Nutrition and Metabolism NUTRITION AND METABOLISM 2 1. The Normal Process of the Cellular Uptake of Cholesterol.4 2. The Genetic Causes of HeFH
VerifiedAdded on 2023/04/23
|15
|4218
|363
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: NUTRITION AND METABOLISM
Nutrition and Metabolism
Nutrition and Metabolism
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

NUTRITION AND METABOLISM 2
Table of Contents
1. Describe the normal process of the cellular uptake of cholesterol.........................................3
2. Briefly explain the proposed genetic causes of HeFH...........................................................4
3. Discuss the range of symptoms of FH, which characterize this condition............................5
4. Critically explain which of the specific parameters from the Simon Broome criteria, might
the clinicians have applied to make Carol’s and David’s diagnosis..........................................5
5. What specific dietary advice do Carol and her family need to follow, with the aim of
reducing their LDL-cholesterol by half?....................................................................................8
6. Apart from dietary factors, how might FH be managed in adults and children?.................10
7. What are the major health consequences for patients if FH remains undiagnosed? Give a
brief, critical explanation of why these consequences occur...................................................11
References................................................................................................................................13
Table of Contents
1. Describe the normal process of the cellular uptake of cholesterol.........................................3
2. Briefly explain the proposed genetic causes of HeFH...........................................................4
3. Discuss the range of symptoms of FH, which characterize this condition............................5
4. Critically explain which of the specific parameters from the Simon Broome criteria, might
the clinicians have applied to make Carol’s and David’s diagnosis..........................................5
5. What specific dietary advice do Carol and her family need to follow, with the aim of
reducing their LDL-cholesterol by half?....................................................................................8
6. Apart from dietary factors, how might FH be managed in adults and children?.................10
7. What are the major health consequences for patients if FH remains undiagnosed? Give a
brief, critical explanation of why these consequences occur...................................................11
References................................................................................................................................13

NUTRITION AND METABOLISM 3
1. Describe the normal process of the cellular uptake of cholesterol
The procedure of cellular ingestion of cholesterol in Carol and David can be categorized into
four different process.
Steps 1:
There is a reductase enzyme named HMG CoA that helps to synthesize the cholesterol in ER
(endoplasmic reticulum). Hence, there is need to consider the enzyme of HMG CoA and it is
known as hydroxymethylglutaryl coenzyme. The integral membrane of protein is attached
with ER (endoplasmic reticulum). Along with this, this membrane consists two types of
protein such as, SREBP and SCAP (Baffi, et. al., 2016). SREBP is called as sterol regulatory
element that binds the protein whereas SCAP is a Sterol cleavage-activating protein. In the
procedure of biosynthesis cholesterol, enzymes are considered named reductase enzyme
HMG-CoA. Gene expression of these involved enzymes is controlled by SREBP and SCAP
(Xu, Wang, Li, and Feng, 2016). Cholesterol can be transported via the bloodstream in the
form of lipoprotein particles and the most common is known as low-density lipoprotein, or
LDL.
Steps 2:
Cholesterol that contains low-density lipoprotein (LDL) are attached with LDL-R (low-
density lipoprotein receptor). This cholesterol is transported within Carol and David through
the endosomal pathway that consists of early endosomes, late endosomes, lysosomes,
andclathrin- coated pits (Brown, et. al., 2017). MLN64 assists in the transportation of
cholesterol from late endosomes and lysosomes to the mitochondria. It also supports the
transferring of cholesterol from the lysosomes, with the aids of NPC1 (NiemanPicktype C1)
and NPC2 (Nieman-Pick type C2) (Courtney, and Landreth, 2016).
The key information about this process came from the study of patients with inherited disease
and it is called familial hypercholesterolemia. Patients with this disease have high degree of
1. Describe the normal process of the cellular uptake of cholesterol
The procedure of cellular ingestion of cholesterol in Carol and David can be categorized into
four different process.
Steps 1:
There is a reductase enzyme named HMG CoA that helps to synthesize the cholesterol in ER
(endoplasmic reticulum). Hence, there is need to consider the enzyme of HMG CoA and it is
known as hydroxymethylglutaryl coenzyme. The integral membrane of protein is attached
with ER (endoplasmic reticulum). Along with this, this membrane consists two types of
protein such as, SREBP and SCAP (Baffi, et. al., 2016). SREBP is called as sterol regulatory
element that binds the protein whereas SCAP is a Sterol cleavage-activating protein. In the
procedure of biosynthesis cholesterol, enzymes are considered named reductase enzyme
HMG-CoA. Gene expression of these involved enzymes is controlled by SREBP and SCAP
(Xu, Wang, Li, and Feng, 2016). Cholesterol can be transported via the bloodstream in the
form of lipoprotein particles and the most common is known as low-density lipoprotein, or
LDL.
Steps 2:
Cholesterol that contains low-density lipoprotein (LDL) are attached with LDL-R (low-
density lipoprotein receptor). This cholesterol is transported within Carol and David through
the endosomal pathway that consists of early endosomes, late endosomes, lysosomes,
andclathrin- coated pits (Brown, et. al., 2017). MLN64 assists in the transportation of
cholesterol from late endosomes and lysosomes to the mitochondria. It also supports the
transferring of cholesterol from the lysosomes, with the aids of NPC1 (NiemanPicktype C1)
and NPC2 (Nieman-Pick type C2) (Courtney, and Landreth, 2016).
The key information about this process came from the study of patients with inherited disease
and it is called familial hypercholesterolemia. Patients with this disease have high degree of

NUTRITION AND METABOLISM 4
serum cholesterol and suffer heart attacks early such as father, grandfather and uncle of Carol
had already had heart attacks at a young age (Xu, Wang, Li, and Feng, 2016). It is assessed
that cells of these patients were unable to internalise the LDL from extracellular fluids and its
resultant in the accumulation of high levels of cholesterol in the circulation. Moreover, cell of
normal individual posses a receptor for LDL and it concentrates on coated pits and that
familial hypercholesterolemia consequences from inherited mutations in LDL
receptor(Behzadi, et. al., 2017).
Steps 3:
Cholesterol could be supplied in the plasma membrane from HDL (high-density lipoprotein)
and may be transferred to cytoplasm through SR-B1 (scavenger receptor class B, type I) in
Carol and David. This receptor is contained in caveolin-rich domains which are known as
caveolae or lipid rafts (France, et. al., 2016). There is an enzyme of Carol and David that
could be utilized for steroidogenesis, named cholesteryl esterase also known as HSL
(hormone-sensitive lipase). This enzyme converts CE (esterified cholesterol) to FC (free
cholesterol) in Carol and David. Esterified cholesterol stored in the plasma membrane and
could also store in LD (lipid droplet)of Carol and David (Lillis, et. al., 2015).
Steps 4:
LD (lipid droplets), hormone-sensitive lipase (HSL) and CE (esterified cholesterol) produces
FC (free cholesterol) that is used in the process of steroidogenesis. It is seen in the case of
Carol and David. Nonesterified FC (free cholesterol) could be stored in the Lipid droplets
after esterification (Lillis, et. al., 2015). It could be done by ACAT (acyl cholesterol acyl
transferase) and steroidogenesis, which transports the free cholesterol into mitochondria
(Behzadi, et. al., 2017).
2. Briefly explain the proposed genetic causes of HeFH.
Proposed genetic causes of HeFH
serum cholesterol and suffer heart attacks early such as father, grandfather and uncle of Carol
had already had heart attacks at a young age (Xu, Wang, Li, and Feng, 2016). It is assessed
that cells of these patients were unable to internalise the LDL from extracellular fluids and its
resultant in the accumulation of high levels of cholesterol in the circulation. Moreover, cell of
normal individual posses a receptor for LDL and it concentrates on coated pits and that
familial hypercholesterolemia consequences from inherited mutations in LDL
receptor(Behzadi, et. al., 2017).
Steps 3:
Cholesterol could be supplied in the plasma membrane from HDL (high-density lipoprotein)
and may be transferred to cytoplasm through SR-B1 (scavenger receptor class B, type I) in
Carol and David. This receptor is contained in caveolin-rich domains which are known as
caveolae or lipid rafts (France, et. al., 2016). There is an enzyme of Carol and David that
could be utilized for steroidogenesis, named cholesteryl esterase also known as HSL
(hormone-sensitive lipase). This enzyme converts CE (esterified cholesterol) to FC (free
cholesterol) in Carol and David. Esterified cholesterol stored in the plasma membrane and
could also store in LD (lipid droplet)of Carol and David (Lillis, et. al., 2015).
Steps 4:
LD (lipid droplets), hormone-sensitive lipase (HSL) and CE (esterified cholesterol) produces
FC (free cholesterol) that is used in the process of steroidogenesis. It is seen in the case of
Carol and David. Nonesterified FC (free cholesterol) could be stored in the Lipid droplets
after esterification (Lillis, et. al., 2015). It could be done by ACAT (acyl cholesterol acyl
transferase) and steroidogenesis, which transports the free cholesterol into mitochondria
(Behzadi, et. al., 2017).
2. Briefly explain the proposed genetic causes of HeFH.
Proposed genetic causes of HeFH
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

NUTRITION AND METABOLISM 5
HeFH is caused due to transformation in parent’s gene that is complex to remove LDL
cholesterol through the bloodstream such Carol’s son David was diagnosed HeFH and it occurs
because his father has same disease (Arca, 2017). HeFH is featured by cholesterol deposits that
affect different parts of Carol and David. These are corneas, extensor tendons, eyelids, hasten
vascular disease, and prominent plasma awareness of low-density lipoprotein (LDL)
cholesterol, particularly coronary artery disease (CAD) (Iacocca, and Hegele, 2017).
Although HeFH is created in David due to genetic heterogeneous but it is highly caused
because of heterozygous mutations in LDLR gene that encode LDL receptor.
3. Discuss the range of symptoms of FH, which characterize this condition
In the early period, there may be no symptoms. But after that, the given below symptoms can
occur in Carol and David:
Sores on the toes that do not heal (Farkas, 2017).
Sudden stroke-related symptoms like trouble in speaking, slumped on one side of the
face, weakness of an arm and leg, as well as loss of balance (Berberich, and Hegele,
2018).
Full of fat skin deposits called xanthomas over parts of hands, ankles, knees, elbows,
and around the cornea of the eye (Reinehr, et. al., 2016).
High Cholesterol in eyelids (Stang, and Stotmeister, 2017).
Chest pain and other indication of coronary artery disease might be present at a young
age (Arca, 2017).
Xanthomas such as full of fat deposits highly addressed in tendons and on the elbows,
knees, and buttock (Berberich, and Hegele, 2018).
Cramping of one or both calves during walking
High gray-white cholesterol around the corneas is also known as corneal arcus (Stang,
and Stotmeister, 2017).
HeFH is caused due to transformation in parent’s gene that is complex to remove LDL
cholesterol through the bloodstream such Carol’s son David was diagnosed HeFH and it occurs
because his father has same disease (Arca, 2017). HeFH is featured by cholesterol deposits that
affect different parts of Carol and David. These are corneas, extensor tendons, eyelids, hasten
vascular disease, and prominent plasma awareness of low-density lipoprotein (LDL)
cholesterol, particularly coronary artery disease (CAD) (Iacocca, and Hegele, 2017).
Although HeFH is created in David due to genetic heterogeneous but it is highly caused
because of heterozygous mutations in LDLR gene that encode LDL receptor.
3. Discuss the range of symptoms of FH, which characterize this condition
In the early period, there may be no symptoms. But after that, the given below symptoms can
occur in Carol and David:
Sores on the toes that do not heal (Farkas, 2017).
Sudden stroke-related symptoms like trouble in speaking, slumped on one side of the
face, weakness of an arm and leg, as well as loss of balance (Berberich, and Hegele,
2018).
Full of fat skin deposits called xanthomas over parts of hands, ankles, knees, elbows,
and around the cornea of the eye (Reinehr, et. al., 2016).
High Cholesterol in eyelids (Stang, and Stotmeister, 2017).
Chest pain and other indication of coronary artery disease might be present at a young
age (Arca, 2017).
Xanthomas such as full of fat deposits highly addressed in tendons and on the elbows,
knees, and buttock (Berberich, and Hegele, 2018).
Cramping of one or both calves during walking
High gray-white cholesterol around the corneas is also known as corneal arcus (Stang,
and Stotmeister, 2017).

NUTRITION AND METABOLISM 6
4. Critically explain which of the specific parameters from the Simon Broome criteria,
might the clinicians have applied to make Carol’s and David’s diagnosis
Diagnosis
As per the case study, Carol and his 15-year old son David is suffering from HeFH. After
DNA based evidence test of Carol and David, positive identification of HeFH is found within
them (Martini, et. al., 2018). For addressing the diagnosed of Carol and David, clinicians
have applied Simon Broome criteria (Berberich, and Hegele, 2018).
The Simon Broome criterion is as follow:
The featured of lipid profile in FH
A family history related to hypercholesterolemia and premature ischaemic heart
disease (Weickert, and Pfeiffer, 2018).
This issue is diagnosed due to the availability of tendon xanthomata where
xanthelasmas and premature corneal arcus may be demonstrated but cannot diagnostic
(Reinehr, et. al., 2016).
As per Simon Broome criteria, Carol’s son David is facing this issue because his father had
FH disorder. This criterion also defines that Carol and David have hyperlipidemia and FH
issue such as common hypercholesterolemia and familial integrated hyperlipidemia. In case
of Carol and David, genetic testing is used for different key mutations, which have currently
been addressed as the reason for FH. In addition, in Carol and David, specific clinical
diagnoses of FH were diagnosed and it is favorable for one of these three transformations
(Lozano, et. al., 2016).
Simon Broome Register Group of FH
4. Critically explain which of the specific parameters from the Simon Broome criteria,
might the clinicians have applied to make Carol’s and David’s diagnosis
Diagnosis
As per the case study, Carol and his 15-year old son David is suffering from HeFH. After
DNA based evidence test of Carol and David, positive identification of HeFH is found within
them (Martini, et. al., 2018). For addressing the diagnosed of Carol and David, clinicians
have applied Simon Broome criteria (Berberich, and Hegele, 2018).
The Simon Broome criterion is as follow:
The featured of lipid profile in FH
A family history related to hypercholesterolemia and premature ischaemic heart
disease (Weickert, and Pfeiffer, 2018).
This issue is diagnosed due to the availability of tendon xanthomata where
xanthelasmas and premature corneal arcus may be demonstrated but cannot diagnostic
(Reinehr, et. al., 2016).
As per Simon Broome criteria, Carol’s son David is facing this issue because his father had
FH disorder. This criterion also defines that Carol and David have hyperlipidemia and FH
issue such as common hypercholesterolemia and familial integrated hyperlipidemia. In case
of Carol and David, genetic testing is used for different key mutations, which have currently
been addressed as the reason for FH. In addition, in Carol and David, specific clinical
diagnoses of FH were diagnosed and it is favorable for one of these three transformations
(Lozano, et. al., 2016).
Simon Broome Register Group of FH

NUTRITION AND METABOLISM 7
a) Total cholesterol level above 7.5 mmol/L (290 mg/dl) in adults or a total cholesterol level
above 6.7 mmol/L (260 mg/dl) for children under 16 OR LDL-c levels above 4.9 mmol/L (190 mg/dL)
in adults (4.0 mmol/L in children) (either pretreatment or highest on treatment)
b) tendon xanthomas in patient or relative (parent, child, sibling, grandparent, aunt, uncle) OR
c) DNA-based evidence of an LDL receptor mutation or familial defective apo B-100
(Sources: Reinehr, et. al., 2016).
Dutch Lipid Clinic network diagnosis of FH
Family History
A) First degree relative with known premature (<55 years men; < 60 years women)
coronary disease and vascular disease OR LDL-c > 95th percentile
B) First degree relative with tendon xanthomata and/or arcuscornealis OR childhood
(<18 years) with LDL-c > 95th percentile
(Sources: Farkas, 2017).
Clinical history
A Patient with premature CAD (men <55, women <60 years)
B Patient with premature cerebral or PVD (men <55, women <60 years)
Physical Examination
A Tendon xanthomas
B Premature arcus
Lab analysis
A LDL – c > 8.5 mmol/L
B LDL-c 6.5–8.4 mmol/L
C LDL- c 5.0–6.4 mmol/L
D LDL – c 4.0–4.9 mmol/L
Functional
DNA mutations
Definite FH: > 8 points, Probable FH: 6–8 points, Possible FH: 3–5
a) Total cholesterol level above 7.5 mmol/L (290 mg/dl) in adults or a total cholesterol level
above 6.7 mmol/L (260 mg/dl) for children under 16 OR LDL-c levels above 4.9 mmol/L (190 mg/dL)
in adults (4.0 mmol/L in children) (either pretreatment or highest on treatment)
b) tendon xanthomas in patient or relative (parent, child, sibling, grandparent, aunt, uncle) OR
c) DNA-based evidence of an LDL receptor mutation or familial defective apo B-100
(Sources: Reinehr, et. al., 2016).
Dutch Lipid Clinic network diagnosis of FH
Family History
A) First degree relative with known premature (<55 years men; < 60 years women)
coronary disease and vascular disease OR LDL-c > 95th percentile
B) First degree relative with tendon xanthomata and/or arcuscornealis OR childhood
(<18 years) with LDL-c > 95th percentile
(Sources: Farkas, 2017).
Clinical history
A Patient with premature CAD (men <55, women <60 years)
B Patient with premature cerebral or PVD (men <55, women <60 years)
Physical Examination
A Tendon xanthomas
B Premature arcus
Lab analysis
A LDL – c > 8.5 mmol/L
B LDL-c 6.5–8.4 mmol/L
C LDL- c 5.0–6.4 mmol/L
D LDL – c 4.0–4.9 mmol/L
Functional
DNA mutations
Definite FH: > 8 points, Probable FH: 6–8 points, Possible FH: 3–5
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

NUTRITION AND METABOLISM 8
(Sources: Xu, et. al., 2016).
As per the case study, it is stated that David who is 15 years old was suffering from FH and
his total cholesterol was averaged 9mmol/L and his low-density lipoprotein (LDL) measured
6mmol/L. Carol who was 45 years old was diagnosed FH with total cholesterol was high;
around 10mmol/L. These criteria is matched with the Simon Criteria table. It indicated that
both was FH and they faced this issue due to family history.
It is significant to prohibit the secondary causes of hypercholesterolemia, which can be
created as a consequence of hypothyroidism, diabetes, liver or kidney disease, and obesity.
There is some medication that can increase cholesterol levels in Carol and David such as
ciclosporin, atypical antipsychotics, and ciclosporin (Xu, et. al., 2016). Carol and David
needs to increase the awareness regarding the cardiovascular disease risk assessment
techniques like Framingham cannot be applied for those individuals with FH because they are
already at significantly gained risk of CHD (Farkas, 2017).
NHS North of Tyne has gained local guidelines as per the NICE document to assist in the
discovery of FH and the management of a patient who has high serum lipids. This instruction
can be summarised in the procedure (Weickert, and Pfeiffer, 2018).
NHS has developed a local instruction as per the NICE document for assisting and detecting
the FH and managing Carol and David those have high serum lipids. This guideline can be
summarised in algorithm (Reinehr, et. al., 2016).
5. What specific dietary advice do Carol and her family need to follow, with the aim of
reducing their LDL-cholesterol by half?
Avoid these foods
Cholesterol extents can be increased due to high diet in saturated fats as well as Trans fatty
acids is also illustrated as trans-fat. Diets with high type of fat are contributed to high extents
of low-density lipoprotein (LDL) cholesterol (France, et. al., 2016).
(Sources: Xu, et. al., 2016).
As per the case study, it is stated that David who is 15 years old was suffering from FH and
his total cholesterol was averaged 9mmol/L and his low-density lipoprotein (LDL) measured
6mmol/L. Carol who was 45 years old was diagnosed FH with total cholesterol was high;
around 10mmol/L. These criteria is matched with the Simon Criteria table. It indicated that
both was FH and they faced this issue due to family history.
It is significant to prohibit the secondary causes of hypercholesterolemia, which can be
created as a consequence of hypothyroidism, diabetes, liver or kidney disease, and obesity.
There is some medication that can increase cholesterol levels in Carol and David such as
ciclosporin, atypical antipsychotics, and ciclosporin (Xu, et. al., 2016). Carol and David
needs to increase the awareness regarding the cardiovascular disease risk assessment
techniques like Framingham cannot be applied for those individuals with FH because they are
already at significantly gained risk of CHD (Farkas, 2017).
NHS North of Tyne has gained local guidelines as per the NICE document to assist in the
discovery of FH and the management of a patient who has high serum lipids. This instruction
can be summarised in the procedure (Weickert, and Pfeiffer, 2018).
NHS has developed a local instruction as per the NICE document for assisting and detecting
the FH and managing Carol and David those have high serum lipids. This guideline can be
summarised in algorithm (Reinehr, et. al., 2016).
5. What specific dietary advice do Carol and her family need to follow, with the aim of
reducing their LDL-cholesterol by half?
Avoid these foods
Cholesterol extents can be increased due to high diet in saturated fats as well as Trans fatty
acids is also illustrated as trans-fat. Diets with high type of fat are contributed to high extents
of low-density lipoprotein (LDL) cholesterol (France, et. al., 2016).
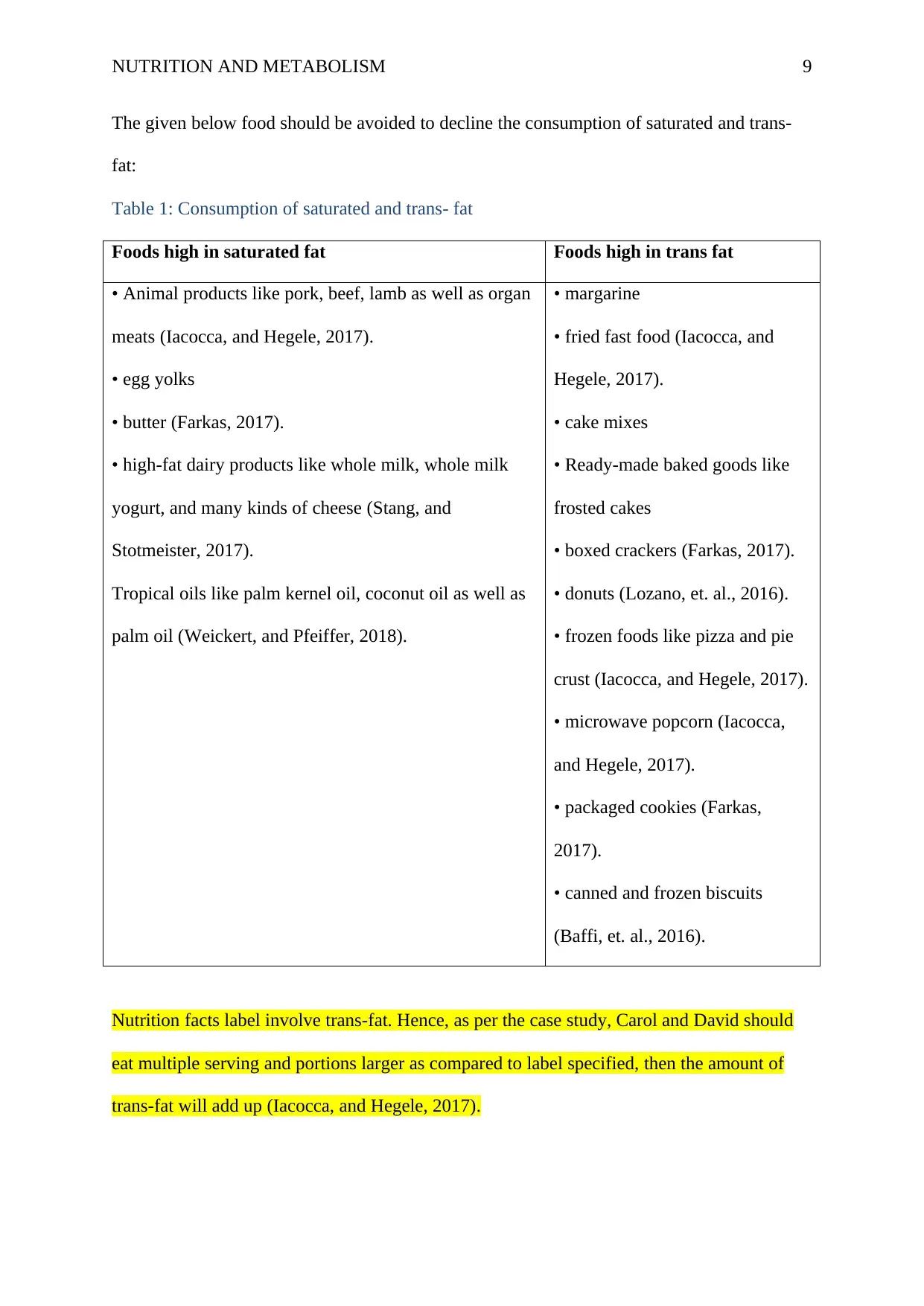
NUTRITION AND METABOLISM 9
The given below food should be avoided to decline the consumption of saturated and trans-
fat:
Table 1: Consumption of saturated and trans- fat
Foods high in saturated fat Foods high in trans fat
• Animal products like pork, beef, lamb as well as organ
meats (Iacocca, and Hegele, 2017).
• egg yolks
• butter (Farkas, 2017).
• high-fat dairy products like whole milk, whole milk
yogurt, and many kinds of cheese (Stang, and
Stotmeister, 2017).
Tropical oils like palm kernel oil, coconut oil as well as
palm oil (Weickert, and Pfeiffer, 2018).
• margarine
• fried fast food (Iacocca, and
Hegele, 2017).
• cake mixes
• Ready-made baked goods like
frosted cakes
• boxed crackers (Farkas, 2017).
• donuts (Lozano, et. al., 2016).
• frozen foods like pizza and pie
crust (Iacocca, and Hegele, 2017).
• microwave popcorn (Iacocca,
and Hegele, 2017).
• packaged cookies (Farkas,
2017).
• canned and frozen biscuits
(Baffi, et. al., 2016).
Nutrition facts label involve trans-fat. Hence, as per the case study, Carol and David should
eat multiple serving and portions larger as compared to label specified, then the amount of
trans-fat will add up (Iacocca, and Hegele, 2017).
The given below food should be avoided to decline the consumption of saturated and trans-
fat:
Table 1: Consumption of saturated and trans- fat
Foods high in saturated fat Foods high in trans fat
• Animal products like pork, beef, lamb as well as organ
meats (Iacocca, and Hegele, 2017).
• egg yolks
• butter (Farkas, 2017).
• high-fat dairy products like whole milk, whole milk
yogurt, and many kinds of cheese (Stang, and
Stotmeister, 2017).
Tropical oils like palm kernel oil, coconut oil as well as
palm oil (Weickert, and Pfeiffer, 2018).
• margarine
• fried fast food (Iacocca, and
Hegele, 2017).
• cake mixes
• Ready-made baked goods like
frosted cakes
• boxed crackers (Farkas, 2017).
• donuts (Lozano, et. al., 2016).
• frozen foods like pizza and pie
crust (Iacocca, and Hegele, 2017).
• microwave popcorn (Iacocca,
and Hegele, 2017).
• packaged cookies (Farkas,
2017).
• canned and frozen biscuits
(Baffi, et. al., 2016).
Nutrition facts label involve trans-fat. Hence, as per the case study, Carol and David should
eat multiple serving and portions larger as compared to label specified, then the amount of
trans-fat will add up (Iacocca, and Hegele, 2017).

NUTRITION AND METABOLISM 10
Along with this, sugar in the diet can be promoted inflammation and it can accelerate plaque
development as well as heart disease (Iacocca, and Hegele, 2017). Carol and David should
decline the added sugars such as table sugar, maple syrup, high fructose corn syrup, and
agave nectar to less than 24 g per day for women or 36 g per day for men (Martini, et. al.,
2018).
Eat more of these foods
Healthy diets can rich in high-fiber foods and the ‘good’ fats such as polyunsaturated and
monounsaturated fats can also support to decline the cholesterol levels of Carol and David
(Farkas, 2017).
Fiber
A daily intake of minimum 25 g of fiber may decline the risk of heart disease. In addition,
soluble fiber can aid to decline the LDL levels in Carol and David by shifting cholesterol out
of digestive tract rapidly. There are different sources of Fiber such as beans, whole grains,
vegetables and fruits that could be taken by Carol and David (Veale, et. al., 2018).
Polyunsaturated fat
Polyunsaturated fat can be considered into healthy fat and it should be eliminated by Carol
and David. It involves salmon, avocados, nuts, seeds and plant-based oils like olive oil,
avocado oil, sunflower oil, and grapeseed oil, and tofu (Iacocca, and Hegele, 2017).
Monounsaturated fat
The same plant has relied on oil with polyunsaturated fat are a good source for
monounsaturated fat in Carol and David. In spite of saturated and trans-fat, these types of oils
have health advantageous while eaten moderation food. Foods that have high
monounsaturated fat involves the olives, avocados, walnuts, and peanut or almond butter
(Waters, and Lemaire, 2016) that should be avoided by Carol and David.
Along with this, sugar in the diet can be promoted inflammation and it can accelerate plaque
development as well as heart disease (Iacocca, and Hegele, 2017). Carol and David should
decline the added sugars such as table sugar, maple syrup, high fructose corn syrup, and
agave nectar to less than 24 g per day for women or 36 g per day for men (Martini, et. al.,
2018).
Eat more of these foods
Healthy diets can rich in high-fiber foods and the ‘good’ fats such as polyunsaturated and
monounsaturated fats can also support to decline the cholesterol levels of Carol and David
(Farkas, 2017).
Fiber
A daily intake of minimum 25 g of fiber may decline the risk of heart disease. In addition,
soluble fiber can aid to decline the LDL levels in Carol and David by shifting cholesterol out
of digestive tract rapidly. There are different sources of Fiber such as beans, whole grains,
vegetables and fruits that could be taken by Carol and David (Veale, et. al., 2018).
Polyunsaturated fat
Polyunsaturated fat can be considered into healthy fat and it should be eliminated by Carol
and David. It involves salmon, avocados, nuts, seeds and plant-based oils like olive oil,
avocado oil, sunflower oil, and grapeseed oil, and tofu (Iacocca, and Hegele, 2017).
Monounsaturated fat
The same plant has relied on oil with polyunsaturated fat are a good source for
monounsaturated fat in Carol and David. In spite of saturated and trans-fat, these types of oils
have health advantageous while eaten moderation food. Foods that have high
monounsaturated fat involves the olives, avocados, walnuts, and peanut or almond butter
(Waters, and Lemaire, 2016) that should be avoided by Carol and David.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

NUTRITION AND METABOLISM 11
6. Apart from dietary factors, how might FH be managed in adults and children?
Apart from the dietary factor, FH can be managed in Carol and David. They can encourage a
heart-healthy lifestyle by exercise, low-fat diet, and not smoking. But, this condition may
create at birth along with; high cholesterol can be founded from one day in Carol and David
with FH. Diet alone is rarely enough. Statin medication is related to the cornerstone of
treatment for Carol and David however, it is not enough (Veale, et. al., 2018).
PCSK9 inhibitors such as Praluent may decline the amount of LDL cholesterol in circulation
and lower total cholesterol. There are other medications such as ezetimibe Zetia that decline
the absorption in the intestine as well as bile acid sequestrants like cholestyramine or
colestipol could be practiced by Carol and David. There are some patients such as Carol and
David cannot tolerate medication due to high cholesterol (France, et. al., 2016). For them,
LDL apheresis is a process to eliminate cholesterol from the blood such as dialysis and is
found in Carol and David from one to two weeks. Along with this, new medications were
approved by the FDA in the context of extraordinary cases such as Kynamro injection, and
Juxtapid. However, it has side effects (Martini, et. al., 2018).
7. What are the major health consequences for patients if FH remains undiagnosed?
Give a brief, critical explanation of why these consequences occur.
Carol and David with untreated FH have regarding 20 times greater risk for building early
heart disease. Along with this, high ’bad’ cholesterol is a major risk factor for atherosclerosis
such as plaque build-up within the artery wall can lead to CVD.
When FH is remained untreated, then the potential risk for the coronary event is:
• 50% for men by 50 years of age
• 30% for women by 60 years of age (Neely, Cramb, and Shoulders, 2016).
In several nations, an estimated 14-34 million people have FH. There are about 200,000
people who die from CVD each year. The existing condition of Carol and David is
6. Apart from dietary factors, how might FH be managed in adults and children?
Apart from the dietary factor, FH can be managed in Carol and David. They can encourage a
heart-healthy lifestyle by exercise, low-fat diet, and not smoking. But, this condition may
create at birth along with; high cholesterol can be founded from one day in Carol and David
with FH. Diet alone is rarely enough. Statin medication is related to the cornerstone of
treatment for Carol and David however, it is not enough (Veale, et. al., 2018).
PCSK9 inhibitors such as Praluent may decline the amount of LDL cholesterol in circulation
and lower total cholesterol. There are other medications such as ezetimibe Zetia that decline
the absorption in the intestine as well as bile acid sequestrants like cholestyramine or
colestipol could be practiced by Carol and David. There are some patients such as Carol and
David cannot tolerate medication due to high cholesterol (France, et. al., 2016). For them,
LDL apheresis is a process to eliminate cholesterol from the blood such as dialysis and is
found in Carol and David from one to two weeks. Along with this, new medications were
approved by the FDA in the context of extraordinary cases such as Kynamro injection, and
Juxtapid. However, it has side effects (Martini, et. al., 2018).
7. What are the major health consequences for patients if FH remains undiagnosed?
Give a brief, critical explanation of why these consequences occur.
Carol and David with untreated FH have regarding 20 times greater risk for building early
heart disease. Along with this, high ’bad’ cholesterol is a major risk factor for atherosclerosis
such as plaque build-up within the artery wall can lead to CVD.
When FH is remained untreated, then the potential risk for the coronary event is:
• 50% for men by 50 years of age
• 30% for women by 60 years of age (Neely, Cramb, and Shoulders, 2016).
In several nations, an estimated 14-34 million people have FH. There are about 200,000
people who die from CVD each year. The existing condition of Carol and David is

NUTRITION AND METABOLISM 12
underdiagnosis partly because of incomplete adoption related to current cholesterol
screening. It is recommended Carol and David should take US preventive services to
overcome their disease (Lozano, et. al., 2016). The consequences are created in all adults to
start at the age 35 years in men, age 45 years in women, and age 20 years when there is a
family history of heart disease however these guidelines are not highly implemented
(Weickert, and Pfeiffer, 2018).
Carol and David are suffering from FH have an increased occurrence of atherosclerotic
cardiovascular disease (CVD), specifically coronary artery disease (CAD). Along with this,
when the left is untreated, then FH confers a 100-fold excess uncertainty of CAD in young
men (Reinehr, et. al., 2016). Women with FH is also substantial uncertainty of
CAD,however, the uncertainty is lower as compared to that for men, and on overage, women
create the disease with 10 years later (Baffi, et. al., 2016).
underdiagnosis partly because of incomplete adoption related to current cholesterol
screening. It is recommended Carol and David should take US preventive services to
overcome their disease (Lozano, et. al., 2016). The consequences are created in all adults to
start at the age 35 years in men, age 45 years in women, and age 20 years when there is a
family history of heart disease however these guidelines are not highly implemented
(Weickert, and Pfeiffer, 2018).
Carol and David are suffering from FH have an increased occurrence of atherosclerotic
cardiovascular disease (CVD), specifically coronary artery disease (CAD). Along with this,
when the left is untreated, then FH confers a 100-fold excess uncertainty of CAD in young
men (Reinehr, et. al., 2016). Women with FH is also substantial uncertainty of
CAD,however, the uncertainty is lower as compared to that for men, and on overage, women
create the disease with 10 years later (Baffi, et. al., 2016).

NUTRITION AND METABOLISM 13
References
Arca, M., 2017.Old challenges and new opportunities in the clinical management of
heterozygous familial hypercholesterolemia (HeFH): The promises of PCSK9
inhibitors. Atherosclerosis, 256, pp.134-145.
Baffi, C.W., Wood, L., Winnica, D., StrolloJr, P.J., Gladwin, M.T., Que, L.G. and Holguin,
F., 2016.Metabolic syndrome and the lung. Chest, 149(6), pp.1525-1534.
Behzadi, S., Serpooshan, V., Tao, W., Hamaly, M.A., Alkawareek, M.Y., Dreaden, E.C.,
Brown, D., Alkilany, A.M., Farokhzad, O.C. and Mahmoudi, M., 2017. Cellular uptake of
nanoparticles: a journey inside the cell. Chemical Society Reviews, 46(14), pp.4218-4244.
Berberich, A.J. and Hegele, R.A., 2018. The complex molecular genetics of familial
hypercholesterolemia. Nature Reviews Cardiology, p.1.
Courtney, R. and Landreth, G.E., 2016. LXR regulation of brain cholesterol: from
development to disease. Trends in Endocrinology & Metabolism, 27(6), pp.404-414.
Farkas, C.A. ed., 2017. Reading the psychosomatic in medical and popular culture:
Something.Nothing.Everything.UK: Routledge.
France, M., Rees, A., Datta, D., Thompson, G., Capps, N., Ferns, G., Ramaswami, U., Seed,
M., Neely, D., Cramb, R. and Shoulders, C., 2016.HEART UK statement on the management
of homozygous familial hypercholesterolemia in the United Kingdom. Atherosclerosis, 255,
pp.128-139.
Iacocca, M.A., andHegele, R.A., 2017. Recent advances in genetic testing for familial
hypercholesterolemia. Expert review of molecular diagnostics, 17(7), pp.641-651.
Lillis, A.P., Muratoglu, S.C., Au, D.T., Migliorini, M., Lee, M.J., Fried, S.K., Mikhailenko, I.
and Strickland, D.K., 2015. LDL receptor-related protein-1 (LRP1) regulates cholesterol
accumulation in macrophages. PloS one, 10(6), p.e0128903.
References
Arca, M., 2017.Old challenges and new opportunities in the clinical management of
heterozygous familial hypercholesterolemia (HeFH): The promises of PCSK9
inhibitors. Atherosclerosis, 256, pp.134-145.
Baffi, C.W., Wood, L., Winnica, D., StrolloJr, P.J., Gladwin, M.T., Que, L.G. and Holguin,
F., 2016.Metabolic syndrome and the lung. Chest, 149(6), pp.1525-1534.
Behzadi, S., Serpooshan, V., Tao, W., Hamaly, M.A., Alkawareek, M.Y., Dreaden, E.C.,
Brown, D., Alkilany, A.M., Farokhzad, O.C. and Mahmoudi, M., 2017. Cellular uptake of
nanoparticles: a journey inside the cell. Chemical Society Reviews, 46(14), pp.4218-4244.
Berberich, A.J. and Hegele, R.A., 2018. The complex molecular genetics of familial
hypercholesterolemia. Nature Reviews Cardiology, p.1.
Courtney, R. and Landreth, G.E., 2016. LXR regulation of brain cholesterol: from
development to disease. Trends in Endocrinology & Metabolism, 27(6), pp.404-414.
Farkas, C.A. ed., 2017. Reading the psychosomatic in medical and popular culture:
Something.Nothing.Everything.UK: Routledge.
France, M., Rees, A., Datta, D., Thompson, G., Capps, N., Ferns, G., Ramaswami, U., Seed,
M., Neely, D., Cramb, R. and Shoulders, C., 2016.HEART UK statement on the management
of homozygous familial hypercholesterolemia in the United Kingdom. Atherosclerosis, 255,
pp.128-139.
Iacocca, M.A., andHegele, R.A., 2017. Recent advances in genetic testing for familial
hypercholesterolemia. Expert review of molecular diagnostics, 17(7), pp.641-651.
Lillis, A.P., Muratoglu, S.C., Au, D.T., Migliorini, M., Lee, M.J., Fried, S.K., Mikhailenko, I.
and Strickland, D.K., 2015. LDL receptor-related protein-1 (LRP1) regulates cholesterol
accumulation in macrophages. PloS one, 10(6), p.e0128903.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

NUTRITION AND METABOLISM 14
Lozano, P., Henrikson, N.B., Dunn, J., Morrison, C.C., Nguyen, M., Blasi, P.R., Anderson,
M.L. and Whitlock, E.P., 2016.Lipid screening in childhood and adolescence for detection of
familial hypercholesterolemia: evidence report and systematic review for the US Preventive
Services Task Force. Jama, 316(6), pp.645-655.
Martini, D., Guareschi, C., Biasini, B., Bedogni, G., Galli, C., Angelino, D., Marchi, L.,
Zavaroni, I., Pruneti, C., Ventura, M. and Galli, D., 2018. Claimed effects, outcome
variables, and methods of measurement for health claims proposed under Regulation (EC)
1924/2006 in the framework of bone health. PharmaNutrition, 6(1), pp.17-36.
Reinehr, T., Lass, N., Toschke, C., Rothermel, J., Lanzinger, S. and Holl, R.W., 2016. Which
amount of BMI-SDS reduction is necessary to improve cardiovascular risk factors in
overweight children?. The Journal of Clinical Endocrinology & Metabolism, 101(8),
pp.3171-3179.
Stang, J.S. and Stotmeister, B., 2017. Nutrition in adolescence.In the Nutrition Guide for
Physicians and Related Healthcare Professionals (pp. 29-39).USA: Humana Press, Cham.
Veale, E.L., Stewart, A.J., Mathie, A., Lall, S.K., Rees-Roberts, M., Savickas, V., Bhamra,
S.K. and Corlett, S.A., 2018.Pharmacists detecting atrial fibrillation (PDAF) in primary care
during the influenza vaccination season: a multisite, cross-sectional screening protocol. BMJ
Open, 8(3), p.e021121.
Waters, A. and Lemaire, M., 2016.Genetic Diagnosis of Renal Diseases: Basic Concepts and
Testing.In Pediatric Kidney Disease (pp. 107-149).Springer, Berlin, Heidelberg.
Weickert, M.O. and Pfeiffer, A.F., 2018. Impact of dietary fiber consumption on insulin
resistance and the prevention of type 2 diabetes. The Journal of nutrition, 148(1), pp.7-12.
Xu, L., Wang, Y.R., Li, P.C. and Feng, B., 2016. Advanced glycationend products increase
lipids accumulation in macrophages through upregulation of receptor of advanced
Lozano, P., Henrikson, N.B., Dunn, J., Morrison, C.C., Nguyen, M., Blasi, P.R., Anderson,
M.L. and Whitlock, E.P., 2016.Lipid screening in childhood and adolescence for detection of
familial hypercholesterolemia: evidence report and systematic review for the US Preventive
Services Task Force. Jama, 316(6), pp.645-655.
Martini, D., Guareschi, C., Biasini, B., Bedogni, G., Galli, C., Angelino, D., Marchi, L.,
Zavaroni, I., Pruneti, C., Ventura, M. and Galli, D., 2018. Claimed effects, outcome
variables, and methods of measurement for health claims proposed under Regulation (EC)
1924/2006 in the framework of bone health. PharmaNutrition, 6(1), pp.17-36.
Reinehr, T., Lass, N., Toschke, C., Rothermel, J., Lanzinger, S. and Holl, R.W., 2016. Which
amount of BMI-SDS reduction is necessary to improve cardiovascular risk factors in
overweight children?. The Journal of Clinical Endocrinology & Metabolism, 101(8),
pp.3171-3179.
Stang, J.S. and Stotmeister, B., 2017. Nutrition in adolescence.In the Nutrition Guide for
Physicians and Related Healthcare Professionals (pp. 29-39).USA: Humana Press, Cham.
Veale, E.L., Stewart, A.J., Mathie, A., Lall, S.K., Rees-Roberts, M., Savickas, V., Bhamra,
S.K. and Corlett, S.A., 2018.Pharmacists detecting atrial fibrillation (PDAF) in primary care
during the influenza vaccination season: a multisite, cross-sectional screening protocol. BMJ
Open, 8(3), p.e021121.
Waters, A. and Lemaire, M., 2016.Genetic Diagnosis of Renal Diseases: Basic Concepts and
Testing.In Pediatric Kidney Disease (pp. 107-149).Springer, Berlin, Heidelberg.
Weickert, M.O. and Pfeiffer, A.F., 2018. Impact of dietary fiber consumption on insulin
resistance and the prevention of type 2 diabetes. The Journal of nutrition, 148(1), pp.7-12.
Xu, L., Wang, Y.R., Li, P.C. and Feng, B., 2016. Advanced glycationend products increase
lipids accumulation in macrophages through upregulation of receptor of advanced

NUTRITION AND METABOLISM 15
glycationend products: increasing uptake, esterification and decreasing efflux of
cholesterol. Lipids in health and disease, 15(1), p.161.
glycationend products: increasing uptake, esterification and decreasing efflux of
cholesterol. Lipids in health and disease, 15(1), p.161.
1 out of 15
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.