Comparative Analysis of Boiling Points in Organic Chemistry: A Report
VerifiedAdded on 2020/03/28
|7
|1498
|818
Report
AI Summary
This report delves into the boiling points of various organic compounds, specifically comparing 1-pentanol, 2-pentanol, 2-pentanone, and 1-hexanol. The introduction defines boiling point and explains the influence of intermolecular forces like hydrogen bonds, dipole-dipole interactions, and Van der Waals forces on the boiling points of organic compounds. The report then compares the boiling points of the four compounds, correlating them with their molecular structures and the strength of intermolecular forces. It highlights that alcohols have higher boiling points due to hydrogen bonding, with primary alcohols exhibiting higher boiling points than secondary alcohols due to greater exposure of the O-H group. Ketones, lacking hydrogen bonds, demonstrate lower boiling points than alcohols. The report concludes by summarizing the order of boiling points and the underlying reasons for these differences, referencing the molecular structures and intermolecular forces involved. The analysis underscores how the nature and strength of intermolecular forces, which are influenced by the polarity and structure of the molecules, dictate the boiling points of organic compounds. The report utilizes the provided data to explain the observed trends in boiling points.

Running head: ORGANIC CHEMISTRY 1
Organic Chemistry
Professor’s Name:
Name:
Date:
Organic Chemistry
Professor’s Name:
Name:
Date:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ORGANIC CHEMISTRY 2
Introduction
The boiling point is defined as the temperature at which a pure substance under normal pressure
of 1 atmosphere changes from liquid phase to gaseous phase (Ophardt, 2003). The molecules of
a liquid are packed closely and held together by inter-molecular forces of attraction. When a
liquid is heated, the molecules acquire kinetic energy resulting to increased vibrations. As more
energy is supplied, the vibrations become intense enough to overcome the intermolecular forces
and the molecules break free becoming a gas (Ophardt, 2003). Gas molecules are not in contact
with each other. The boiling points of organic compounds depend on the strength of the inter-
molecular forces between the molecules. Stronger intermolecular forces require a lot of energy to
overcome resulting to higher boiling points. Among the common inter-molecular forces include
hydrogen bonds, dipole-dipole interactions and Van der Waals (London dispersion forces)
(Reusch, 1999). The strongest intermolecular forces are Hydrogen bonds while the weakest are
London dispersion forces (Clayden, 2012)
The nature of inter-molecular forces of attraction between molecules is dependent on the polarity
of the molecules (Ophardt, 2003). Highly polar molecules are held together by very strong
intermolecular forces of attraction (Hydrogen bonds and dipole-dipole interactions) leading to
high boiling points while non-polar molecules have very weak intermolecular associations
(London dispersion forces) leading to low boiling point. The degree of polarity of a molecule is
determined by the nature of the functional group present (Ophardt, 2003).
Comparison of the boiling points of given compounds
.The boiling points of 1-pentanol, 2-pentanol, 2-pentanone and 1-hexanol together with their
formulae and molar masses are as tabulated in table 1.
Introduction
The boiling point is defined as the temperature at which a pure substance under normal pressure
of 1 atmosphere changes from liquid phase to gaseous phase (Ophardt, 2003). The molecules of
a liquid are packed closely and held together by inter-molecular forces of attraction. When a
liquid is heated, the molecules acquire kinetic energy resulting to increased vibrations. As more
energy is supplied, the vibrations become intense enough to overcome the intermolecular forces
and the molecules break free becoming a gas (Ophardt, 2003). Gas molecules are not in contact
with each other. The boiling points of organic compounds depend on the strength of the inter-
molecular forces between the molecules. Stronger intermolecular forces require a lot of energy to
overcome resulting to higher boiling points. Among the common inter-molecular forces include
hydrogen bonds, dipole-dipole interactions and Van der Waals (London dispersion forces)
(Reusch, 1999). The strongest intermolecular forces are Hydrogen bonds while the weakest are
London dispersion forces (Clayden, 2012)
The nature of inter-molecular forces of attraction between molecules is dependent on the polarity
of the molecules (Ophardt, 2003). Highly polar molecules are held together by very strong
intermolecular forces of attraction (Hydrogen bonds and dipole-dipole interactions) leading to
high boiling points while non-polar molecules have very weak intermolecular associations
(London dispersion forces) leading to low boiling point. The degree of polarity of a molecule is
determined by the nature of the functional group present (Ophardt, 2003).
Comparison of the boiling points of given compounds
.The boiling points of 1-pentanol, 2-pentanol, 2-pentanone and 1-hexanol together with their
formulae and molar masses are as tabulated in table 1.
ORGANIC CHEMISTRY 3
Table1: formula, mass and boiling point of given organic compounds (Lide, 2005; O’Neil, 2001;
Haynes, 2014)
Compound Molecular formula Structural formula Molar mass (g) Boiling point (0C)
1-pentanol C5H12O CH3(CH2)3CH2OH 88.15 138
2-pentanol C5H12O CH3(CH2)2CHOHCH3 88.15 119
2-pentanone C5H10O CH3(CH2)2COCH3 86.134 102
1-hexanol C6H14O CH3(CH2)4CH2OH 102.177 157
The boiling points of the four compounds increase in the order: 2-pentanone<2-pentanol<1-
pentanol<1-hexanol. 2-pentanone has the lowest boiling point while 1-hexanol has the highest
boiling point. The differences in the boiling points arise due to differences in the molecular
structures of the compounds (Brown, 2000) and the type of intermolecular forces existing
between the molecules of each compound (Carrey, 2001). Boiling points of organic compounds
increase with increase in molecular mass (Brown, 2000). For compounds with comparable
molecular masses, the boiling points depend on the nature of the inter-molecular forces between
the molecules.
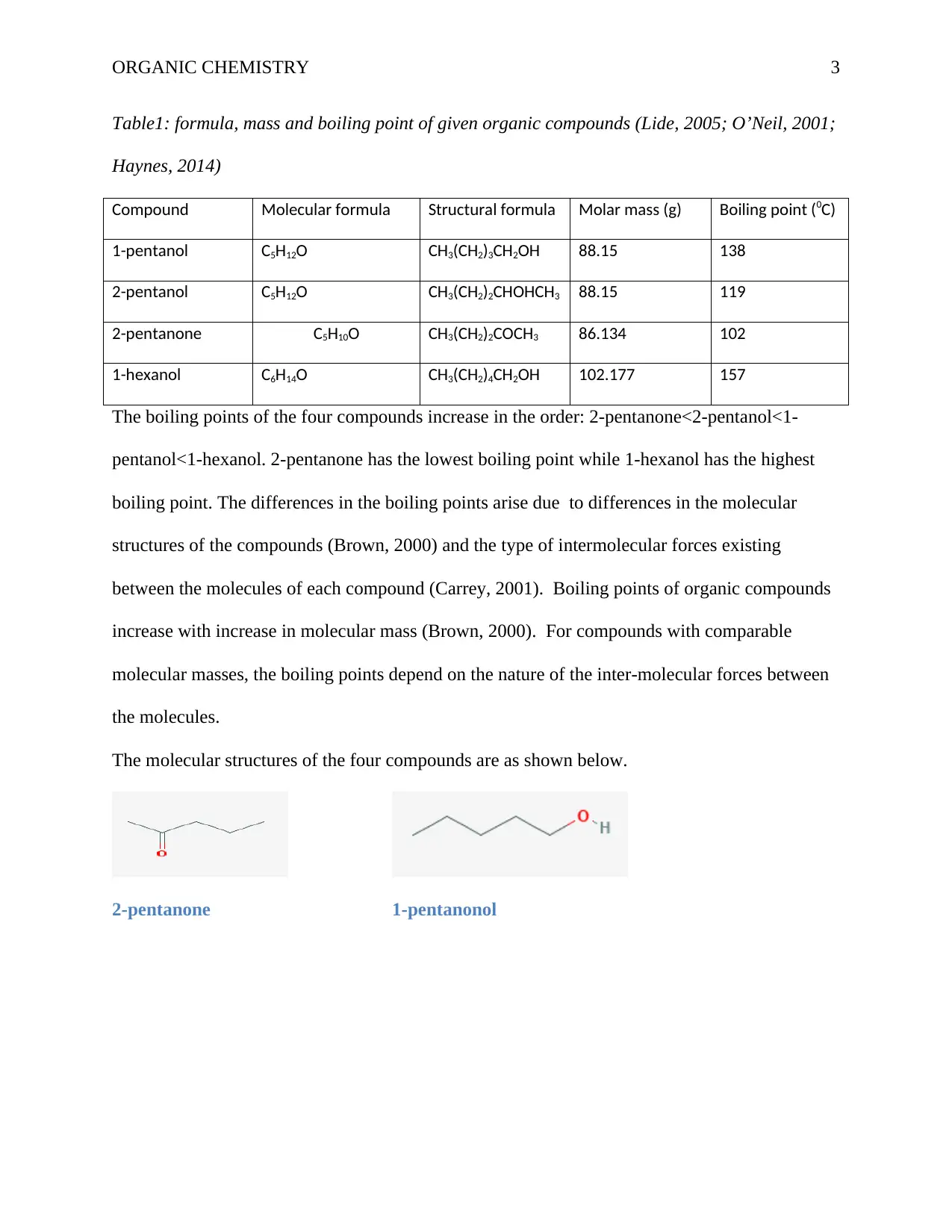
The molecular structures of the four compounds are as shown below.
2-pentanone 1-pentanonol
Table1: formula, mass and boiling point of given organic compounds (Lide, 2005; O’Neil, 2001;
Haynes, 2014)
Compound Molecular formula Structural formula Molar mass (g) Boiling point (0C)
1-pentanol C5H12O CH3(CH2)3CH2OH 88.15 138
2-pentanol C5H12O CH3(CH2)2CHOHCH3 88.15 119
2-pentanone C5H10O CH3(CH2)2COCH3 86.134 102
1-hexanol C6H14O CH3(CH2)4CH2OH 102.177 157
The boiling points of the four compounds increase in the order: 2-pentanone<2-pentanol<1-
pentanol<1-hexanol. 2-pentanone has the lowest boiling point while 1-hexanol has the highest
boiling point. The differences in the boiling points arise due to differences in the molecular
structures of the compounds (Brown, 2000) and the type of intermolecular forces existing
between the molecules of each compound (Carrey, 2001). Boiling points of organic compounds
increase with increase in molecular mass (Brown, 2000). For compounds with comparable
molecular masses, the boiling points depend on the nature of the inter-molecular forces between
the molecules.
The molecular structures of the four compounds are as shown below.
2-pentanone 1-pentanonol
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
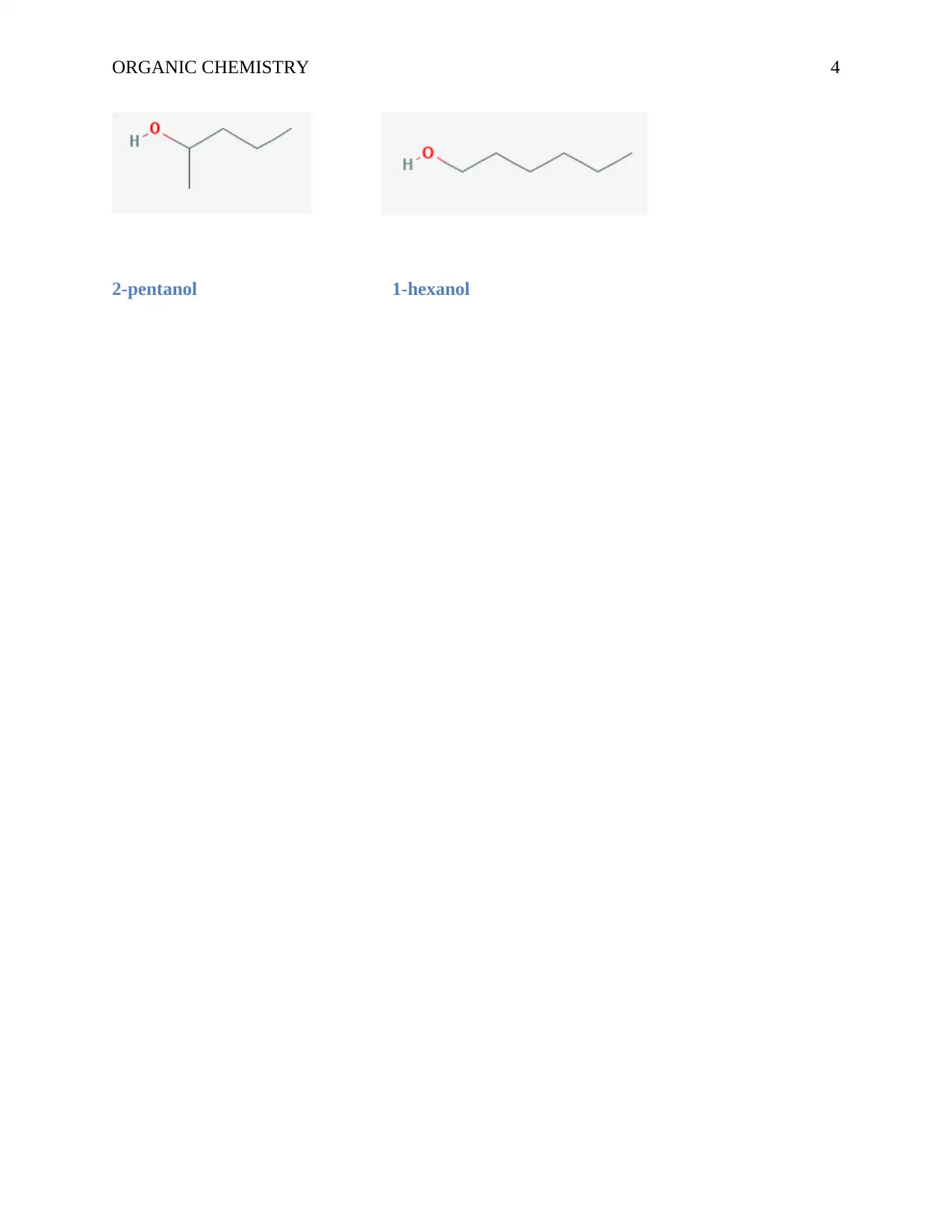
ORGANIC CHEMISTRY 4
2-pentanol 1-hexanol
2-pentanol 1-hexanol
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

ORGANIC CHEMISTRY 5
1-pentanol, 2-pentanol and 1-hexanol are all alcohols. In alcohols, the oxygen atom is directly
bonded to a hydrogen atom. Because O is highly electronegative, it attracts the electrons of the
O-H bond, acquiring a partial negative charge and the H acquires a partial positive charged. The
positive H attracts lone-electrons from O atom of neighboring molecules resulting to the
formation of hydrogen bonds. Hydrogen bonds are the strongest intermolecular forces and this
explains why alcohols have unusually high boiling points when compared to other organic
compounds of comparable molecular size (Solomons, 2008).
Among alcohols, the boiling point increase with increase in molecular weight. As the carbon
chain increases, the van der Waals dispersion forces between the molecules become stronger due
to additional electrons and these results to increased boiling point for longer chain alcohols
(wade, 2014). 1-pentanol and 2-pentanol have same molecular mass (88.15 g) because they both
have 5 carbon atoms. 1-hexanol on the other hand, has 6 carbon atoms and a higher molecular
mass (102.177 g). The boiling point of 1-hexanol (157 0C) is higher than that of both 1-pentanol
(138 0C) and 2-pentanol (119 0C) due to stronger van der Waals arising from the increased length
of the carbon chain.
For alcohols with the same molecular weight, the boiling points vary with the strength of
hydrogen bonds which is directly impacted by the extent of exposure of the O-H bond.
Generally, primary alcohols have higher boiling points, followed by secondary alcohols and
tertiary alcohols have the lowest boiling points (Wade, 2014). In primary alcohols, the O-H is
more exposed and can readily interact with other molecules leading to stronger hydrogen bonds
and higher boiling point.
1-pentanol, 2-pentanol and 1-hexanol are all alcohols. In alcohols, the oxygen atom is directly
bonded to a hydrogen atom. Because O is highly electronegative, it attracts the electrons of the
O-H bond, acquiring a partial negative charge and the H acquires a partial positive charged. The
positive H attracts lone-electrons from O atom of neighboring molecules resulting to the
formation of hydrogen bonds. Hydrogen bonds are the strongest intermolecular forces and this
explains why alcohols have unusually high boiling points when compared to other organic
compounds of comparable molecular size (Solomons, 2008).
Among alcohols, the boiling point increase with increase in molecular weight. As the carbon
chain increases, the van der Waals dispersion forces between the molecules become stronger due
to additional electrons and these results to increased boiling point for longer chain alcohols
(wade, 2014). 1-pentanol and 2-pentanol have same molecular mass (88.15 g) because they both
have 5 carbon atoms. 1-hexanol on the other hand, has 6 carbon atoms and a higher molecular
mass (102.177 g). The boiling point of 1-hexanol (157 0C) is higher than that of both 1-pentanol
(138 0C) and 2-pentanol (119 0C) due to stronger van der Waals arising from the increased length
of the carbon chain.
For alcohols with the same molecular weight, the boiling points vary with the strength of
hydrogen bonds which is directly impacted by the extent of exposure of the O-H bond.
Generally, primary alcohols have higher boiling points, followed by secondary alcohols and
tertiary alcohols have the lowest boiling points (Wade, 2014). In primary alcohols, the O-H is
more exposed and can readily interact with other molecules leading to stronger hydrogen bonds
and higher boiling point.

ORGANIC CHEMISTRY 6
1-pentanol and 2-pentanol have the same molecular mass. However, 1-pentanol is a primary
alcohol and 2-pentanol is a secondary alcohol. In 1-pentanol, the O-H group is located at the
terminal end of the chain and linked with only 1 alkyl group. The O-H group is more exposed
and can interact with the O-H of many neighboring molecules leading to strong hydrogen bonds
and this explains why the boiling point of 1-pentanol is higher than that of 2-pentanol. In 2-
pentanol, the O-H is linked to two alkyl groups. The presence of many alky groups hinders the
interaction of the O-H group with many adjacent molecules, leading to relatively weaker
hydrogen bonds as compared to those formed by 1-pentanol. Consequently, the boiling point of
2-pentanol is relatively lower than that of 1-pentanol.
2-pentanone has the lowest boiling point when compared to the rest of the compounds which are
alcohols with comparable masses. 2-pentanone is a ketone with 5 carbon atoms. In 2-pentanone,
carbonyl bond (O=C) is highly polar. Since O is more electronegative than C, it pulls the
electrons of the bond towards itself, making the O end of the bond partially negatively charged
and the C end partially positive. This results to the formation of a dipole. Positive end of one
molecule attracts the negative end of neighboring molecules and thus, the molecules are held
together by dipole-dipole forces of attraction between the molecules (Solomon, 2008). Dipole-
dipole attractions are relatively strong and this gives 2-pentanone the significantly high boiling
point (102 0C). The boiling point of 2-pentanone is lower than that of the corresponding alcohols
because the dipole-dipole attractions are not as strong as the hydrogen bonding found in
alcohols.
Conclusion
1-pentanol and 2-pentanol have the same molecular mass. However, 1-pentanol is a primary
alcohol and 2-pentanol is a secondary alcohol. In 1-pentanol, the O-H group is located at the
terminal end of the chain and linked with only 1 alkyl group. The O-H group is more exposed
and can interact with the O-H of many neighboring molecules leading to strong hydrogen bonds
and this explains why the boiling point of 1-pentanol is higher than that of 2-pentanol. In 2-
pentanol, the O-H is linked to two alkyl groups. The presence of many alky groups hinders the
interaction of the O-H group with many adjacent molecules, leading to relatively weaker
hydrogen bonds as compared to those formed by 1-pentanol. Consequently, the boiling point of
2-pentanol is relatively lower than that of 1-pentanol.
2-pentanone has the lowest boiling point when compared to the rest of the compounds which are
alcohols with comparable masses. 2-pentanone is a ketone with 5 carbon atoms. In 2-pentanone,
carbonyl bond (O=C) is highly polar. Since O is more electronegative than C, it pulls the
electrons of the bond towards itself, making the O end of the bond partially negatively charged
and the C end partially positive. This results to the formation of a dipole. Positive end of one
molecule attracts the negative end of neighboring molecules and thus, the molecules are held
together by dipole-dipole forces of attraction between the molecules (Solomon, 2008). Dipole-
dipole attractions are relatively strong and this gives 2-pentanone the significantly high boiling
point (102 0C). The boiling point of 2-pentanone is lower than that of the corresponding alcohols
because the dipole-dipole attractions are not as strong as the hydrogen bonding found in
alcohols.
Conclusion
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

ORGANIC CHEMISTRY 7
The boiling points of the given compounds are in the order: 2-pentanone<2-pentanol<1-
pentanol<1-hexanol. 2-pentanone has the lowest boiling point while 1-hexanol has the highest
boiling point. Alcohols have high boiling point because of the hydrogen bonding between O and
H of neighboring molecules. Primary alcohols have higher boiling point than secondary alcohols
because the O-H is more exposed in 1o alcohol forming stronger hydrogen bonds. Ketones have
lower boiling point than alcohols because they lack hydrogen bonds.
The boiling points of the given compounds are in the order: 2-pentanone<2-pentanol<1-
pentanol<1-hexanol. 2-pentanone has the lowest boiling point while 1-hexanol has the highest
boiling point. Alcohols have high boiling point because of the hydrogen bonding between O and
H of neighboring molecules. Primary alcohols have higher boiling point than secondary alcohols
because the O-H is more exposed in 1o alcohol forming stronger hydrogen bonds. Ketones have
lower boiling point than alcohols because they lack hydrogen bonds.
1 out of 7
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





