No Possible Isomerism l Question Answers
Added on 2022-09-15
5 Pages834 Words17 Views
Question 2
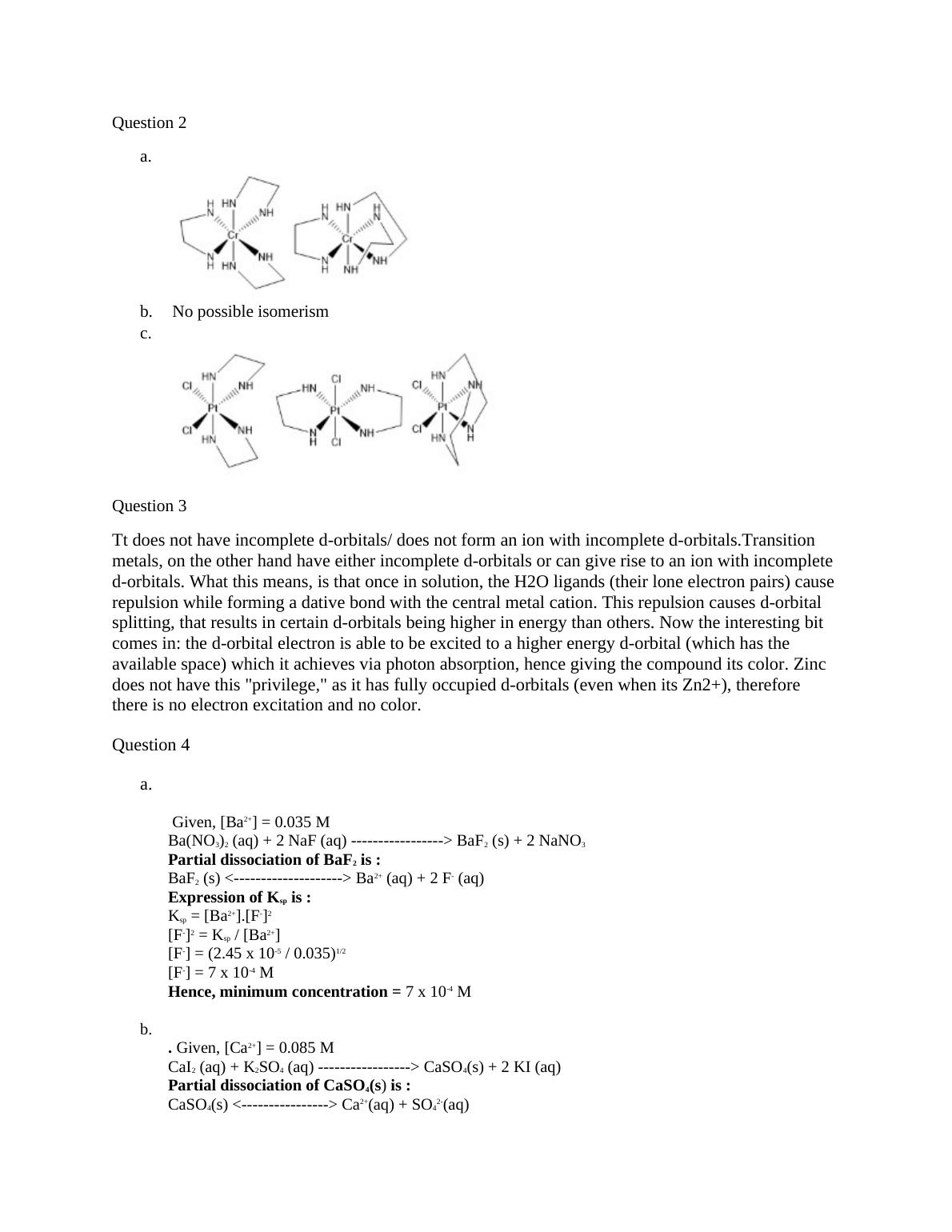
a.
b. No possible isomerism
c.
Question 3
Tt does not have incomplete d-orbitals/ does not form an ion with incomplete d-orbitals.Transition
metals, on the other hand have either incomplete d-orbitals or can give rise to an ion with incomplete
d-orbitals. What this means, is that once in solution, the H2O ligands (their lone electron pairs) cause
repulsion while forming a dative bond with the central metal cation. This repulsion causes d-orbital
splitting, that results in certain d-orbitals being higher in energy than others. Now the interesting bit
comes in: the d-orbital electron is able to be excited to a higher energy d-orbital (which has the
available space) which it achieves via photon absorption, hence giving the compound its color. Zinc
does not have this "privilege," as it has fully occupied d-orbitals (even when its Zn2+), therefore
there is no electron excitation and no color.
Question 4
a.
Given, [Ba2+] = 0.035 M
Ba(NO3)2 (aq) + 2 NaF (aq) -----------------> BaF2 (s) + 2 NaNO3
Partial dissociation of BaF2 is :
BaF2 (s) <--------------------> Ba2+ (aq) + 2 F- (aq)
Expression of Ksp is :
Ksp = [Ba2+].[F-]2
[F-]2 = Ksp / [Ba2+]
[F-] = (2.45 x 10-5 / 0.035)1/2
[F-] = 7 x 10-4 M
Hence, minimum concentration = 7 x 10-4 M
b.
. Given, [Ca2+] = 0.085 M
CaI2 (aq) + K2SO4 (aq) -----------------> CaSO4(s) + 2 KI (aq)
Partial dissociation of CaSO4(s) is :
CaSO4(s) <----------------> Ca2+(aq) + SO42-(aq)
a.
b. No possible isomerism
c.
Question 3
Tt does not have incomplete d-orbitals/ does not form an ion with incomplete d-orbitals.Transition
metals, on the other hand have either incomplete d-orbitals or can give rise to an ion with incomplete
d-orbitals. What this means, is that once in solution, the H2O ligands (their lone electron pairs) cause
repulsion while forming a dative bond with the central metal cation. This repulsion causes d-orbital
splitting, that results in certain d-orbitals being higher in energy than others. Now the interesting bit
comes in: the d-orbital electron is able to be excited to a higher energy d-orbital (which has the
available space) which it achieves via photon absorption, hence giving the compound its color. Zinc
does not have this "privilege," as it has fully occupied d-orbitals (even when its Zn2+), therefore
there is no electron excitation and no color.
Question 4
a.
Given, [Ba2+] = 0.035 M
Ba(NO3)2 (aq) + 2 NaF (aq) -----------------> BaF2 (s) + 2 NaNO3
Partial dissociation of BaF2 is :
BaF2 (s) <--------------------> Ba2+ (aq) + 2 F- (aq)
Expression of Ksp is :
Ksp = [Ba2+].[F-]2
[F-]2 = Ksp / [Ba2+]
[F-] = (2.45 x 10-5 / 0.035)1/2
[F-] = 7 x 10-4 M
Hence, minimum concentration = 7 x 10-4 M
b.
. Given, [Ca2+] = 0.085 M
CaI2 (aq) + K2SO4 (aq) -----------------> CaSO4(s) + 2 KI (aq)
Partial dissociation of CaSO4(s) is :
CaSO4(s) <----------------> Ca2+(aq) + SO42-(aq)
Expression of Ksp is :
Ksp = [Ca2+].[SO42-]
[SO42-] = Ksp / [Ca2+]
[SO42-] = (7.10 x 10-5 / 8.5 x 10-2)
[SO42-] = 8.35 x 10-4 M
Hence, minimum concentration = 8.35 x 10-4 M
c. Because of equal valences,
minimum concentration= 1. 77 ×10−10
0 . 0018 =9. 833 ×10−8 M
Question 8
Question 9
Ksp = [Ca2+].[SO42-]
[SO42-] = Ksp / [Ca2+]
[SO42-] = (7.10 x 10-5 / 8.5 x 10-2)
[SO42-] = 8.35 x 10-4 M
Hence, minimum concentration = 8.35 x 10-4 M
c. Because of equal valences,
minimum concentration= 1. 77 ×10−10
0 . 0018 =9. 833 ×10−8 M
Question 8
Question 9

End of preview
Want to access all the pages? Upload your documents or become a member.
