Solutions to Chemistry Homework Assignment on Coordination Chemistry
VerifiedAdded on 2022/09/15
|5
|834
|17
Homework Assignment
AI Summary
This document provides detailed solutions to a chemistry homework assignment. The solutions cover various topics including the absence of isomerism in a compound, the lack of color in zinc compounds explained by the absence of electron excitation due to fully occupied d-orbitals. The assignment i...
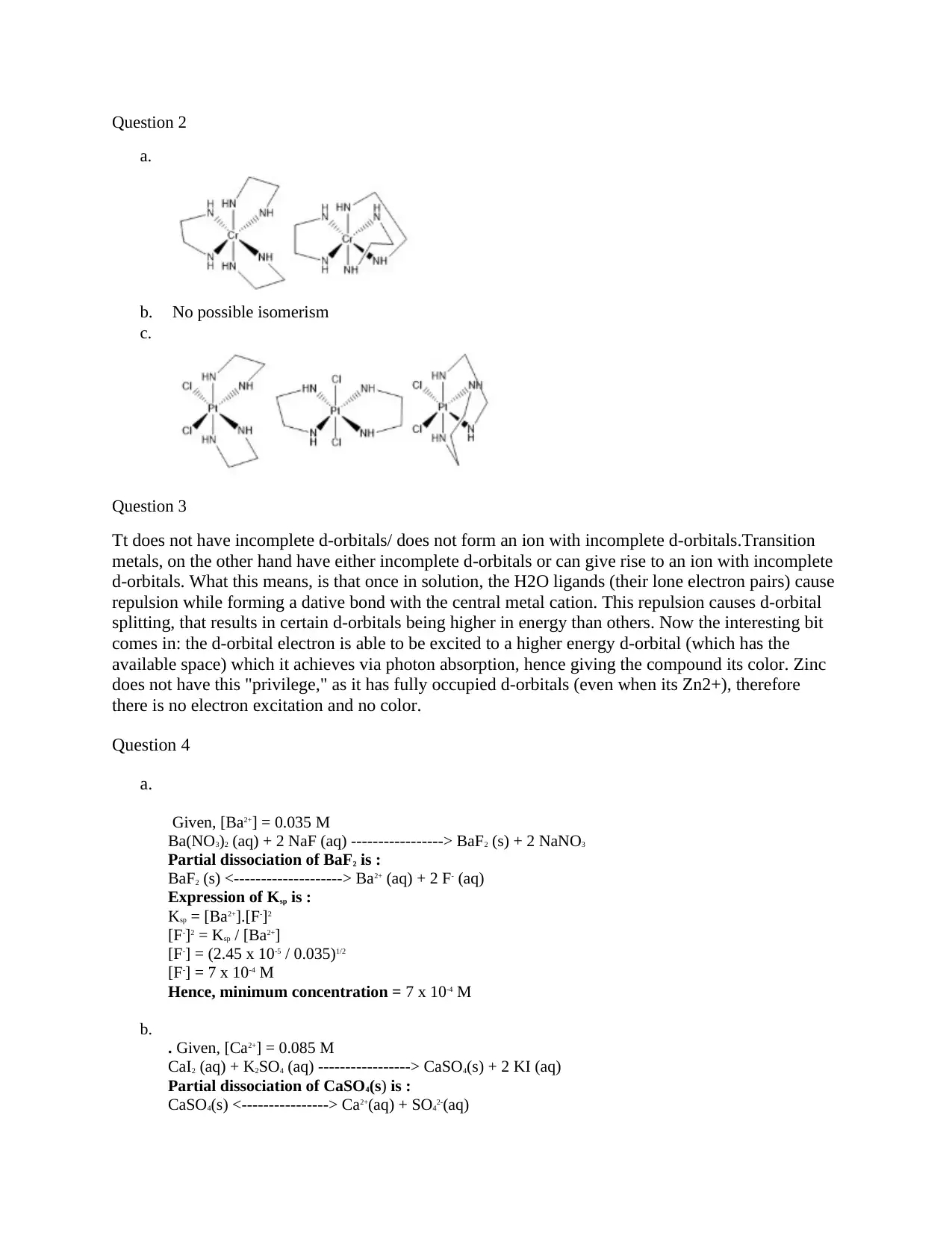
Question 2
a.
b. No possible isomerism
c.
Question 3
Tt does not have incomplete d-orbitals/ does not form an ion with incomplete d-orbitals.Transition
metals, on the other hand have either incomplete d-orbitals or can give rise to an ion with incomplete
d-orbitals. What this means, is that once in solution, the H2O ligands (their lone electron pairs) cause
repulsion while forming a dative bond with the central metal cation. This repulsion causes d-orbital
splitting, that results in certain d-orbitals being higher in energy than others. Now the interesting bit
comes in: the d-orbital electron is able to be excited to a higher energy d-orbital (which has the
available space) which it achieves via photon absorption, hence giving the compound its color. Zinc
does not have this "privilege," as it has fully occupied d-orbitals (even when its Zn2+), therefore
there is no electron excitation and no color.
Question 4
a.
Given, [Ba2+] = 0.035 M
Ba(NO3)2 (aq) + 2 NaF (aq) -----------------> BaF2 (s) + 2 NaNO3
Partial dissociation of BaF2 is :
BaF2 (s) <--------------------> Ba2+ (aq) + 2 F- (aq)
Expression of Ksp is :
Ksp = [Ba2+].[F-]2
[F-]2 = Ksp / [Ba2+]
[F-] = (2.45 x 10-5 / 0.035)1/2
[F-] = 7 x 10-4 M
Hence, minimum concentration = 7 x 10-4 M
b.
. Given, [Ca2+] = 0.085 M
CaI2 (aq) + K2SO4 (aq) -----------------> CaSO4(s) + 2 KI (aq)
Partial dissociation of CaSO4(s) is :
CaSO4(s) <----------------> Ca2+(aq) + SO42-(aq)
a.
b. No possible isomerism
c.
Question 3
Tt does not have incomplete d-orbitals/ does not form an ion with incomplete d-orbitals.Transition
metals, on the other hand have either incomplete d-orbitals or can give rise to an ion with incomplete
d-orbitals. What this means, is that once in solution, the H2O ligands (their lone electron pairs) cause
repulsion while forming a dative bond with the central metal cation. This repulsion causes d-orbital
splitting, that results in certain d-orbitals being higher in energy than others. Now the interesting bit
comes in: the d-orbital electron is able to be excited to a higher energy d-orbital (which has the
available space) which it achieves via photon absorption, hence giving the compound its color. Zinc
does not have this "privilege," as it has fully occupied d-orbitals (even when its Zn2+), therefore
there is no electron excitation and no color.
Question 4
a.
Given, [Ba2+] = 0.035 M
Ba(NO3)2 (aq) + 2 NaF (aq) -----------------> BaF2 (s) + 2 NaNO3
Partial dissociation of BaF2 is :
BaF2 (s) <--------------------> Ba2+ (aq) + 2 F- (aq)
Expression of Ksp is :
Ksp = [Ba2+].[F-]2
[F-]2 = Ksp / [Ba2+]
[F-] = (2.45 x 10-5 / 0.035)1/2
[F-] = 7 x 10-4 M
Hence, minimum concentration = 7 x 10-4 M
b.
. Given, [Ca2+] = 0.085 M
CaI2 (aq) + K2SO4 (aq) -----------------> CaSO4(s) + 2 KI (aq)
Partial dissociation of CaSO4(s) is :
CaSO4(s) <----------------> Ca2+(aq) + SO42-(aq)
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Expression of Ksp is :
Ksp = [Ca2+].[SO42-]
[SO42-] = Ksp / [Ca2+]
[SO42-] = (7.10 x 10-5 / 8.5 x 10-2)
[SO42-] = 8.35 x 10-4 M
Hence, minimum concentration = 8.35 x 10-4 M
c. Because of equal valences,
minimum concentration= 1. 77 ×10−10
0 . 0018 =9. 833 ×10−8 M
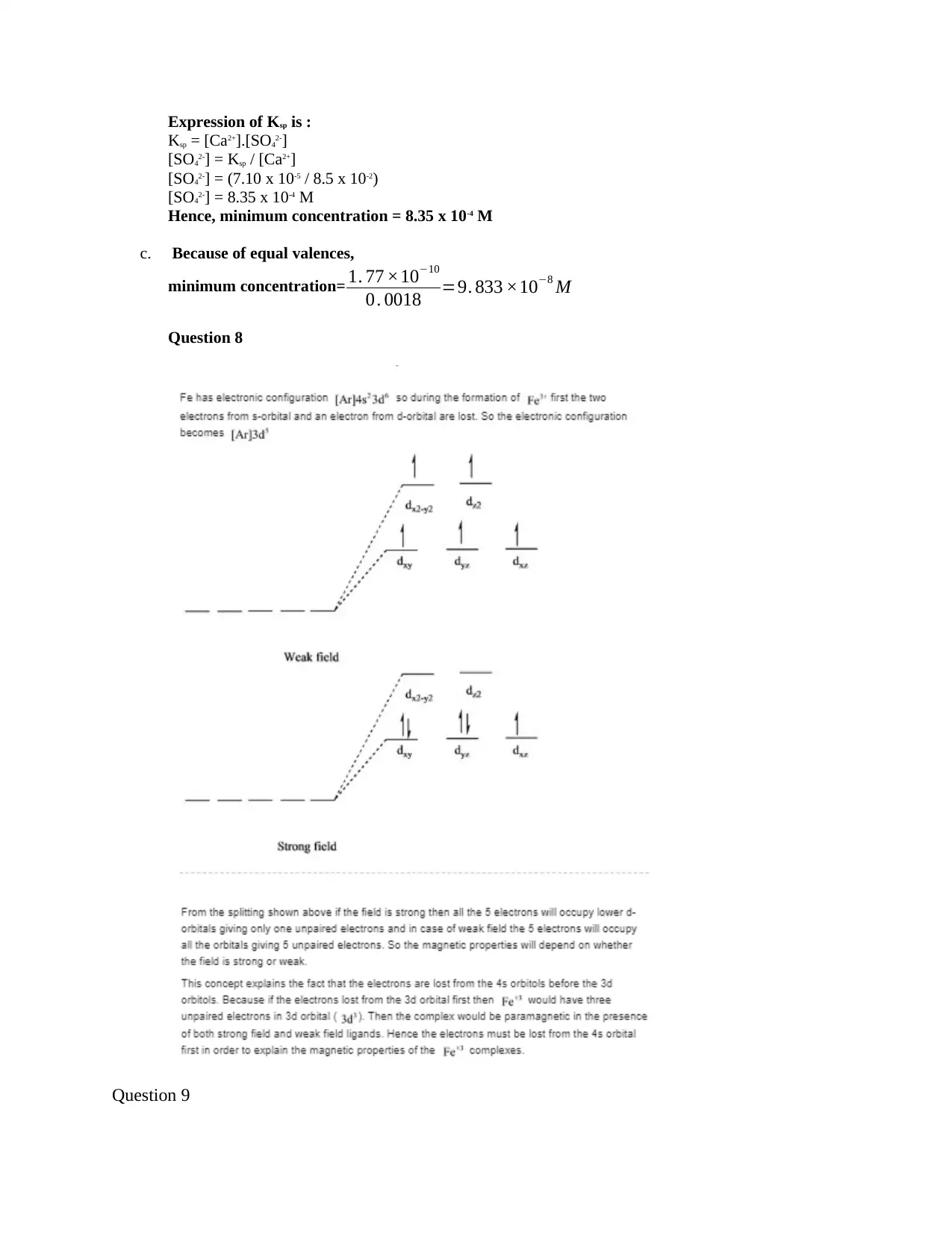
Question 8
Question 9
Ksp = [Ca2+].[SO42-]
[SO42-] = Ksp / [Ca2+]
[SO42-] = (7.10 x 10-5 / 8.5 x 10-2)
[SO42-] = 8.35 x 10-4 M
Hence, minimum concentration = 8.35 x 10-4 M
c. Because of equal valences,
minimum concentration= 1. 77 ×10−10
0 . 0018 =9. 833 ×10−8 M
Question 8
Question 9

Blue color (A) has strong ligand because different ligands are associated with either high or
low spin—a "strong field" ligand results in a large ∆o and a low spin configuration, while
a "weak field" ligand results in a small ∆o and a high spin configuration since blue light
has a much higher frequency and more energy than purple light. The diagram for the 2
cases are shown below
Purple
blue
Question 10
HNO3 + NH3 → NH4NO
Kw =¿
¿
pH=-log5.5556−10=9.26
From the problem statement,
Question 11
Expected to have high spin.
The Fe2+ ion in [Fe(H2O)6]2+ has outer electronic configuration of 3d6 . The complex is a
high spin complex. It contains 4 unpaired electrons
low spin—a "strong field" ligand results in a large ∆o and a low spin configuration, while
a "weak field" ligand results in a small ∆o and a high spin configuration since blue light
has a much higher frequency and more energy than purple light. The diagram for the 2
cases are shown below
Purple
blue
Question 10
HNO3 + NH3 → NH4NO
Kw =¿
¿
pH=-log5.5556−10=9.26
From the problem statement,
Question 11
Expected to have high spin.
The Fe2+ ion in [Fe(H2O)6]2+ has outer electronic configuration of 3d6 . The complex is a
high spin complex. It contains 4 unpaired electrons
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Question 12
a. The buffer is basic (Acid/base=0.5)
b. Greater than 9.23
c. pH = p K A + log Cbase
Cacid =10+ log ( 0.5 )=9.699
Question 15
For dimethylamine:
Acidity (pKa) 10.64
Basicity (pKb) 3.36
At this point have a short think: If the molar ratio of dimethylamine : dimethylammonium chloride was 1 :1 you
would be at the half equivalence point , and pH would equal pKa . The pH would be 10.64.
But you want a buffer with pH = 10.43. Therefore there must be a molar excess of the dimethylammonium chloride.
Use the H-H equation:
pH = pKa + log ( [[base]/[salt]]
10.43 = 10.64 + log ([base[/[salt])
log ([base[/]salt]) = 10-43 - 10.64
log ([base]/[salt] = -0.21
[base]/[salt] = 10^-0.21
[base]/[salt] = 0.62
The buffer must be prepared by mixing 0.62mol dimethylamine and 1.0mol dimethylammonium chloride.
Mass ratio as required:
Molar mass dimethylammonium chloride 81.55g/mol
Molar mass dimethylamine = 45.08g/mol - 0.62 mol = 27.95g
Mass ratio of dimethylammonium chloride : dimethylamine = 81.55 : 27.95
OR Ratio = 81.55/27.95 = 2.92:1.0
Question 16
a. Red colour (intensely coloured)
b.
The given absorbance spectrum shows the complex absorbs 480 nm( not accurate)
480 nm = 10000000 / 480 cm-1
480 nm = 20,833.33 cm-1
Since 1 kJ mol-1 = 83.7 cm-1
a. The buffer is basic (Acid/base=0.5)
b. Greater than 9.23
c. pH = p K A + log Cbase
Cacid =10+ log ( 0.5 )=9.699
Question 15
For dimethylamine:
Acidity (pKa) 10.64
Basicity (pKb) 3.36
At this point have a short think: If the molar ratio of dimethylamine : dimethylammonium chloride was 1 :1 you
would be at the half equivalence point , and pH would equal pKa . The pH would be 10.64.
But you want a buffer with pH = 10.43. Therefore there must be a molar excess of the dimethylammonium chloride.
Use the H-H equation:
pH = pKa + log ( [[base]/[salt]]
10.43 = 10.64 + log ([base[/[salt])
log ([base[/]salt]) = 10-43 - 10.64
log ([base]/[salt] = -0.21
[base]/[salt] = 10^-0.21
[base]/[salt] = 0.62
The buffer must be prepared by mixing 0.62mol dimethylamine and 1.0mol dimethylammonium chloride.
Mass ratio as required:
Molar mass dimethylammonium chloride 81.55g/mol
Molar mass dimethylamine = 45.08g/mol - 0.62 mol = 27.95g
Mass ratio of dimethylammonium chloride : dimethylamine = 81.55 : 27.95
OR Ratio = 81.55/27.95 = 2.92:1.0
Question 16
a. Red colour (intensely coloured)
b.
The given absorbance spectrum shows the complex absorbs 480 nm( not accurate)
480 nm = 10000000 / 480 cm-1
480 nm = 20,833.33 cm-1
Since 1 kJ mol-1 = 83.7 cm-1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Thus, crystal field splitting energy = 20833.33 / 83.7
= 248.9047 kJ mol-1
= 248 kJ mol-1
c.
= 248.9047 kJ mol-1
= 248 kJ mol-1
c.
1 out of 5
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.