CHEM 201 Lab: Selective Reductions of m-Nitroacetophenone Experiment
VerifiedAdded on 2021/04/17
|9
|1125
|378
Practical Assignment
AI Summary
This assignment details a chemistry experiment focused on the selective reduction of m-nitroacetophenone. The experiment explores the use of tin and hydrochloric acid, as well as sodium borohydride, as reducing agents. The objectives include determining the reduction of m-nitroacetophenone with these agents. The methodology involves weighing and reacting the compound with the reducing agents, followed by refluxing, cooling, filtration, and analysis via IR spectroscopy. The results are presented, followed by a discussion of the reaction mechanisms, including the conversion of nitrobenzene to aniline and the role of tin in the reduction process. The assignment concludes with a summary of the findings, supporting the theory of reduction and oxidation in organic compounds.

Running head: ORGANIC CHEMISTRY
ORGANIC CHEMISTRY
[Author Name(s), First M. Last, Omit Titles and Degrees]
[Institutional Affiliation(s)]
ORGANIC CHEMISTRY
[Author Name(s), First M. Last, Omit Titles and Degrees]
[Institutional Affiliation(s)]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
2
ORGANIC CHEMISTRY
Title: Selective Reductions of m-Nitroacetophenone with Tin and Sodium Borohydride
Objectives
To determine the reduction of m-nitroacetophenone with tin
To determine the reduction of m-nitroacetophenone with sodium borohydride
Introduction
Reduction and oxidation is defined in organic chemistry as a gain in hydrogen atoms and oxygen
atoms respectively. For this experiment, tin (Lewis acid) and sodium borohydride (NaBH4) are
used to reduce m-nitroacetophenone. Lewis acid refers to a metal that is able to donate electrons
when reducing organic compounds. In the first part of this experiment, tin and hydrochloric acid
are used as the reducing agents to reduce m-nitroacetophenone (Dewick, 2013). The metal, tin,
donates its electrons to the organic compound hence the metal becomes oxidized to forming an
ion with the formula Sn2+. By the end of the first part of the experiment, the resulting solution is
found to be basic and tin changes to tin oxide, SnO. The process of reduction in the reduction
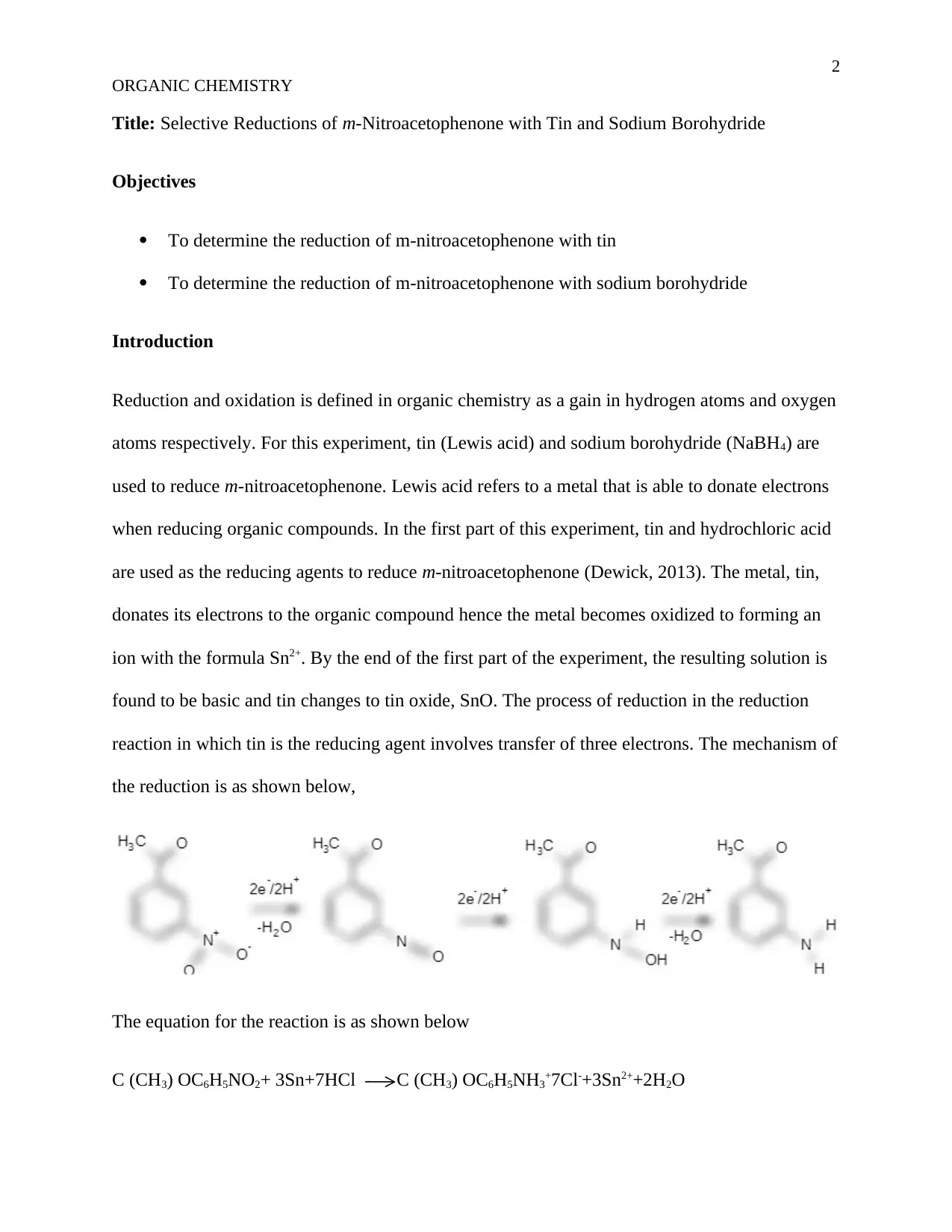
reaction in which tin is the reducing agent involves transfer of three electrons. The mechanism of
the reduction is as shown below,
The equation for the reaction is as shown below
C (CH3) OC6H5NO2+ 3Sn+7HCl C (CH3) OC6H5NH3+7Cl-+3Sn2++2H2O
ORGANIC CHEMISTRY
Title: Selective Reductions of m-Nitroacetophenone with Tin and Sodium Borohydride
Objectives
To determine the reduction of m-nitroacetophenone with tin
To determine the reduction of m-nitroacetophenone with sodium borohydride
Introduction
Reduction and oxidation is defined in organic chemistry as a gain in hydrogen atoms and oxygen
atoms respectively. For this experiment, tin (Lewis acid) and sodium borohydride (NaBH4) are
used to reduce m-nitroacetophenone. Lewis acid refers to a metal that is able to donate electrons
when reducing organic compounds. In the first part of this experiment, tin and hydrochloric acid
are used as the reducing agents to reduce m-nitroacetophenone (Dewick, 2013). The metal, tin,
donates its electrons to the organic compound hence the metal becomes oxidized to forming an
ion with the formula Sn2+. By the end of the first part of the experiment, the resulting solution is
found to be basic and tin changes to tin oxide, SnO. The process of reduction in the reduction
reaction in which tin is the reducing agent involves transfer of three electrons. The mechanism of
the reduction is as shown below,
The equation for the reaction is as shown below
C (CH3) OC6H5NO2+ 3Sn+7HCl C (CH3) OC6H5NH3+7Cl-+3Sn2++2H2O
3
ORGANIC CHEMISTRY
Sodium borohydride is preferred for use as a reducing agent in comparison with other reducing
agents due to its less reactive nature. It is usable in the reduction of ketones and aldehydes while
on the other hand aluminium hydride can be used in the reduction of carboxylic acid and esters
among other organic compounds. In the subsequent part of the experiment, sodium borohydride
is being used to reduce m-nitroacetophenone. The functional group of ketone is reduced to an
alcohol branch through the transfer of the hydride to the m-nitroacetophenone (Philippa B.
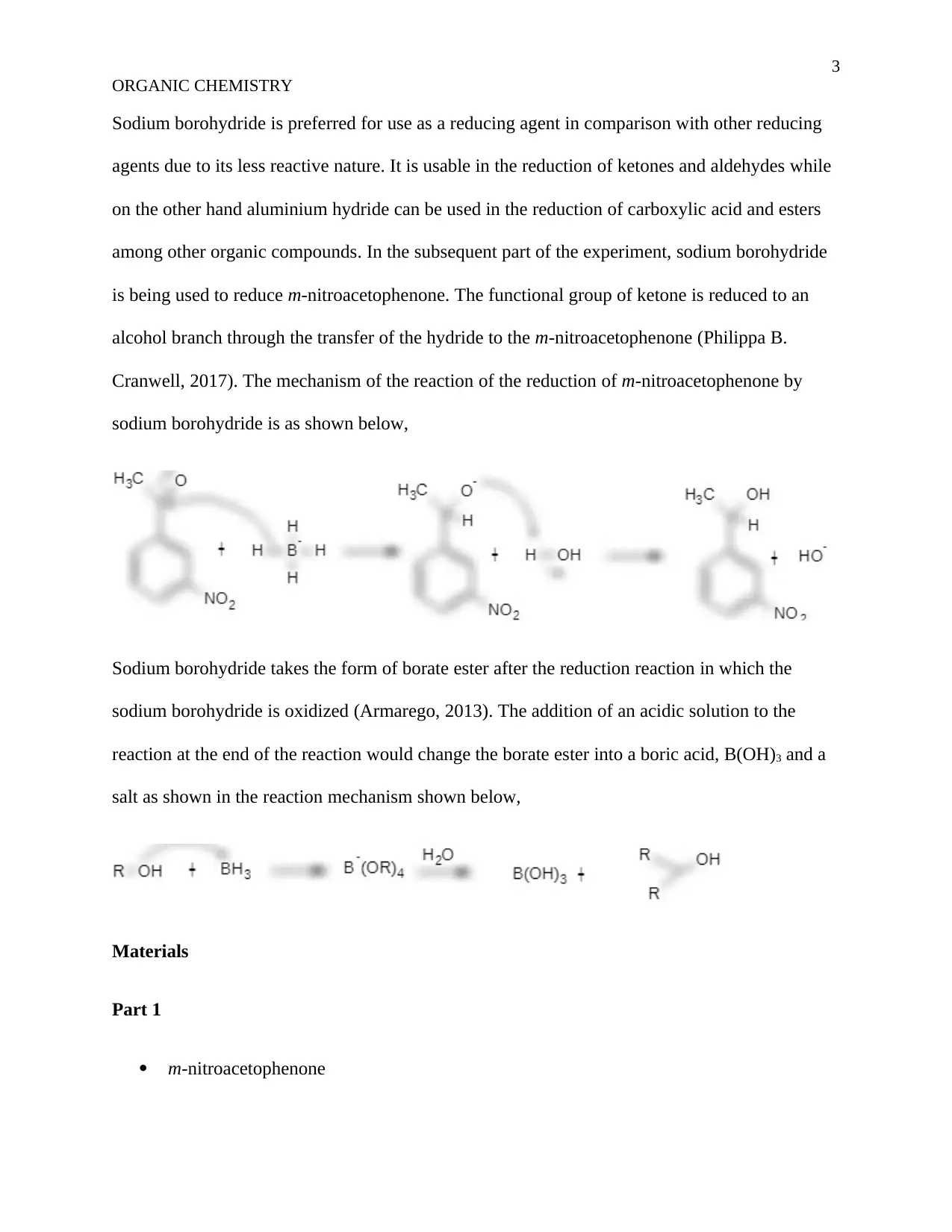
Cranwell, 2017). The mechanism of the reaction of the reduction of m-nitroacetophenone by
sodium borohydride is as shown below,
Sodium borohydride takes the form of borate ester after the reduction reaction in which the
sodium borohydride is oxidized (Armarego, 2013). The addition of an acidic solution to the
reaction at the end of the reaction would change the borate ester into a boric acid, B(OH)3 and a
salt as shown in the reaction mechanism shown below,
Materials
Part 1
m-nitroacetophenone
ORGANIC CHEMISTRY
Sodium borohydride is preferred for use as a reducing agent in comparison with other reducing
agents due to its less reactive nature. It is usable in the reduction of ketones and aldehydes while
on the other hand aluminium hydride can be used in the reduction of carboxylic acid and esters
among other organic compounds. In the subsequent part of the experiment, sodium borohydride
is being used to reduce m-nitroacetophenone. The functional group of ketone is reduced to an
alcohol branch through the transfer of the hydride to the m-nitroacetophenone (Philippa B.
Cranwell, 2017). The mechanism of the reaction of the reduction of m-nitroacetophenone by
sodium borohydride is as shown below,
Sodium borohydride takes the form of borate ester after the reduction reaction in which the
sodium borohydride is oxidized (Armarego, 2013). The addition of an acidic solution to the
reaction at the end of the reaction would change the borate ester into a boric acid, B(OH)3 and a
salt as shown in the reaction mechanism shown below,
Materials
Part 1
m-nitroacetophenone
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

4
ORGANIC CHEMISTRY
6M HCl
Granulated tin
0.5g of sodium hydroxide
2.5 ml absolute ethanol
Water
Ethyl acetate
Dichloromethane
Sodium hydrogen carbonate
Part 2
m-nitroacetophenone
Water
Sodium borohydride
HCl
Anhydrous ethanol
Dichloromethane
Methodology
Part 1
1. Weigh m-nitroacetophenone (0.1 g, 0.605 mmol) into a 10 ml pear-shaped flask
2. Weigh 0.2 g, 1.96 mmol granulated tin on an ordinary top loader (to the nearest 0.01 g)
and add it to the flask through a small funnel
3. Add 2 ml of HCl
4. Add one magnetic stirrer into the contents of the flask
ORGANIC CHEMISTRY
6M HCl
Granulated tin
0.5g of sodium hydroxide
2.5 ml absolute ethanol
Water
Ethyl acetate
Dichloromethane
Sodium hydrogen carbonate
Part 2
m-nitroacetophenone
Water
Sodium borohydride
HCl
Anhydrous ethanol
Dichloromethane
Methodology
Part 1
1. Weigh m-nitroacetophenone (0.1 g, 0.605 mmol) into a 10 ml pear-shaped flask
2. Weigh 0.2 g, 1.96 mmol granulated tin on an ordinary top loader (to the nearest 0.01 g)
and add it to the flask through a small funnel
3. Add 2 ml of HCl
4. Add one magnetic stirrer into the contents of the flask
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

5
ORGANIC CHEMISTRY
5. Slowly swirl the mixture
6. Set up the condenser and reflux the mixture for 30 minutes to dissolve all the tin
7. Add about 0.5 g of sodium hydroxide to 1.2 ml of water in a beaker and set it aside to
cool
8. Cool the reaction solution after the reflux and add the sodium hydroxide solution in ice
for a few minutes
9. Add all the aqueous base to the reaction solution to precipitate the product and convert
Sn(IV) to a soluble stannate
10. Shake the pale yellow slurry to ensure thorough mixing and cool the pale yellow
suspension in ice
11. Filter the pale yellow precipitate through vacuum filtration, rinse the last bit of solid from
the flask with a small amount of ice water and wash the filtered solid with a few drops of
ice water
12. Allow the product to dry and label it
Obtain an IR spectrum of the product.
Part 2
1. Dissolve 0.2 g (1.21 mmol) of m-nitroacetophenone in 2.5 ml of absolute ethanol in a
conical flask
2. Heat the mixture on a steam bath for 5 minutes
3. Cool the solution
4. Add 0.05 g (1.32 mmol) of sodium borohydride
5. Slowly swirl the suspension for 1-2 minutes. The solution will turn yellow to light brown
ORGANIC CHEMISTRY
5. Slowly swirl the mixture
6. Set up the condenser and reflux the mixture for 30 minutes to dissolve all the tin
7. Add about 0.5 g of sodium hydroxide to 1.2 ml of water in a beaker and set it aside to
cool
8. Cool the reaction solution after the reflux and add the sodium hydroxide solution in ice
for a few minutes
9. Add all the aqueous base to the reaction solution to precipitate the product and convert
Sn(IV) to a soluble stannate
10. Shake the pale yellow slurry to ensure thorough mixing and cool the pale yellow
suspension in ice
11. Filter the pale yellow precipitate through vacuum filtration, rinse the last bit of solid from
the flask with a small amount of ice water and wash the filtered solid with a few drops of
ice water
12. Allow the product to dry and label it
Obtain an IR spectrum of the product.
Part 2
1. Dissolve 0.2 g (1.21 mmol) of m-nitroacetophenone in 2.5 ml of absolute ethanol in a
conical flask
2. Heat the mixture on a steam bath for 5 minutes
3. Cool the solution
4. Add 0.05 g (1.32 mmol) of sodium borohydride
5. Slowly swirl the suspension for 1-2 minutes. The solution will turn yellow to light brown

6
ORGANIC CHEMISTRY
6. Add 2 ml of water
7. Heat the solution on a steam bath for 5 minutes to destroy excess borohydride
8. Allow the solution to cool for a few minutes until hydrogen evolution has essentially
stopped
9. Add 5 ml of water when the solution is at room temperature
10. Extract the mixture with 10 ml of dichloromethane
11. Separate the organic phase
12. Add anhydrous sodium sulphate
13. Filter the solution via gravity filtration and evaporate the solvent on a steam bath
14. Cool the product in a refrigerator. Yellow crystals will be observed
Obtain an IR spectrum of the product.
Results
ORGANIC CHEMISTRY
6. Add 2 ml of water
7. Heat the solution on a steam bath for 5 minutes to destroy excess borohydride
8. Allow the solution to cool for a few minutes until hydrogen evolution has essentially
stopped
9. Add 5 ml of water when the solution is at room temperature
10. Extract the mixture with 10 ml of dichloromethane
11. Separate the organic phase
12. Add anhydrous sodium sulphate
13. Filter the solution via gravity filtration and evaporate the solvent on a steam bath
14. Cool the product in a refrigerator. Yellow crystals will be observed
Obtain an IR spectrum of the product.
Results
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
7
ORGANIC CHEMISTRY
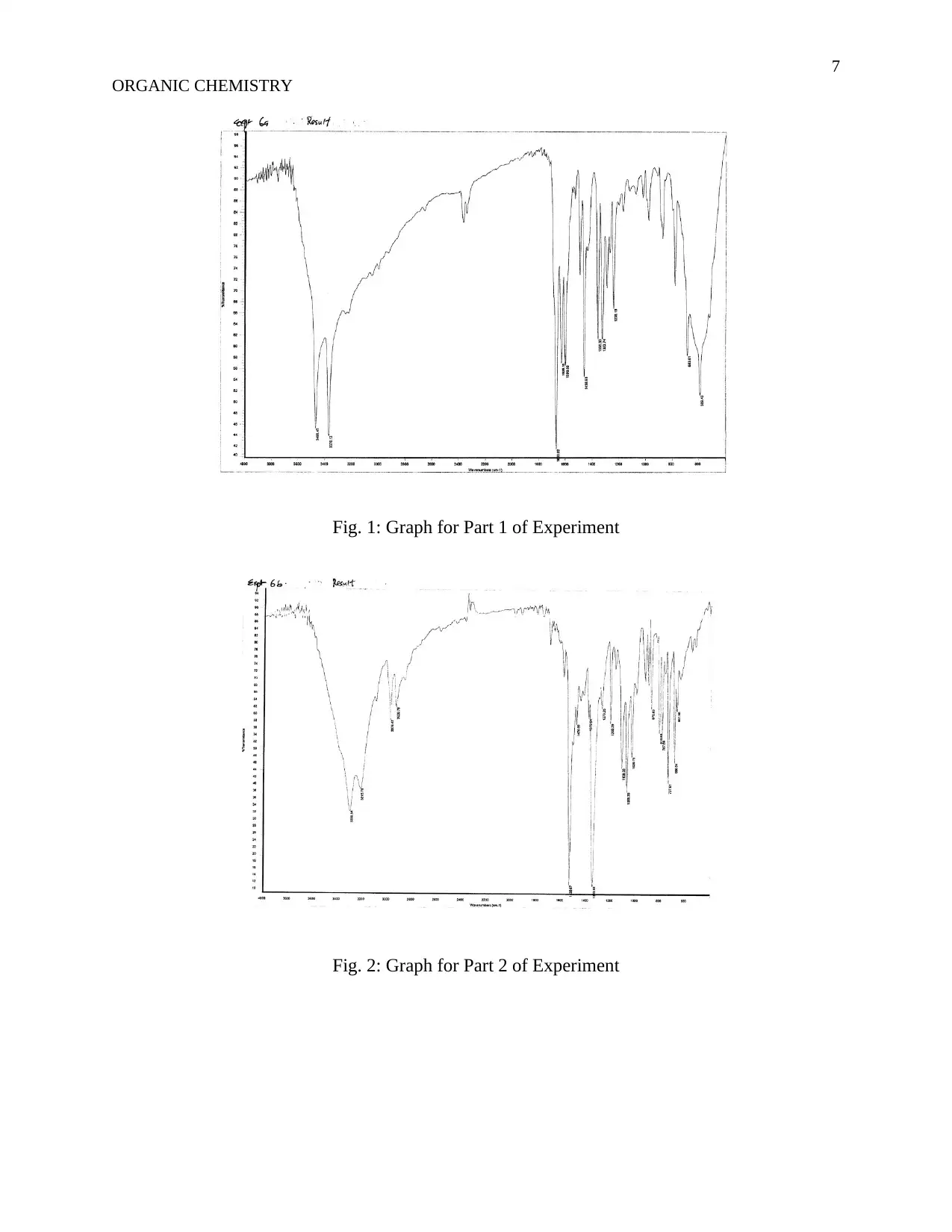
Fig. 1: Graph for Part 1 of Experiment
Fig. 2: Graph for Part 2 of Experiment
ORGANIC CHEMISTRY
Fig. 1: Graph for Part 1 of Experiment
Fig. 2: Graph for Part 2 of Experiment
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
8
ORGANIC CHEMISTRY
Discussion
In the presence of tin and hydrochloric acid, nitrobenzene is reduced to ailine by irons in an
aqueous acid as shown in the equation below
C6H5NO2+ 6H C6H5 NH2 + 2H2O
The conversion of nitrobenzene to aniline occurs in two stages with the first stage involving the
conversion of nitrobenzene to phenyl ammonium ions and the second stage involving the
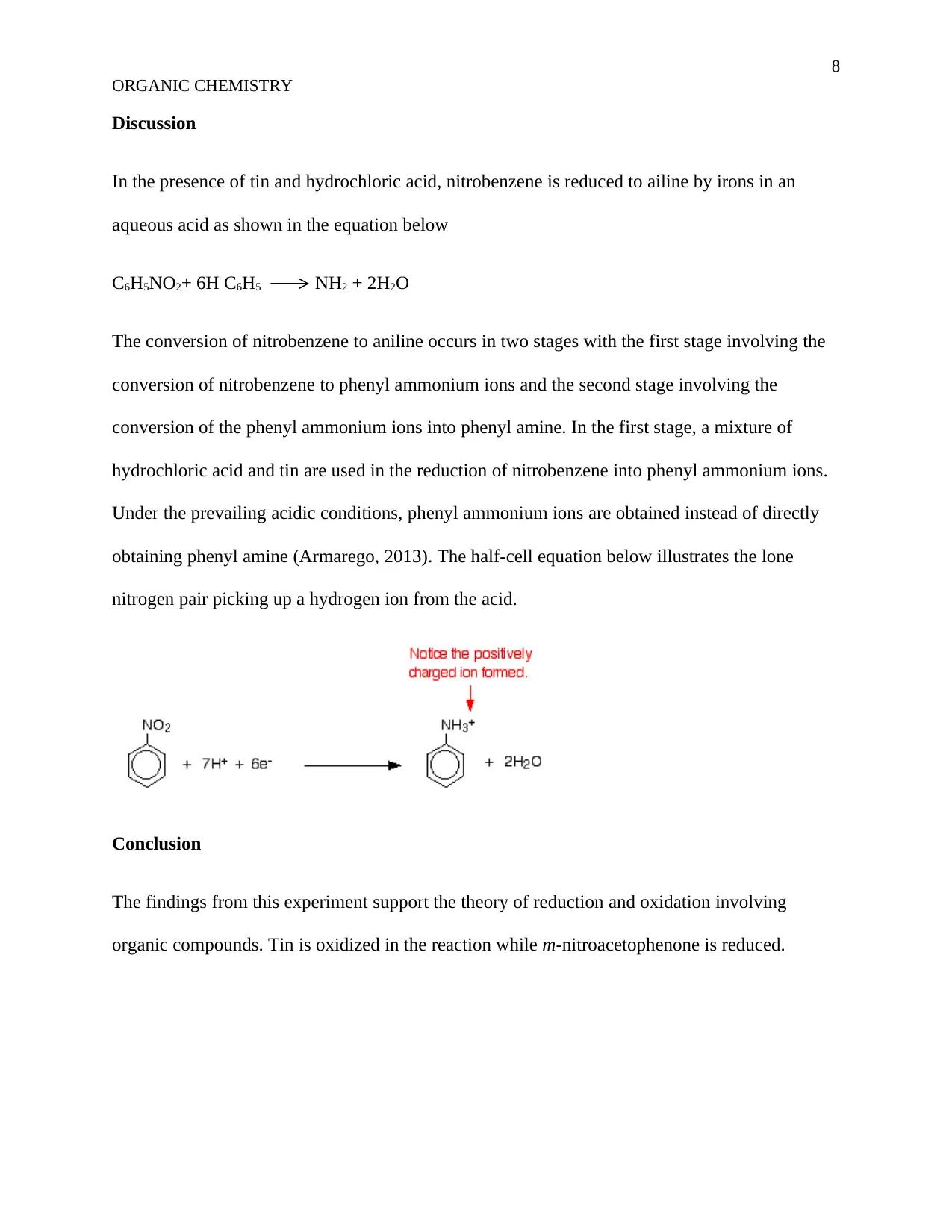
conversion of the phenyl ammonium ions into phenyl amine. In the first stage, a mixture of
hydrochloric acid and tin are used in the reduction of nitrobenzene into phenyl ammonium ions.
Under the prevailing acidic conditions, phenyl ammonium ions are obtained instead of directly
obtaining phenyl amine (Armarego, 2013). The half-cell equation below illustrates the lone
nitrogen pair picking up a hydrogen ion from the acid.
Conclusion
The findings from this experiment support the theory of reduction and oxidation involving
organic compounds. Tin is oxidized in the reaction while m-nitroacetophenone is reduced.
ORGANIC CHEMISTRY
Discussion
In the presence of tin and hydrochloric acid, nitrobenzene is reduced to ailine by irons in an
aqueous acid as shown in the equation below
C6H5NO2+ 6H C6H5 NH2 + 2H2O
The conversion of nitrobenzene to aniline occurs in two stages with the first stage involving the
conversion of nitrobenzene to phenyl ammonium ions and the second stage involving the
conversion of the phenyl ammonium ions into phenyl amine. In the first stage, a mixture of
hydrochloric acid and tin are used in the reduction of nitrobenzene into phenyl ammonium ions.
Under the prevailing acidic conditions, phenyl ammonium ions are obtained instead of directly
obtaining phenyl amine (Armarego, 2013). The half-cell equation below illustrates the lone
nitrogen pair picking up a hydrogen ion from the acid.
Conclusion
The findings from this experiment support the theory of reduction and oxidation involving
organic compounds. Tin is oxidized in the reaction while m-nitroacetophenone is reduced.

9
ORGANIC CHEMISTRY
References
Armarego, W. L. (2013). Purification of Laboratory Chemicals. Beijing: Butterworth-
Heinemann.
Bruckner, R. (2010). Organic Mechanisms: Reactions, Stereochemistry and Synthesis. London:
Springer Science & Business Media.
Dewick, P. M. (2013). Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal
Chemistry and Biological Chemistry. New York: John Wiley & Sons.
Philippa B. Cranwell, L. M. (2017). Experimental Organic Chemistry. New York: John Wiley &
Sons.
ORGANIC CHEMISTRY
References
Armarego, W. L. (2013). Purification of Laboratory Chemicals. Beijing: Butterworth-
Heinemann.
Bruckner, R. (2010). Organic Mechanisms: Reactions, Stereochemistry and Synthesis. London:
Springer Science & Business Media.
Dewick, P. M. (2013). Essentials of Organic Chemistry: For Students of Pharmacy, Medicinal
Chemistry and Biological Chemistry. New York: John Wiley & Sons.
Philippa B. Cranwell, L. M. (2017). Experimental Organic Chemistry. New York: John Wiley &
Sons.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
1 out of 9
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.


