Assignment On No Kinetic And Potential Energy Change
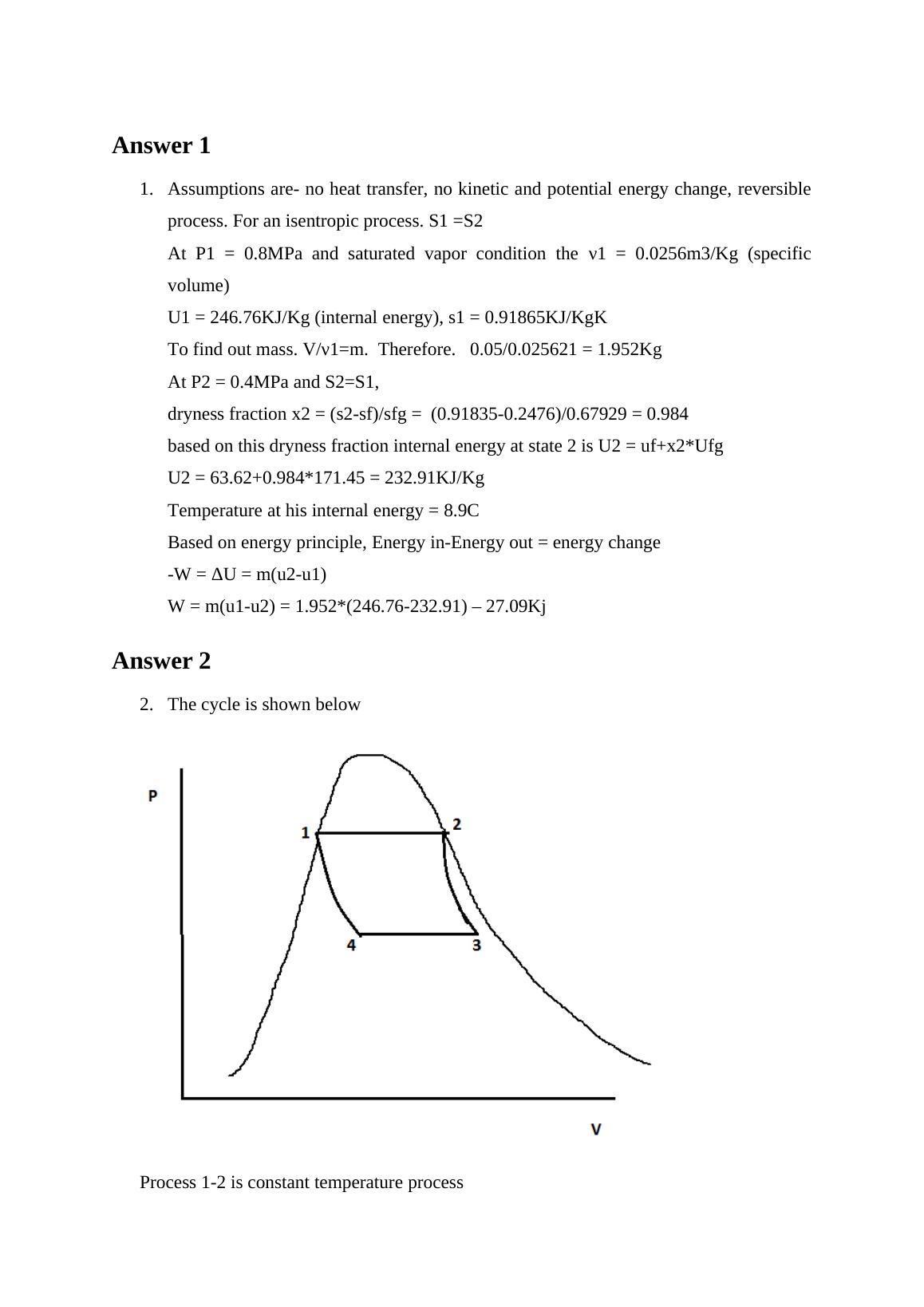
Analyze two problems related to thermodynamics and determine final temperature and work done in the first problem, and draw a Carnot heat-engine cycle using water as the working fluid in the second problem.
3 Pages456 Words14 Views
Added on 2022-10-04
Assignment On No Kinetic And Potential Energy Change
Analyze two problems related to thermodynamics and determine final temperature and work done in the first problem, and draw a Carnot heat-engine cycle using water as the working fluid in the second problem.
Added on 2022-10-04
ShareRelated Documents
End of preview
Want to access all the pages? Upload your documents or become a member.
Engineering Thermodynamics Assignment
|7
|754
|129