NURS90076: Applied Pathophysiology - Diabetic Ketoacidosis Case Study
VerifiedAdded on 2020/03/04
|11
|2709
|310
Case Study
AI Summary
This case study examines the presentation of a 12-year-old girl, Carly, to the emergency department with Diabetic Ketoacidosis (DKA). The assignment details her symptoms, including polydipsia, polyuria, fever, abdominal pain, nausea, and weight loss. Upon admission, Carly presented with tachycardia, orthostatic hypotension, and altered mental status. The paper provides a concept map linking the patient's presentation to the underlying pathophysiological processes. The pathophysiology of DKA is explained, highlighting the role of insulin deficiency, hyperglycemia, ketogenesis, and metabolic acidosis. The paper rationalizes the changes in Carly's vital signs and neurological status, including the development of cerebral edema and the subsequent treatment with Mannitol. The discussion covers the impact of osmotic diuresis, electrolyte imbalances, and the systemic inflammatory response. The paper also discusses the symptoms of DKA, such as nausea, vomiting, polydipsia, polyuria, and acetone-smelling breath, and their relation to the disease process, along with a conclusion summarizing the case study.

Running Head: APPLIED PATHOPHYSIOLOGY
Applied pathophysiology
Name of the Student
Name of the University
Author Note
Applied pathophysiology
Name of the Student
Name of the University
Author Note
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

1APPLIED PATHOPHYSIOLOGY
Introduction
The paper deals with the case study of Carly, a12 year old girl who presents to the
emergency department for treatment of Diabetic ketoacidosis. She was experiencing polydipsia,
polyuria and a fever for last two weeks. The patient was suffering from abdominal pain and
nausea at the time of admission. After admission she was found tachycardic. Carly has
orthostatic hypotension. Over the next three to four hours gradual clinical and biochemical
improvement was observed. Six hours into her resuscitation and treatment, the patient was found
with noticeable neurological deterioration. Later she was administered Mannitol. With the
improvement in the GCS, she was discharged. The paper presents a concept map in response to
the case study. The concept map links the presentation and pathophysiological processes that
caused Carly’s illness. Further, the paper explains the pathophysiology of the disease in details
and rationalises the changes in the vital signs found in this patient, when present to an emergency
department.
Introduction
The paper deals with the case study of Carly, a12 year old girl who presents to the
emergency department for treatment of Diabetic ketoacidosis. She was experiencing polydipsia,
polyuria and a fever for last two weeks. The patient was suffering from abdominal pain and
nausea at the time of admission. After admission she was found tachycardic. Carly has
orthostatic hypotension. Over the next three to four hours gradual clinical and biochemical
improvement was observed. Six hours into her resuscitation and treatment, the patient was found
with noticeable neurological deterioration. Later she was administered Mannitol. With the
improvement in the GCS, she was discharged. The paper presents a concept map in response to
the case study. The concept map links the presentation and pathophysiological processes that
caused Carly’s illness. Further, the paper explains the pathophysiology of the disease in details
and rationalises the changes in the vital signs found in this patient, when present to an emergency
department.

2APPLIED PATHOPHYSIOLOGY
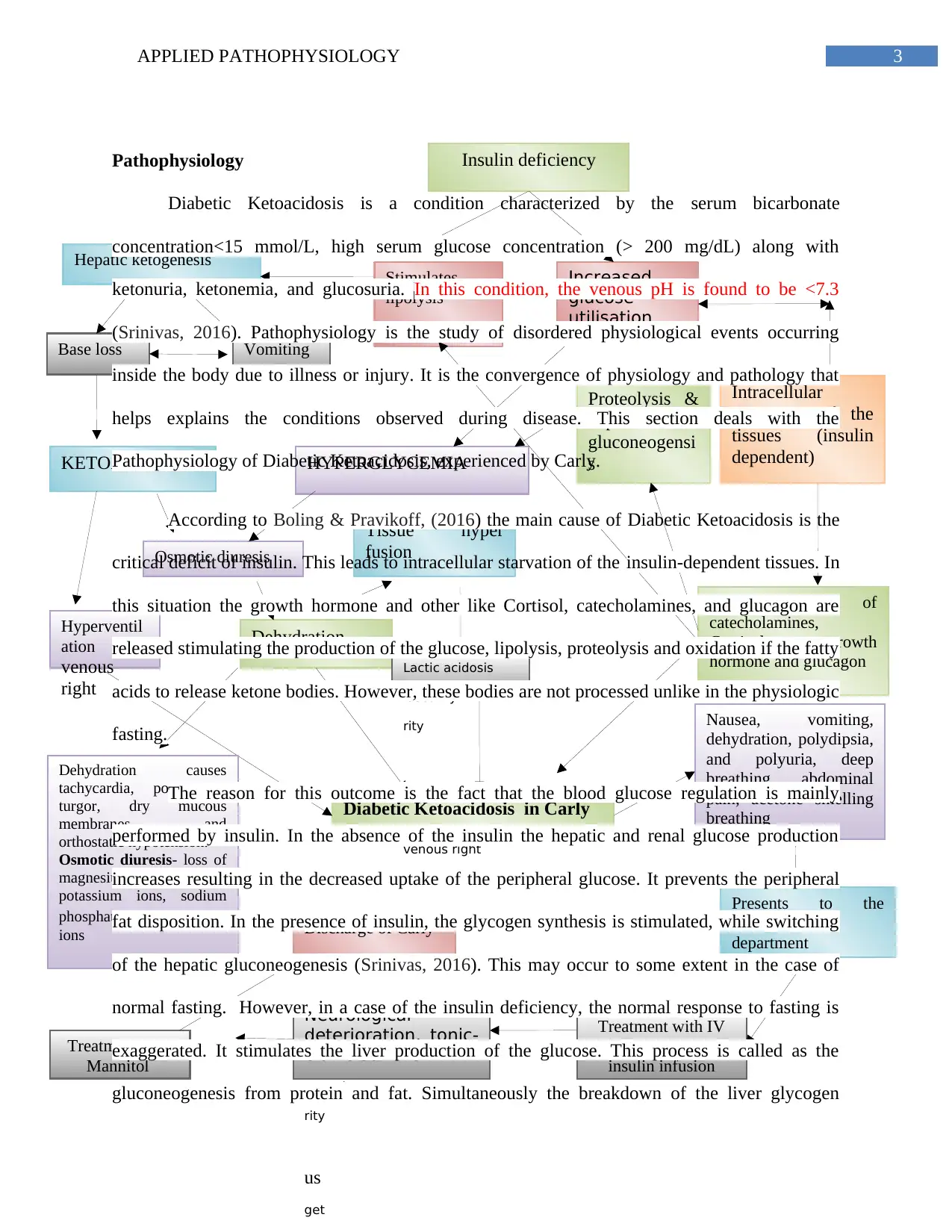
Concept map
Concept map
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
3APPLIED PATHOPHYSIOLOGY
Intracellular
starvation of the
tissues (insulin
dependent)
Hyperventil
ation
venous
right
HYPERGLYCEMIAKETOACIDOSIS
Hepatic ketogenesis
Dehydration
Release of
catecholamines,
Cortisol, growth
hormone and glucagon
Base loss Vomiting
Insulin deficiency
Stimulates
lipolysis
Increased
glucose
utilisation
o
Proteolysis &
hepatic
gluconeogensi
s
Tissue hyper
fusionOsmotic diuresis
Lactic acidosis
security
rity
us
get
venous right
Diabetic Ketoacidosis in Carly
Nausea, vomiting,
dehydration, polydipsia,
and polyuria, deep
breathing, abdominal
pain, acetone smelling
breathing
Presents to the
emergency
department
Dehydration causes
tachycardia, poor skin
turgor, dry mucous
membranes, and
orthostatic hypotension.
Osmotic diuresis- loss of
magnesium ions,
potassium ions, sodium
phosphate, and phosphate
ions
Treatment with IV
fluids and an IV
insulin infusion
Neurological
deterioration, tonic-
clonic seizure
security
rity
us
get
Treatment with
Mannitol
Discharge of Carly
Pathophysiology
Diabetic Ketoacidosis is a condition characterized by the serum bicarbonate
concentration<15 mmol/L, high serum glucose concentration (> 200 mg/dL) along with
ketonuria, ketonemia, and glucosuria. In this condition, the venous pH is found to be <7.3
(Srinivas, 2016). Pathophysiology is the study of disordered physiological events occurring
inside the body due to illness or injury. It is the convergence of physiology and pathology that
helps explains the conditions observed during disease. This section deals with the
Pathophysiology of Diabetic Ketoacidosis, experienced by Carly.
According to Boling & Pravikoff, (2016) the main cause of Diabetic Ketoacidosis is the
critical deficit of insulin. This leads to intracellular starvation of the insulin-dependent tissues. In
this situation the growth hormone and other like Cortisol, catecholamines, and glucagon are
released stimulating the production of the glucose, lipolysis, proteolysis and oxidation if the fatty
acids to release ketone bodies. However, these bodies are not processed unlike in the physiologic
fasting.
The reason for this outcome is the fact that the blood glucose regulation is mainly
performed by insulin. In the absence of the insulin the hepatic and renal glucose production
increases resulting in the decreased uptake of the peripheral glucose. It prevents the peripheral
fat disposition. In the presence of insulin, the glycogen synthesis is stimulated, while switching
of the hepatic gluconeogenesis (Srinivas, 2016). This may occur to some extent in the case of
normal fasting. However, in a case of the insulin deficiency, the normal response to fasting is
exaggerated. It stimulates the liver production of the glucose. This process is called as the
gluconeogenesis from protein and fat. Simultaneously the breakdown of the liver glycogen
Intracellular
starvation of the
tissues (insulin
dependent)
Hyperventil
ation
venous
right
HYPERGLYCEMIAKETOACIDOSIS
Hepatic ketogenesis
Dehydration
Release of
catecholamines,
Cortisol, growth
hormone and glucagon
Base loss Vomiting
Insulin deficiency
Stimulates
lipolysis
Increased
glucose
utilisation
o
Proteolysis &
hepatic
gluconeogensi
s
Tissue hyper
fusionOsmotic diuresis
Lactic acidosis
security
rity
us
get
venous right
Diabetic Ketoacidosis in Carly
Nausea, vomiting,
dehydration, polydipsia,
and polyuria, deep
breathing, abdominal
pain, acetone smelling
breathing
Presents to the
emergency
department
Dehydration causes
tachycardia, poor skin
turgor, dry mucous
membranes, and
orthostatic hypotension.
Osmotic diuresis- loss of
magnesium ions,
potassium ions, sodium
phosphate, and phosphate
ions
Treatment with IV
fluids and an IV
insulin infusion
Neurological
deterioration, tonic-
clonic seizure
security
rity
us
get
Treatment with
Mannitol
Discharge of Carly
Pathophysiology
Diabetic Ketoacidosis is a condition characterized by the serum bicarbonate
concentration<15 mmol/L, high serum glucose concentration (> 200 mg/dL) along with
ketonuria, ketonemia, and glucosuria. In this condition, the venous pH is found to be <7.3
(Srinivas, 2016). Pathophysiology is the study of disordered physiological events occurring
inside the body due to illness or injury. It is the convergence of physiology and pathology that
helps explains the conditions observed during disease. This section deals with the
Pathophysiology of Diabetic Ketoacidosis, experienced by Carly.
According to Boling & Pravikoff, (2016) the main cause of Diabetic Ketoacidosis is the
critical deficit of insulin. This leads to intracellular starvation of the insulin-dependent tissues. In
this situation the growth hormone and other like Cortisol, catecholamines, and glucagon are
released stimulating the production of the glucose, lipolysis, proteolysis and oxidation if the fatty
acids to release ketone bodies. However, these bodies are not processed unlike in the physiologic
fasting.
The reason for this outcome is the fact that the blood glucose regulation is mainly
performed by insulin. In the absence of the insulin the hepatic and renal glucose production
increases resulting in the decreased uptake of the peripheral glucose. It prevents the peripheral
fat disposition. In the presence of insulin, the glycogen synthesis is stimulated, while switching
of the hepatic gluconeogenesis (Srinivas, 2016). This may occur to some extent in the case of
normal fasting. However, in a case of the insulin deficiency, the normal response to fasting is
exaggerated. It stimulates the liver production of the glucose. This process is called as the
gluconeogenesis from protein and fat. Simultaneously the breakdown of the liver glycogen
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

4APPLIED PATHOPHYSIOLOGY
occurs by glycogenolysis. Hence, the peripheral glucose uptake is inhibited. This is followed by
the various metabolic consequences (Boling & Pravikoff, 2016).
The lipolysis stimulated by the insulin deficiency leads to hepatic ketogenesis. It causes
vomiting, base loss (deficit of Na+, K+) and acidosis. Base loss accelerates ketoacidosis due to
overproduction of ketone bodies (Kamel & Halperin, 2015). The two outcomes of the
ketoacidosis are osmotic diuresis and hyperventilation, both of which cumulatively result in
dehydration followed by tissue perfusion and lactic acidosis (Shah et al., 2017). The glucose
utilisation caused by the insulin deficiency leads to hyperglycemia. On the other hand, the
release of the hormones such as glucagon, catecholamine, Cortisol, and growth hormone are
responsible for the stimulation of proteolysis and hepatic gluconeogenesis (Nyenwe & Kitabchi,
2016). Hyperglycemia is also caused by proteolysis and hepatic gluconeogenesis.
Hyperglycemia also contributes to osmotic diuresis as it impairs the uptake of the peripheral
glucose. Hyperglycemia also hampers the synthesis of any residual insulin synthesis (Wolfsdorf
et al., 2014).
This phenomenon is responsible for Carly’s serum glucose to be 48 mmol/L. She had the
elevated β-hydroxybutyrate (β-HBO) level of 1.9 mmol/L. With the treatment of the Diabetic
Ketoacidosis, CSF exhibits a change in acid-base status. The CSF pH falls even without
bicarbonate administration. The ventilatory response results in this fall. CSF pH fall is
characterised by the sudden rise in Pco2 (Srinivas, 2016). This was responsible for the arterial
blood gas pH of 7.06 and PaCO2 of 10 mmHg.
The common symptoms of Diabetic ketoacidosis are nausea, vomiting, polydipsia, and
polyurea (Boling & Pravikoff, 2016). Polydipsia is the condition characterised by the excessive
occurs by glycogenolysis. Hence, the peripheral glucose uptake is inhibited. This is followed by
the various metabolic consequences (Boling & Pravikoff, 2016).
The lipolysis stimulated by the insulin deficiency leads to hepatic ketogenesis. It causes
vomiting, base loss (deficit of Na+, K+) and acidosis. Base loss accelerates ketoacidosis due to
overproduction of ketone bodies (Kamel & Halperin, 2015). The two outcomes of the
ketoacidosis are osmotic diuresis and hyperventilation, both of which cumulatively result in
dehydration followed by tissue perfusion and lactic acidosis (Shah et al., 2017). The glucose
utilisation caused by the insulin deficiency leads to hyperglycemia. On the other hand, the
release of the hormones such as glucagon, catecholamine, Cortisol, and growth hormone are
responsible for the stimulation of proteolysis and hepatic gluconeogenesis (Nyenwe & Kitabchi,
2016). Hyperglycemia is also caused by proteolysis and hepatic gluconeogenesis.
Hyperglycemia also contributes to osmotic diuresis as it impairs the uptake of the peripheral
glucose. Hyperglycemia also hampers the synthesis of any residual insulin synthesis (Wolfsdorf
et al., 2014).
This phenomenon is responsible for Carly’s serum glucose to be 48 mmol/L. She had the
elevated β-hydroxybutyrate (β-HBO) level of 1.9 mmol/L. With the treatment of the Diabetic
Ketoacidosis, CSF exhibits a change in acid-base status. The CSF pH falls even without
bicarbonate administration. The ventilatory response results in this fall. CSF pH fall is
characterised by the sudden rise in Pco2 (Srinivas, 2016). This was responsible for the arterial
blood gas pH of 7.06 and PaCO2 of 10 mmHg.
The common symptoms of Diabetic ketoacidosis are nausea, vomiting, polydipsia, and
polyurea (Boling & Pravikoff, 2016). Polydipsia is the condition characterised by the excessive

5APPLIED PATHOPHYSIOLOGY
thirst which occurs together with polyurea. Dehydration is the main cause of this condition.
Hyperventilation and vomiting are other contributing factors. Ketoacidosis is responsible for
hyperventilation and eventually polydipsia in Carly. Polyurea is also caused by decreased kidney
perfusion, which stimulates rennin and Angiotensin, which signals brain for excess thirst (Kamel
& Halperin, 2015). Consequently, the blood glucose level increases above the threshold of the
renal glucose reabsorption, resulting in the osmotic diuresis. A significant amount of water is lost
by osmotic diuresis. It results in significant loss of magnesium ions, potassium ions, sodium
phosphate, and phosphate ions. However, in patients with high osmotic diuresis with poor fluid
compensation serum sodium can be increased (Boling& Pravikoff, 2016). This explains Carly’s
serum Na+ measured at 128 mmol/L. Her serum K+ measured at 6.3 m. High level of K+ may
indicate reinforcement of potassium level by protein catabolism due to insulin depletion.
According to Wolfsdorf et al. (2014), in many cases patients with DKA may present initially
with low, normal, or even high serum potassium or sodium levels. Nevertheless, a normal serum
potassium level in DKA indicates a large body potassium deficit.
This condition in Carly has resulted due to insulin deficiency. After the two weeks of this
persistent condition, she was admitted to the emergency department. In the last hours of
admission, the patient complained of vomiting, abdominal pain and nausea (Boling& Pravikoff,
2016). The vomiting can be explained by the lipolysis and hepatic ketogenesis. Due to the base
loss, the patient was experiencing loss of weight in last few months (Shah et al., 2017). Diabetic
ketoacidosis is associated with deep and laboured breathing that explains Carly’s deep sighing
respiration. It is evident of hyperventilation and results in the increased depth of respiration. It
reduces the carbon dioxide in the blood. With the metabolic consequences of the acidosis,
breathing became deep and laboured (Nyenwe & Kitabchi, 2016).
thirst which occurs together with polyurea. Dehydration is the main cause of this condition.
Hyperventilation and vomiting are other contributing factors. Ketoacidosis is responsible for
hyperventilation and eventually polydipsia in Carly. Polyurea is also caused by decreased kidney
perfusion, which stimulates rennin and Angiotensin, which signals brain for excess thirst (Kamel
& Halperin, 2015). Consequently, the blood glucose level increases above the threshold of the
renal glucose reabsorption, resulting in the osmotic diuresis. A significant amount of water is lost
by osmotic diuresis. It results in significant loss of magnesium ions, potassium ions, sodium
phosphate, and phosphate ions. However, in patients with high osmotic diuresis with poor fluid
compensation serum sodium can be increased (Boling& Pravikoff, 2016). This explains Carly’s
serum Na+ measured at 128 mmol/L. Her serum K+ measured at 6.3 m. High level of K+ may
indicate reinforcement of potassium level by protein catabolism due to insulin depletion.
According to Wolfsdorf et al. (2014), in many cases patients with DKA may present initially
with low, normal, or even high serum potassium or sodium levels. Nevertheless, a normal serum
potassium level in DKA indicates a large body potassium deficit.
This condition in Carly has resulted due to insulin deficiency. After the two weeks of this
persistent condition, she was admitted to the emergency department. In the last hours of
admission, the patient complained of vomiting, abdominal pain and nausea (Boling& Pravikoff,
2016). The vomiting can be explained by the lipolysis and hepatic ketogenesis. Due to the base
loss, the patient was experiencing loss of weight in last few months (Shah et al., 2017). Diabetic
ketoacidosis is associated with deep and laboured breathing that explains Carly’s deep sighing
respiration. It is evident of hyperventilation and results in the increased depth of respiration. It
reduces the carbon dioxide in the blood. With the metabolic consequences of the acidosis,
breathing became deep and laboured (Nyenwe & Kitabchi, 2016).
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

6APPLIED PATHOPHYSIOLOGY
The acetone smelling breath of Carly can be explained by ketoacidosis. It is the condition
characterized by the breath smelling line acetone with the increase in acetone level in the body to
an unsafe amount. With ketoacidosis the circulating level of the fatty acid increases (Boling&
Pravikoff, 2016). The inhibition of the lipolytic action of the growth hormone and Cortisol by
insulin is prevented by its deficiency. As these fatty acids accumulate, beta-oxidation is
accelerated producing excess ketone bodies. It includes beta hydroxybutyrate and acetoacetate
that forms acetone. This condition is also contributed by the lactic acidosis and poor tissue
perfusion. This is the reason behind Carly’s urinalysis being positive for glucose and ketones
(Boling & Pravikoff, 2016).
The symptoms of orthostatic hypotension and the patient being tachycardic can be
explained by dehydration. Tachycardia is caused by dehydration. It leads to a dry mucous
membrane and poor skin turgor and orthostatic hypotension (Singh et al., 2016). This was the
reason that Carly’s skin was pale and dry. If the onset of this condition is neglected then it many
results in severe acidosis and dehydration. This was the case of Carly as she was admitted two
weeks after suffering from polydipsia, polyuria and a fever (Singh et al., 2016). Carly’s skin was
hot due to fever. Among other symptoms, Fever during Diabetic ketoacidosis may be an outcome
of intercurrent infection (Boling & Pravikoff, 2016). However, CRP, which is elevated as is her
WCC which also shows immature band cells, may occur even in the absence of infection. They
may be elevated due to systemic inflammatory response syndrome (SIRS). In some patients,
CRP is elevated, and immature band cells of WCC is due to severe DKA and its treatment,
which was the case of Carly. Therefore, fever in case of Carly is due to SIRS and not due to
sepsis. It is called as the non-infectious form of systemic inflammatory response syndrome
because CRP level increases here not because of infection but as a side effect of aggressive
The acetone smelling breath of Carly can be explained by ketoacidosis. It is the condition
characterized by the breath smelling line acetone with the increase in acetone level in the body to
an unsafe amount. With ketoacidosis the circulating level of the fatty acid increases (Boling&
Pravikoff, 2016). The inhibition of the lipolytic action of the growth hormone and Cortisol by
insulin is prevented by its deficiency. As these fatty acids accumulate, beta-oxidation is
accelerated producing excess ketone bodies. It includes beta hydroxybutyrate and acetoacetate
that forms acetone. This condition is also contributed by the lactic acidosis and poor tissue
perfusion. This is the reason behind Carly’s urinalysis being positive for glucose and ketones
(Boling & Pravikoff, 2016).
The symptoms of orthostatic hypotension and the patient being tachycardic can be
explained by dehydration. Tachycardia is caused by dehydration. It leads to a dry mucous
membrane and poor skin turgor and orthostatic hypotension (Singh et al., 2016). This was the
reason that Carly’s skin was pale and dry. If the onset of this condition is neglected then it many
results in severe acidosis and dehydration. This was the case of Carly as she was admitted two
weeks after suffering from polydipsia, polyuria and a fever (Singh et al., 2016). Carly’s skin was
hot due to fever. Among other symptoms, Fever during Diabetic ketoacidosis may be an outcome
of intercurrent infection (Boling & Pravikoff, 2016). However, CRP, which is elevated as is her
WCC which also shows immature band cells, may occur even in the absence of infection. They
may be elevated due to systemic inflammatory response syndrome (SIRS). In some patients,
CRP is elevated, and immature band cells of WCC is due to severe DKA and its treatment,
which was the case of Carly. Therefore, fever in case of Carly is due to SIRS and not due to
sepsis. It is called as the non-infectious form of systemic inflammatory response syndrome
because CRP level increases here not because of infection but as a side effect of aggressive
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7APPLIED PATHOPHYSIOLOGY
treatment for Diabetic Ketoacidosis. Rise in bodily temperature in the case of Carly is the marker
of severity of Diabetic Ketoacidosis (Nyenwe & Kitabchi, 2016).
Overall, the process of ketoacidosis and decreased glucose drain the bodily energy. Carly
being the young child is weakened by cumulative effect of dehydration, Polyuria ± polydipsia,
weight loss, Abdominal pain ± vomiting, Rapid, deep sighing, Ketotic breath, and fever. It
results in the overwhelming feeling of sleepiness due to lack of food the body is getting for
compensating for the loss of energy (Gee, 2015). This explains the drowsiness of Carly.
Diabetic Ketoacidosis contributes to cerebral edema. The mechanism is not clear, but
some of the contributing factors include delayed treatment and severity of diabetic ketoacidosis
as is the case of Carly before treatment. Upon intense fluid replacement, and the early
introduction of the insulin therapy cerebral oedema occurs (Cameron et al., 2014). It is also
contributed by the high degree of hyperglycemia. Cerebral oedema occurs within the short time
of start of the treatment. It starts with a headache, urinary incontinence, behavioural changes and
slowly progresses to abrupt neurological deterioration (Wolfsdorf, 2014). This mechanism
explains the noticeable neurological deterioration in Carly such as aggressive behaviour (Watts
& Edge, 2014). However, this treatment (IV fluids and an IV insulin infusion) soon provide
relief and improves the biochemical condition of the body. According to Tasker, and Acerini,
(2014) in children under or 12 years of age, diabetic ketoacidosis commonly leads to cerebral
oedema. This treatment causes generalized tonic–clonic convulsion (Gee, 2015), which was
experienced by Carly. It caused Carly to be unarousable. This condition is marked by GCS on
assessment being 6 and her pupils are dilated but reactive. Mannitol is good to treat cerebral
edema caused by Diabetic ketoacidosis. This hypotonic solution is super in decreasing the effects
treatment for Diabetic Ketoacidosis. Rise in bodily temperature in the case of Carly is the marker
of severity of Diabetic Ketoacidosis (Nyenwe & Kitabchi, 2016).
Overall, the process of ketoacidosis and decreased glucose drain the bodily energy. Carly
being the young child is weakened by cumulative effect of dehydration, Polyuria ± polydipsia,
weight loss, Abdominal pain ± vomiting, Rapid, deep sighing, Ketotic breath, and fever. It
results in the overwhelming feeling of sleepiness due to lack of food the body is getting for
compensating for the loss of energy (Gee, 2015). This explains the drowsiness of Carly.
Diabetic Ketoacidosis contributes to cerebral edema. The mechanism is not clear, but
some of the contributing factors include delayed treatment and severity of diabetic ketoacidosis
as is the case of Carly before treatment. Upon intense fluid replacement, and the early
introduction of the insulin therapy cerebral oedema occurs (Cameron et al., 2014). It is also
contributed by the high degree of hyperglycemia. Cerebral oedema occurs within the short time
of start of the treatment. It starts with a headache, urinary incontinence, behavioural changes and
slowly progresses to abrupt neurological deterioration (Wolfsdorf, 2014). This mechanism
explains the noticeable neurological deterioration in Carly such as aggressive behaviour (Watts
& Edge, 2014). However, this treatment (IV fluids and an IV insulin infusion) soon provide
relief and improves the biochemical condition of the body. According to Tasker, and Acerini,
(2014) in children under or 12 years of age, diabetic ketoacidosis commonly leads to cerebral
oedema. This treatment causes generalized tonic–clonic convulsion (Gee, 2015), which was
experienced by Carly. It caused Carly to be unarousable. This condition is marked by GCS on
assessment being 6 and her pupils are dilated but reactive. Mannitol is good to treat cerebral
edema caused by Diabetic ketoacidosis. This hypotonic solution is super in decreasing the effects

8APPLIED PATHOPHYSIOLOGY
if cerebral oedema (Watts& Edge, 2014). After administering Carly with Mannitol, her GCS
gradually improved. When she was discharged she had no neurological deficits.
Conclusion
The concept map links the pathophysiological processes of diabetic ketoacidosis that
have occurred in Carly, that caused her illness and subsequent deterioration after admission. The
paper highlighted the detailed pathophysiology of diabetic ketoacidosis with relevant literature.
The paper discusses how these pathophysiological disease processes lead to the observed vital
signs, and symptoms in Carly and change in neurological conditions after treatment.
if cerebral oedema (Watts& Edge, 2014). After administering Carly with Mannitol, her GCS
gradually improved. When she was discharged she had no neurological deficits.
Conclusion
The concept map links the pathophysiological processes of diabetic ketoacidosis that
have occurred in Carly, that caused her illness and subsequent deterioration after admission. The
paper highlighted the detailed pathophysiology of diabetic ketoacidosis with relevant literature.
The paper discusses how these pathophysiological disease processes lead to the observed vital
signs, and symptoms in Carly and change in neurological conditions after treatment.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

9APPLIED PATHOPHYSIOLOGY
References
Boling, B., & Pravikoff, D. (2016). Diabetic Ketoacidosis in Children.
Cameron, F. J., Scratch, S. E., Nadebaum, C., Northam, E. A., Koves, I., Jennings, J., ... & Inder,
T. E. (2014). Neurological consequences of diabetic ketoacidosis at initial presentation of
type 1 diabetes in a prospective cohort study of children. Diabetes care, 37(6), 1554-
1562.
Gee, S. W. (2015). The lethargic diabetic: cerebral edema in pediatric patients in diabetic
ketoacidosis. Air medical journal, 34(2), 109-112.
Kamel, K. S., & Halperin, M. L. (2015). Acid–base problems in diabetic ketoacidosis. New
England Journal of Medicine, 372(6), 546-554.
Nyenwe, E. A., & Kitabchi, A. E. (2016). The evolution of diabetic ketoacidosis: an update of its
etiology, pathogenesis and management. Metabolism, 65(4), 507-521.
Shah, I., Hoffman, G. F., Nyhan, W. L., Zschocke, J., Kahler, S. A., Mayatepek, E., ... & Chen,
Y. T. (2017). Lactic Acidosis in Children–A Varied Presentation. Journal of Pediatric
Intensive Care, 6(03), 206-208.
Singh, D., Cantu, M., Marx, M. H., & Akingbola, O. (2016). Diabetic Ketoacidosis and Fluid
Refractory Hypotension. Clinical pediatrics, 55(2), 182-184.
Srinivas, M. (2016). Diabetic Ketoacidosis in Children: A Systematic Review. Journal of
Chalmeda Anand Rao Institute of Medical Sciences Vol, 11(1), 31.
References
Boling, B., & Pravikoff, D. (2016). Diabetic Ketoacidosis in Children.
Cameron, F. J., Scratch, S. E., Nadebaum, C., Northam, E. A., Koves, I., Jennings, J., ... & Inder,
T. E. (2014). Neurological consequences of diabetic ketoacidosis at initial presentation of
type 1 diabetes in a prospective cohort study of children. Diabetes care, 37(6), 1554-
1562.
Gee, S. W. (2015). The lethargic diabetic: cerebral edema in pediatric patients in diabetic
ketoacidosis. Air medical journal, 34(2), 109-112.
Kamel, K. S., & Halperin, M. L. (2015). Acid–base problems in diabetic ketoacidosis. New
England Journal of Medicine, 372(6), 546-554.
Nyenwe, E. A., & Kitabchi, A. E. (2016). The evolution of diabetic ketoacidosis: an update of its
etiology, pathogenesis and management. Metabolism, 65(4), 507-521.
Shah, I., Hoffman, G. F., Nyhan, W. L., Zschocke, J., Kahler, S. A., Mayatepek, E., ... & Chen,
Y. T. (2017). Lactic Acidosis in Children–A Varied Presentation. Journal of Pediatric
Intensive Care, 6(03), 206-208.
Singh, D., Cantu, M., Marx, M. H., & Akingbola, O. (2016). Diabetic Ketoacidosis and Fluid
Refractory Hypotension. Clinical pediatrics, 55(2), 182-184.
Srinivas, M. (2016). Diabetic Ketoacidosis in Children: A Systematic Review. Journal of
Chalmeda Anand Rao Institute of Medical Sciences Vol, 11(1), 31.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

10APPLIED PATHOPHYSIOLOGY
Tasker, R. C., & Acerini, C. L. (2014). Cerebral edema in children with diabetic ketoacidosis:
vasogenic rather than cellular?. Pediatric diabetes, 15(4), 261-270.
Watts, W., & Edge, J. A. (2014). How can cerebral edema during treatment of diabetic
ketoacidosis be avoided?. Pediatric diabetes, 15(4), 271-276.
Wolfsdorf, J. I. (2014). The International Society of Pediatric and Adolescent Diabetes
guidelines for management of diabetic ketoacidosis: do the guidelines need to be
modified?. Pediatric diabetes, 15(4), 277-286.
Wolfsdorf, J. I., Allgrove, J., Craig, M. E., Edge, J., Glaser, N., Jain, V., ... & Hanas, R. (2014).
Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatric
diabetes, 15(S20), 154-179.
Tasker, R. C., & Acerini, C. L. (2014). Cerebral edema in children with diabetic ketoacidosis:
vasogenic rather than cellular?. Pediatric diabetes, 15(4), 261-270.
Watts, W., & Edge, J. A. (2014). How can cerebral edema during treatment of diabetic
ketoacidosis be avoided?. Pediatric diabetes, 15(4), 271-276.
Wolfsdorf, J. I. (2014). The International Society of Pediatric and Adolescent Diabetes
guidelines for management of diabetic ketoacidosis: do the guidelines need to be
modified?. Pediatric diabetes, 15(4), 277-286.
Wolfsdorf, J. I., Allgrove, J., Craig, M. E., Edge, J., Glaser, N., Jain, V., ... & Hanas, R. (2014).
Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatric
diabetes, 15(S20), 154-179.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



