Synthesis of trans-Stilbene via Witting Reaction in Organic Chemistry
VerifiedAdded on 2021/04/16
|8
|865
|397
Practical Assignment
AI Summary
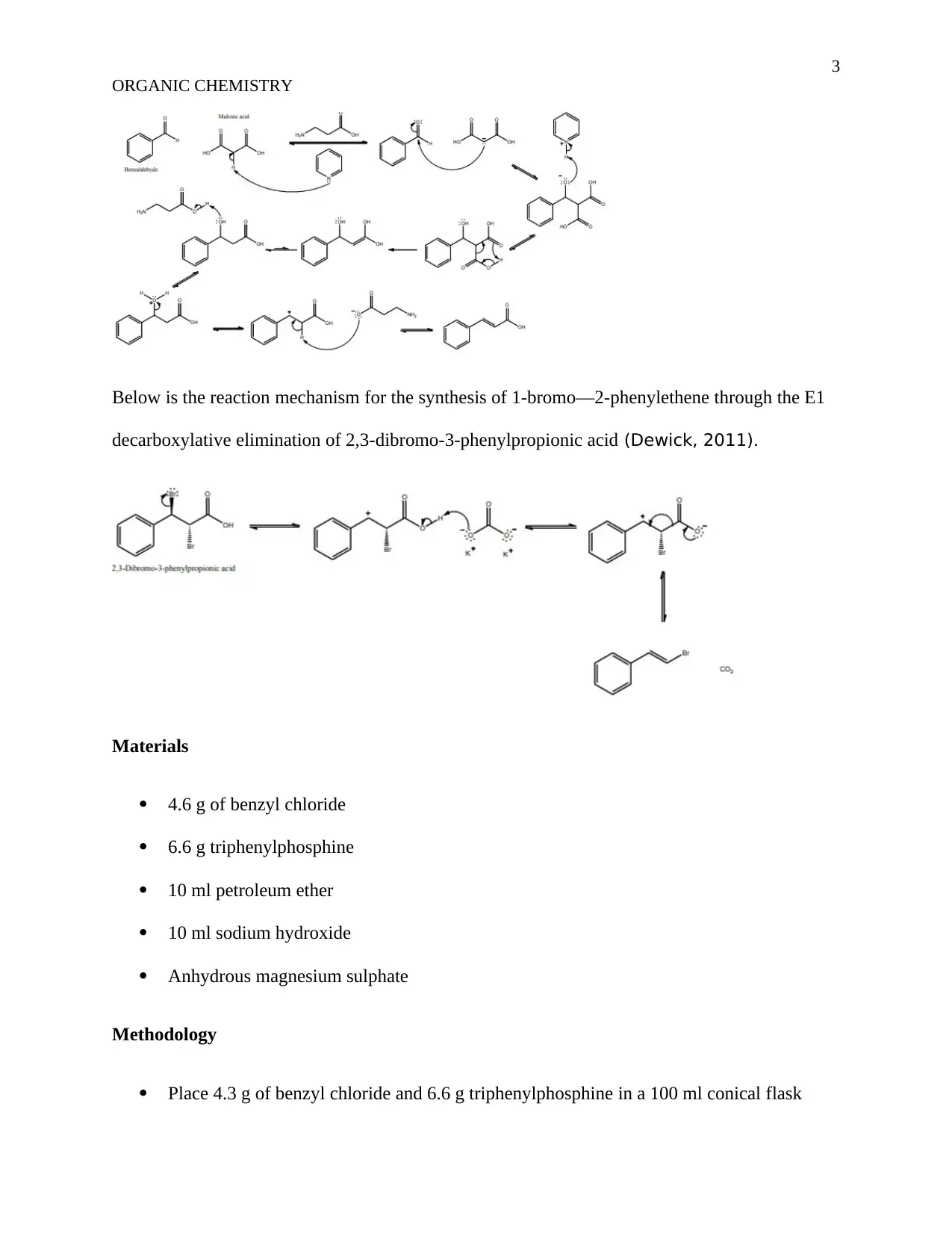
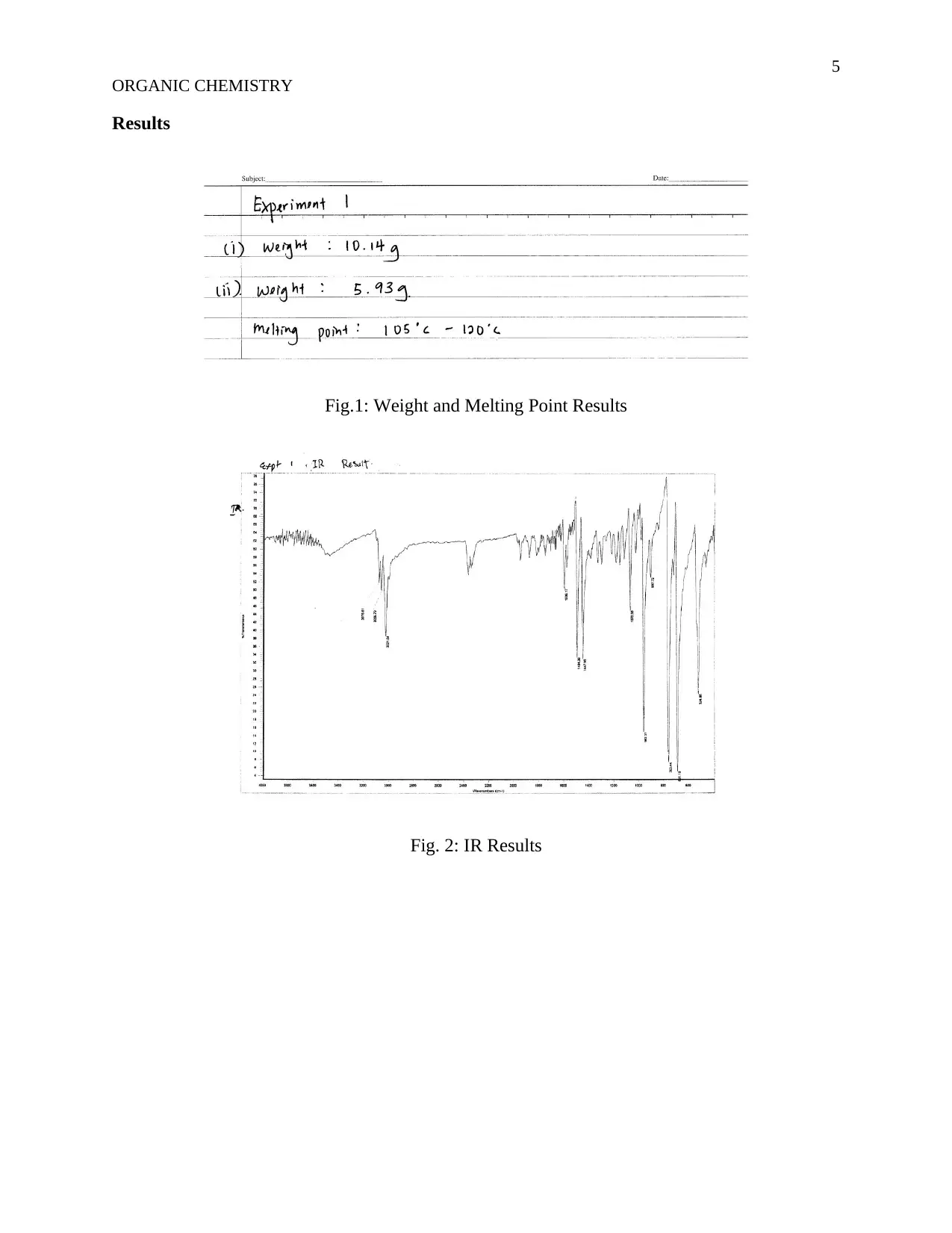
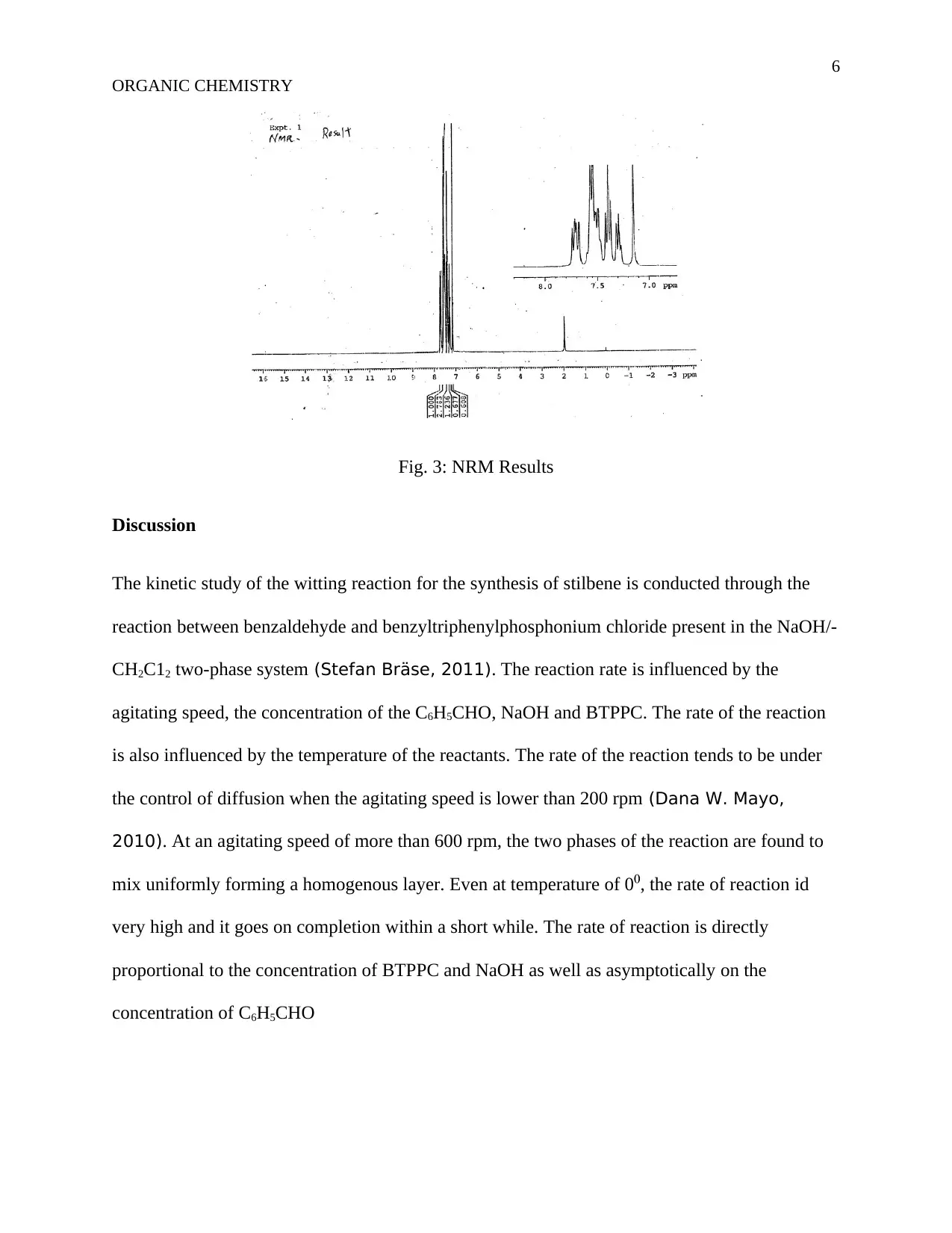
This assignment details an organic chemistry experiment focused on the synthesis of trans-Stilbene using the Wittig reaction. The objective is to prepare trans-Stilbene, with the introduction explaining the reaction mechanism, including the formation of betaine and oxaphosphetane intermediates. The methodology involves reacting benzyl chloride and triphenylphosphine, followed by the addition of benzaldehyde and benzyltriphenylphosphonium chloride with sodium hydroxide. The experiment includes detailed procedures, materials used (benzyl chloride, triphenylphosphine, petroleum ether, sodium hydroxide, and anhydrous magnesium sulphate), and results presented through figures showing weight, melting point, IR spectra, and NMR results. The discussion analyzes the reaction kinetics, the influence of agitation speed, and the concentrations of reactants on the reaction rate. The experiment concludes with a 97% yield of trans-Stilbene, demonstrating the effectiveness of the Wittig reaction. The document references several key sources related to organic synthesis and reaction mechanisms.
1 out of 8










![[object Object]](/_next/static/media/star-bottom.7253800d.svg)