o .. NUCLEI. Atomic mass unit (u) An atomic mass unit i
10 Pages3687 Words2 Views
Added on 2022-11-30
o .. NUCLEI. Atomic mass unit (u) An atomic mass unit i
Added on 2022-11-30
ShareRelated Documents
NUCLEI
Atomic mass unit (u)
An atomic mass unit is defined as 1/12 th the mass of a carbon ( 12
C) atom. According to this-
Isotopes and Average Atomic mass
Isotopes
Atoms with same atomic number (exhibit the same chemical properties) but different mass number are known as
isotopes of each other. For e.g., Chlorine ( 34.98Cl and 36.98Cl), Hydrogen.
Average Atomic mass
Different isotopes of the same element have different relative abundances. Therefore, the atomic mass of such an
element is given by its average atomic mass which is calculated as follows-
For e.g., consider an element A with two isotopes A 1 and A 2 with relative abundance of A 1 being b%. The average
atomic mass of A is given by-
Note: Hydrogen has three isotopes- Protium, Deuterium and Tritium. Tritium nuclei is radioactive and is unstable
therefore, it is not found naturally.
Composition of the nucleus
The nucleus consists of two entities namely, protons and neutrons (collectively known as nucleons). The positive
charge inside the nucleus comes from the protons.
Neutrons are neutral particles. Free neutrons, unlike protons are unstable. It decays into a proton, an electron, and a
antineutrino.
The composition of the nucleus can be described using the following symbols and terms-
Z-atomic number = number of protons
N-neutron number = number if neutrons
A-mass number = Z + N = total number of protons and neutrons.
Note: 1. Notation for nuclear species or nuclides-
Where X is the chemical symbol of the species.
2. Isotopes have the same number of protons but different number of neutrons
3. All nuclides with same mass number are called isobars
4. Nuclides with same neutron number N but different atomic number Z are called isotones
Percentage of A = 100-b2
o.
Atomic mass unit (u)
An atomic mass unit is defined as 1/12 th the mass of a carbon ( 12
C) atom. According to this-
Isotopes and Average Atomic mass
Isotopes
Atoms with same atomic number (exhibit the same chemical properties) but different mass number are known as
isotopes of each other. For e.g., Chlorine ( 34.98Cl and 36.98Cl), Hydrogen.
Average Atomic mass
Different isotopes of the same element have different relative abundances. Therefore, the atomic mass of such an
element is given by its average atomic mass which is calculated as follows-
For e.g., consider an element A with two isotopes A 1 and A 2 with relative abundance of A 1 being b%. The average
atomic mass of A is given by-
Note: Hydrogen has three isotopes- Protium, Deuterium and Tritium. Tritium nuclei is radioactive and is unstable
therefore, it is not found naturally.
Composition of the nucleus
The nucleus consists of two entities namely, protons and neutrons (collectively known as nucleons). The positive
charge inside the nucleus comes from the protons.
Neutrons are neutral particles. Free neutrons, unlike protons are unstable. It decays into a proton, an electron, and a
antineutrino.
The composition of the nucleus can be described using the following symbols and terms-
Z-atomic number = number of protons
N-neutron number = number if neutrons
A-mass number = Z + N = total number of protons and neutrons.
Note: 1. Notation for nuclear species or nuclides-
Where X is the chemical symbol of the species.
2. Isotopes have the same number of protons but different number of neutrons
3. All nuclides with same mass number are called isobars
4. Nuclides with same neutron number N but different atomic number Z are called isotones
Percentage of A = 100-b2
o.

Mass-Energy Equivalence
According to Einstein’s Special Theory of Relativity, mass is another form of energy and one can convert mass-energy
into other forms of energy and vice-versa.
Mass-energy equivalence relation-
Size of the Nucleus (PYQ 2015, 2013)
The radius of a nucleus of mass number A-
Where R˳ = 1.2 × 10 -15 m
This means that the volume of the nucleus is proportional to A and thus the density of the nucleus is independent of
A. Different nuclei are like drops of liquid of constant density
Nuclear Binding Energy (PYQ 2010)
We know that the nucleus is made up of neutrons and protons. Therefore, it is logical to conclude that the mass of
the nucleus is equal to the total mass of the its individual protons and neutrons. However, the nuclear mass M is
always found to be less than this.
The difference in mass of a nucleus and its constituents, ΔM, is called mass defect and is given by-
Meaning of Mass Defect
Since the mass of the nucleus is less than the sum of masses of its constituents, the equivalent energy of the nucleus
is less than the sum of equivalent energies of its constituents. Therefore, if one wants to break a nucleus into
protons and neutrons, the extra energy ΔMc 2 has to be supplied. This energy required E b is related to mass defect-
If a certain number of neutrons and protons are brought together to form a nucleus of a certain charge and mass, an
energy E b will be released in the process. The energy Eb is called the binding energy of the nucleus. If we separate a
nucleus into its nucleons, we would have to supply a total energy equal to E b , to those particles.
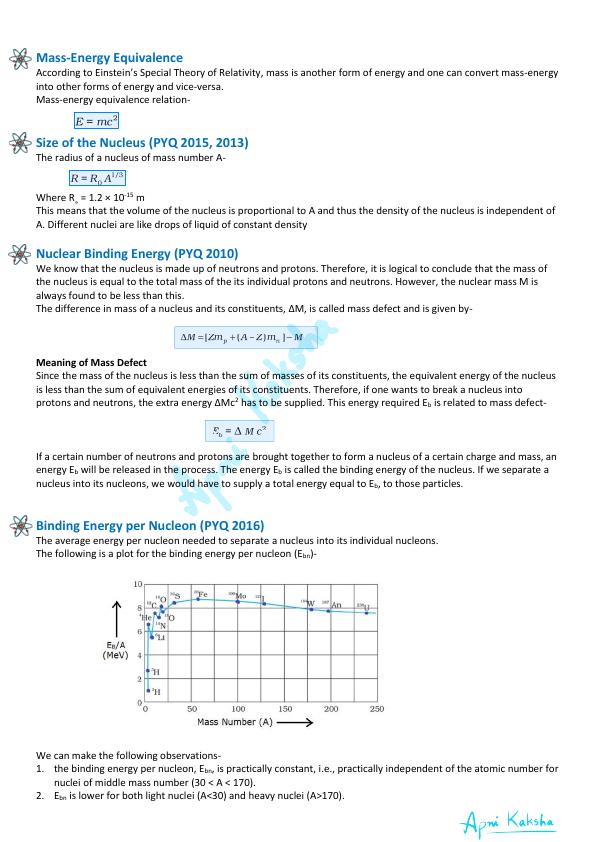
Binding Energy per Nucleon (PYQ 2016)
The average energy per nucleon needed to separate a nucleus into its individual nucleons.
The following is a plot for the binding energy per nucleon (E bn )-
We can make the following observations-
1. the binding energy per nucleon, E bn , is practically constant, i.e., practically independent of the atomic number for
nuclei of middle mass number (30 < A < 170).
2. E bn is lower for both light nuclei (A<30) and heavy nuclei (A>170).
According to Einstein’s Special Theory of Relativity, mass is another form of energy and one can convert mass-energy
into other forms of energy and vice-versa.
Mass-energy equivalence relation-
Size of the Nucleus (PYQ 2015, 2013)
The radius of a nucleus of mass number A-
Where R˳ = 1.2 × 10 -15 m
This means that the volume of the nucleus is proportional to A and thus the density of the nucleus is independent of
A. Different nuclei are like drops of liquid of constant density
Nuclear Binding Energy (PYQ 2010)
We know that the nucleus is made up of neutrons and protons. Therefore, it is logical to conclude that the mass of
the nucleus is equal to the total mass of the its individual protons and neutrons. However, the nuclear mass M is
always found to be less than this.
The difference in mass of a nucleus and its constituents, ΔM, is called mass defect and is given by-
Meaning of Mass Defect
Since the mass of the nucleus is less than the sum of masses of its constituents, the equivalent energy of the nucleus
is less than the sum of equivalent energies of its constituents. Therefore, if one wants to break a nucleus into
protons and neutrons, the extra energy ΔMc 2 has to be supplied. This energy required E b is related to mass defect-
If a certain number of neutrons and protons are brought together to form a nucleus of a certain charge and mass, an
energy E b will be released in the process. The energy Eb is called the binding energy of the nucleus. If we separate a
nucleus into its nucleons, we would have to supply a total energy equal to E b , to those particles.
Binding Energy per Nucleon (PYQ 2016)
The average energy per nucleon needed to separate a nucleus into its individual nucleons.
The following is a plot for the binding energy per nucleon (E bn )-
We can make the following observations-
1. the binding energy per nucleon, E bn , is practically constant, i.e., practically independent of the atomic number for
nuclei of middle mass number (30 < A < 170).
2. E bn is lower for both light nuclei (A<30) and heavy nuclei (A>170).
We can draw the following conclusions from the plot-
1. The force is attractive and sufficiently strong to produce a binding energy of a few MeV per nucleon.
2. The constancy of the binding energy in the range 30 < A < 170 is a consequence of the fact that the nuclear force
is short-ranged. Consider a particular nucleon inside a sufficiently large nucleus. It will be under the influence of
only some of its neighbors, which come within the range of the nuclear force. If any other nucleon is at a
distance more than the range of the nuclear force from the particular nucleon it will have no influence on the
binding energy of the nucleon under consideration. If a nucleon can have a maximum of p neighbors within the
range of nuclear force, its binding energy would be proportional to p. Let the binding energy of the nucleus be
pk, where k is a constant having the dimensions of energy. If we increase A by adding nucleons, they will not
change the binding energy of a nucleon inside. Since most of the nucleons in a large nucleus reside inside it and
not on the surface, the change in binding energy per nucleon would be small. The binding energy per nucleon is
a constant and is approximately equal to pk. This is called saturation property of nuclear force.
3. A very heavy nucleus, say A = 240, has lower binding energy per nucleon compared to that of a nucleus with A =
120. Thus, if a nucleus A = 240 breaks into two A = 120 nuclei, nucleons get more tightly bound. This implies
energy would be released in the process. This is called nuclear fission.
4. Consider two very light nuclei (A ≤ 10) joining to form a heavier nucleus. The binding energy per nucleon of the
fused heavier nuclei is more than the binding energy per nucleon of the lighter nuclei. This means that the final
system is more tightly bound than the initial system. Therefore, energy would be released in such a process of
fusion.
Important PYQs
Ques: A nucleus with mass number A = 240 and B.E./A = 7.6 MeV and breaks into two fragments of each A = 120
with B.E./A = 8.5 MeV. Calculate the released energy (PYQ 2016) [2M]
Ans:
Nuclear Force (PYQ 2018, 2011)
1. The nuclear force is much stronger than the Coulomb force acting between
charges or the gravitational forces between masses.
2. The nuclear force between two nucleons falls rapidly to zero as their distance
is more than a few femtometers. This leads to saturation of forces in a
medium or a large-sized nucleus, which is the reason for the constancy of
the binding energy per nucleon.
3. The nuclear force between neutron-neutron, proton-neutron and
proton-proton is approximately the same. The nuclear force does not depend
on the electric charge.
Radioactivity (PYQ 2020)
Radioactivity is a nuclear phenomenon in which an unstable nucleus undergoes a decay. This is referred to as
radioactive decay. Three types of radioactive decay occur in nature:
1. α-decay in which a helium nucleus 42He is emitted
2. β-decay in which electrons or positrons (particles with the same mass as electrons, but with a charge exactly
opposite to that of electron) are emitted
3. ɣ-decay in which high energy (hundreds of keV or more) photons are emitted.
Ini5al binding energy =
Final Binding energy =
Released energy =
iEf
1. The force is attractive and sufficiently strong to produce a binding energy of a few MeV per nucleon.
2. The constancy of the binding energy in the range 30 < A < 170 is a consequence of the fact that the nuclear force
is short-ranged. Consider a particular nucleon inside a sufficiently large nucleus. It will be under the influence of
only some of its neighbors, which come within the range of the nuclear force. If any other nucleon is at a
distance more than the range of the nuclear force from the particular nucleon it will have no influence on the
binding energy of the nucleon under consideration. If a nucleon can have a maximum of p neighbors within the
range of nuclear force, its binding energy would be proportional to p. Let the binding energy of the nucleus be
pk, where k is a constant having the dimensions of energy. If we increase A by adding nucleons, they will not
change the binding energy of a nucleon inside. Since most of the nucleons in a large nucleus reside inside it and
not on the surface, the change in binding energy per nucleon would be small. The binding energy per nucleon is
a constant and is approximately equal to pk. This is called saturation property of nuclear force.
3. A very heavy nucleus, say A = 240, has lower binding energy per nucleon compared to that of a nucleus with A =
120. Thus, if a nucleus A = 240 breaks into two A = 120 nuclei, nucleons get more tightly bound. This implies
energy would be released in the process. This is called nuclear fission.
4. Consider two very light nuclei (A ≤ 10) joining to form a heavier nucleus. The binding energy per nucleon of the
fused heavier nuclei is more than the binding energy per nucleon of the lighter nuclei. This means that the final
system is more tightly bound than the initial system. Therefore, energy would be released in such a process of
fusion.
Important PYQs
Ques: A nucleus with mass number A = 240 and B.E./A = 7.6 MeV and breaks into two fragments of each A = 120
with B.E./A = 8.5 MeV. Calculate the released energy (PYQ 2016) [2M]
Ans:
Nuclear Force (PYQ 2018, 2011)
1. The nuclear force is much stronger than the Coulomb force acting between
charges or the gravitational forces between masses.
2. The nuclear force between two nucleons falls rapidly to zero as their distance
is more than a few femtometers. This leads to saturation of forces in a
medium or a large-sized nucleus, which is the reason for the constancy of
the binding energy per nucleon.
3. The nuclear force between neutron-neutron, proton-neutron and
proton-proton is approximately the same. The nuclear force does not depend
on the electric charge.
Radioactivity (PYQ 2020)
Radioactivity is a nuclear phenomenon in which an unstable nucleus undergoes a decay. This is referred to as
radioactive decay. Three types of radioactive decay occur in nature:
1. α-decay in which a helium nucleus 42He is emitted
2. β-decay in which electrons or positrons (particles with the same mass as electrons, but with a charge exactly
opposite to that of electron) are emitted
3. ɣ-decay in which high energy (hundreds of keV or more) photons are emitted.
Ini5al binding energy =
Final Binding energy =
Released energy =
iEf

End of preview
Want to access all the pages? Upload your documents or become a member.
