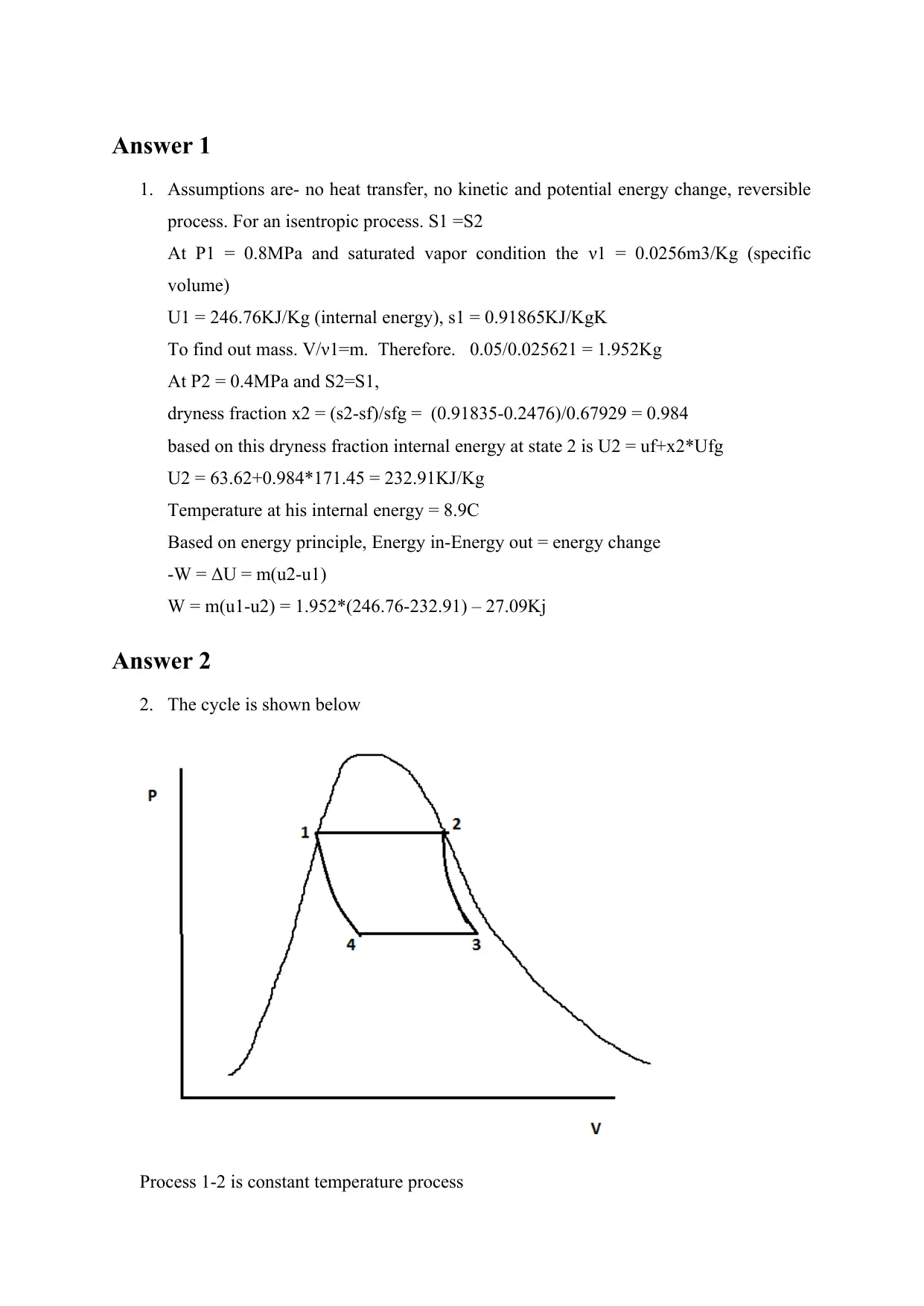
This document provides detailed solutions to MAE 204 Homework #8, focusing on thermodynamics principles. The solutions cover three problems: the first involves an insulated piston-cylinder device containing R-134a undergoing an isentropic expansion, requiring the determination of the final temperature and work done. The second problem analyzes a Carnot heat-engine cycle using water as the working fluid, calculating heat added, work output, and heat rejection. The third problem explores the maximum thermal efficiency of a Carnot cycle, the coefficient of performance (COP) of a heat pump, and the work output of a reversible heat engine, and COP of a refrigerator. The solutions utilize thermodynamic properties, energy principles, and cycle analysis to arrive at the answers, with clear steps and explanations for each problem.
1 out of 3




![[object Object]](/_next/static/media/star-bottom.7253800d.svg)