Organic Chemistry Lab Report: Cycloalkanol Preparation & Analysis
VerifiedAdded on 2023/06/03
|8
|1184
|265
Practical Assignment
AI Summary
This lab report details an organic chemistry experiment focused on the reduction of carbonyl compounds using sodium borohydride. The primary objective was to prepare cyclohexanol through the reduction of cyclohexanone and cyclopentanone oxime. The experiment involved dissolving cyclohexa...

LAB REPORT ON ORGANIC CHEMISTRY
[Author Name(s), First M. Last, Omit Titles and Degrees]
[Institutional Affiliation(s)]
[Author Name(s), First M. Last, Omit Titles and Degrees]
[Institutional Affiliation(s)]
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Topic: REDUCTION OF CARBONYL COMPOUNDS WITH SODIUM
BOROHYDRIDE-PREPARATION OF A CYLOALKANOL
Introduction
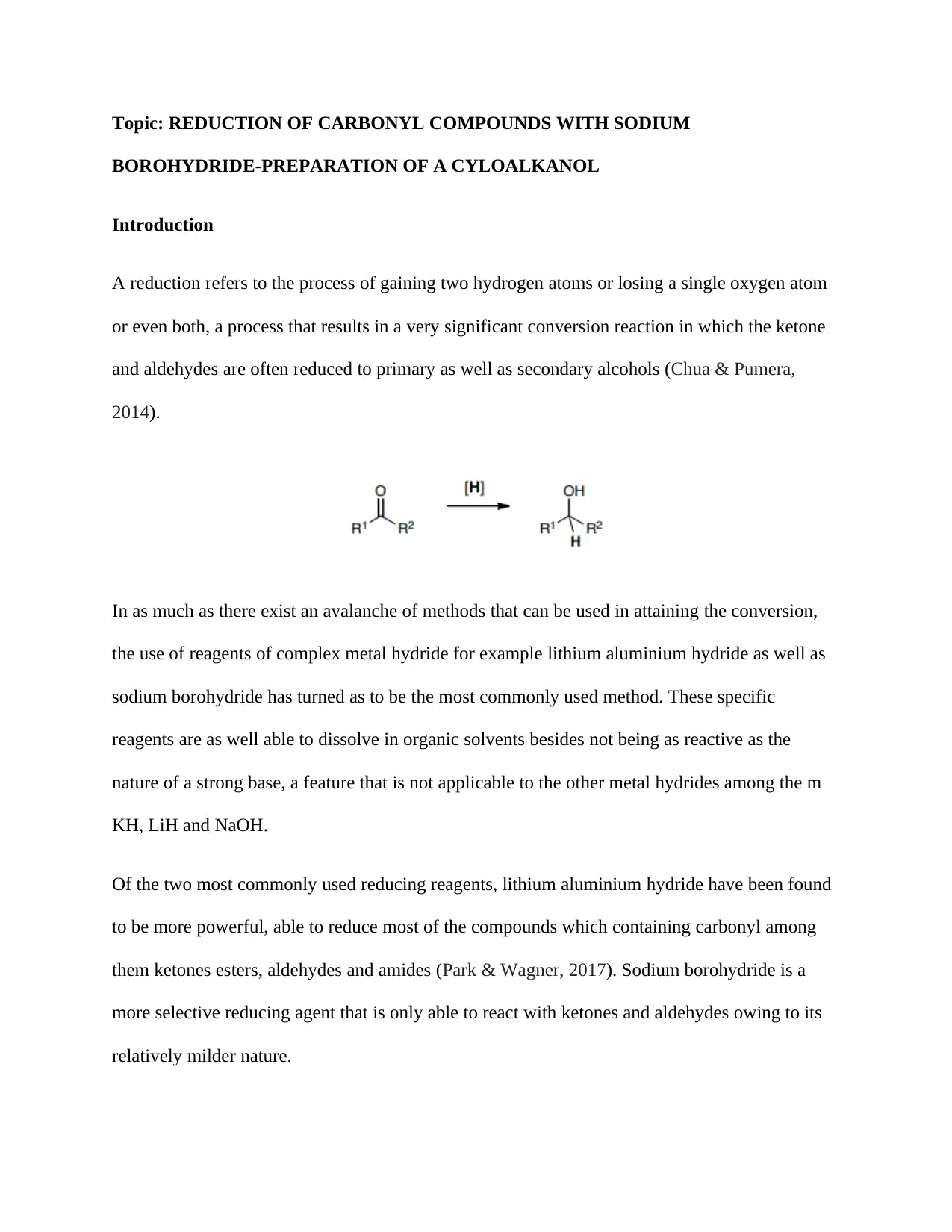
A reduction refers to the process of gaining two hydrogen atoms or losing a single oxygen atom
or even both, a process that results in a very significant conversion reaction in which the ketone
and aldehydes are often reduced to primary as well as secondary alcohols (Chua & Pumera,
2014).
In as much as there exist an avalanche of methods that can be used in attaining the conversion,
the use of reagents of complex metal hydride for example lithium aluminium hydride as well as
sodium borohydride has turned as to be the most commonly used method. These specific
reagents are as well able to dissolve in organic solvents besides not being as reactive as the
nature of a strong base, a feature that is not applicable to the other metal hydrides among the m
KH, LiH and NaOH.
Of the two most commonly used reducing reagents, lithium aluminium hydride have been found
to be more powerful, able to reduce most of the compounds which containing carbonyl among
them ketones esters, aldehydes and amides (Park & Wagner, 2017). Sodium borohydride is a
more selective reducing agent that is only able to react with ketones and aldehydes owing to its
relatively milder nature.
BOROHYDRIDE-PREPARATION OF A CYLOALKANOL
Introduction
A reduction refers to the process of gaining two hydrogen atoms or losing a single oxygen atom
or even both, a process that results in a very significant conversion reaction in which the ketone
and aldehydes are often reduced to primary as well as secondary alcohols (Chua & Pumera,
2014).
In as much as there exist an avalanche of methods that can be used in attaining the conversion,
the use of reagents of complex metal hydride for example lithium aluminium hydride as well as
sodium borohydride has turned as to be the most commonly used method. These specific
reagents are as well able to dissolve in organic solvents besides not being as reactive as the
nature of a strong base, a feature that is not applicable to the other metal hydrides among the m
KH, LiH and NaOH.
Of the two most commonly used reducing reagents, lithium aluminium hydride have been found
to be more powerful, able to reduce most of the compounds which containing carbonyl among
them ketones esters, aldehydes and amides (Park & Wagner, 2017). Sodium borohydride is a
more selective reducing agent that is only able to react with ketones and aldehydes owing to its
relatively milder nature.
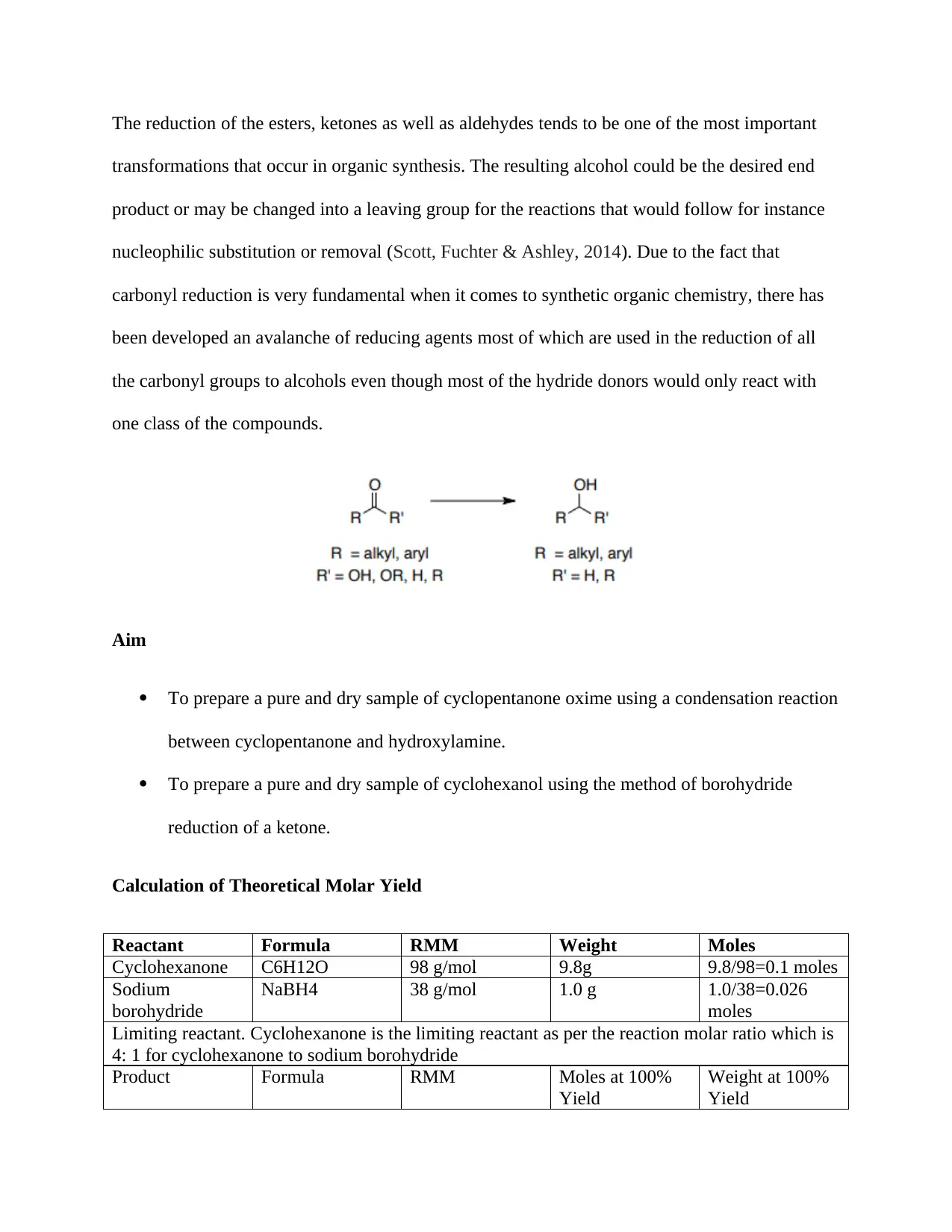
The reduction of the esters, ketones as well as aldehydes tends to be one of the most important
transformations that occur in organic synthesis. The resulting alcohol could be the desired end
product or may be changed into a leaving group for the reactions that would follow for instance
nucleophilic substitution or removal (Scott, Fuchter & Ashley, 2014). Due to the fact that
carbonyl reduction is very fundamental when it comes to synthetic organic chemistry, there has
been developed an avalanche of reducing agents most of which are used in the reduction of all
the carbonyl groups to alcohols even though most of the hydride donors would only react with
one class of the compounds.
Aim
To prepare a pure and dry sample of cyclopentanone oxime using a condensation reaction
between cyclopentanone and hydroxylamine.
To prepare a pure and dry sample of cyclohexanol using the method of borohydride
reduction of a ketone.
Calculation of Theoretical Molar Yield
Reactant Formula RMM Weight Moles
Cyclohexanone C6H12O 98 g/mol 9.8g 9.8/98=0.1 moles
Sodium
borohydride
NaBH4 38 g/mol 1.0 g 1.0/38=0.026
moles
Limiting reactant. Cyclohexanone is the limiting reactant as per the reaction molar ratio which is
4: 1 for cyclohexanone to sodium borohydride
Product Formula RMM Moles at 100%
Yield
Weight at 100%
Yield
transformations that occur in organic synthesis. The resulting alcohol could be the desired end
product or may be changed into a leaving group for the reactions that would follow for instance
nucleophilic substitution or removal (Scott, Fuchter & Ashley, 2014). Due to the fact that
carbonyl reduction is very fundamental when it comes to synthetic organic chemistry, there has
been developed an avalanche of reducing agents most of which are used in the reduction of all
the carbonyl groups to alcohols even though most of the hydride donors would only react with
one class of the compounds.
Aim
To prepare a pure and dry sample of cyclopentanone oxime using a condensation reaction
between cyclopentanone and hydroxylamine.
To prepare a pure and dry sample of cyclohexanol using the method of borohydride
reduction of a ketone.
Calculation of Theoretical Molar Yield
Reactant Formula RMM Weight Moles
Cyclohexanone C6H12O 98 g/mol 9.8g 9.8/98=0.1 moles
Sodium
borohydride
NaBH4 38 g/mol 1.0 g 1.0/38=0.026
moles
Limiting reactant. Cyclohexanone is the limiting reactant as per the reaction molar ratio which is
4: 1 for cyclohexanone to sodium borohydride
Product Formula RMM Moles at 100%
Yield
Weight at 100%
Yield
You're viewing a preview
Unlock full access by subscribing today!

Cyclohexanol C6H12O 100g/mol 0.10 mol 100*0.10=10 g
0.10 moles is the theoretical molar yield while the theoretical weight yield is 10 g
Materials and Methods
Procedure for Preparation of Cyclohexanol
The provided dry freshly distilled cyclohexanone was dissolved in methanol in a 100 ml conical
flask. A solution of sodium methoxide and sodium borohydride was made up in methanol in 100
ml conical flask. The resulting solution was carefully added to the cycloalkanone solution using
a pipette, while swirling and left to stand at room temperature for 5 minutes. The mixture was
poured into a 250 ml flask having ice water, properly mixed and 10 ml of dilute HCl added to it
(Scott, Fuchter & Ashley, 2014). The solution was transferred to a 250 ml separating funnel and
50 ml of ether as well as 50 ml of brine added, the layers gently shaken allowing the frequent
release of pressure. The organic extracts were dried over anhydrous magnesium sulphate for
some minutes, filter off the direr under gravity and the ether/ ethyl acetate stripped off using the
rotary evaporator (Taheri & Berben, 2015). The yield of the product was recorded. The IR of the
product was run as a liquid film
Procedure for Preparation of Cyclopentanone oxime
4.2 g of 0.02 M cyclopentanone, 5.2 g of 0.075 M hydroxylamine hydrochloride and 5.0 g of
0.061 M sodium acetate were dissolve in 20 mL of water and 15 mL if ethanol. The mixture was
then heated under reflux with the aid of a water bath with periodical swirling for half an hour
(Scott, Fuchter & Ashley, 2014). The mixture was then cooled and the crude product filtered off,
washed with 10 ml of water. The pure product was obtained by recrystallizing the 4.62 g of
crude product with 25 mL of ethanol, generating a white crystalline solid.
0.10 moles is the theoretical molar yield while the theoretical weight yield is 10 g
Materials and Methods
Procedure for Preparation of Cyclohexanol
The provided dry freshly distilled cyclohexanone was dissolved in methanol in a 100 ml conical
flask. A solution of sodium methoxide and sodium borohydride was made up in methanol in 100
ml conical flask. The resulting solution was carefully added to the cycloalkanone solution using
a pipette, while swirling and left to stand at room temperature for 5 minutes. The mixture was
poured into a 250 ml flask having ice water, properly mixed and 10 ml of dilute HCl added to it
(Scott, Fuchter & Ashley, 2014). The solution was transferred to a 250 ml separating funnel and
50 ml of ether as well as 50 ml of brine added, the layers gently shaken allowing the frequent
release of pressure. The organic extracts were dried over anhydrous magnesium sulphate for
some minutes, filter off the direr under gravity and the ether/ ethyl acetate stripped off using the
rotary evaporator (Taheri & Berben, 2015). The yield of the product was recorded. The IR of the
product was run as a liquid film
Procedure for Preparation of Cyclopentanone oxime
4.2 g of 0.02 M cyclopentanone, 5.2 g of 0.075 M hydroxylamine hydrochloride and 5.0 g of
0.061 M sodium acetate were dissolve in 20 mL of water and 15 mL if ethanol. The mixture was
then heated under reflux with the aid of a water bath with periodical swirling for half an hour
(Scott, Fuchter & Ashley, 2014). The mixture was then cooled and the crude product filtered off,
washed with 10 ml of water. The pure product was obtained by recrystallizing the 4.62 g of
crude product with 25 mL of ethanol, generating a white crystalline solid.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Results & Discussion
Experiment 1
Boiling point:158-160⁰C
Sample Mass=3.36 g
Tube=18.99 g
Tube+sample=22.35g
Experiment 2
4.62 g of crude oil was yielded
4.1 g of pure recrystallized and air dried product was attained
The recrystallized product had a melting point of 53-55⁰C
The percentage molar yield of the pure product can be estimated as:
(4.1/4.95)*100=83%
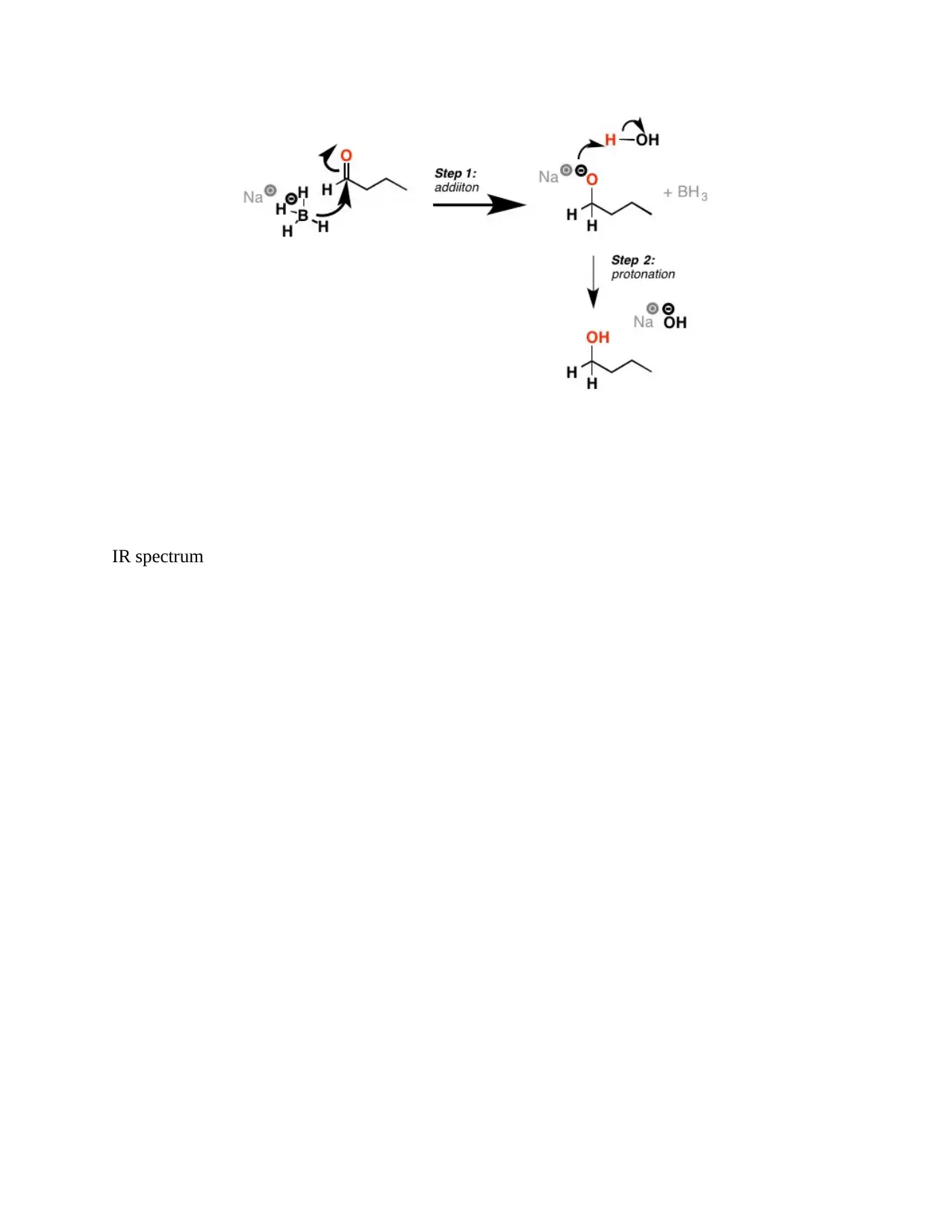
The reaction mechanism of sodium borohydride with ketones and aldehydes continues into two
steps: detachment of the H (-) from the BH4 (-) and adding to the available carbonyl carbon
resulting in the formation of a C-H bond while breakage of a C-O bond and the second step
being the addition of a proton from water to the alkoxide leading to the formation of the alcohol
(Taheri & Berben, 2015)
Experiment 1
Boiling point:158-160⁰C
Sample Mass=3.36 g
Tube=18.99 g
Tube+sample=22.35g
Experiment 2
4.62 g of crude oil was yielded
4.1 g of pure recrystallized and air dried product was attained
The recrystallized product had a melting point of 53-55⁰C
The percentage molar yield of the pure product can be estimated as:
(4.1/4.95)*100=83%
The reaction mechanism of sodium borohydride with ketones and aldehydes continues into two
steps: detachment of the H (-) from the BH4 (-) and adding to the available carbonyl carbon
resulting in the formation of a C-H bond while breakage of a C-O bond and the second step
being the addition of a proton from water to the alkoxide leading to the formation of the alcohol
(Taheri & Berben, 2015)
IR spectrum
You're viewing a preview
Unlock full access by subscribing today!
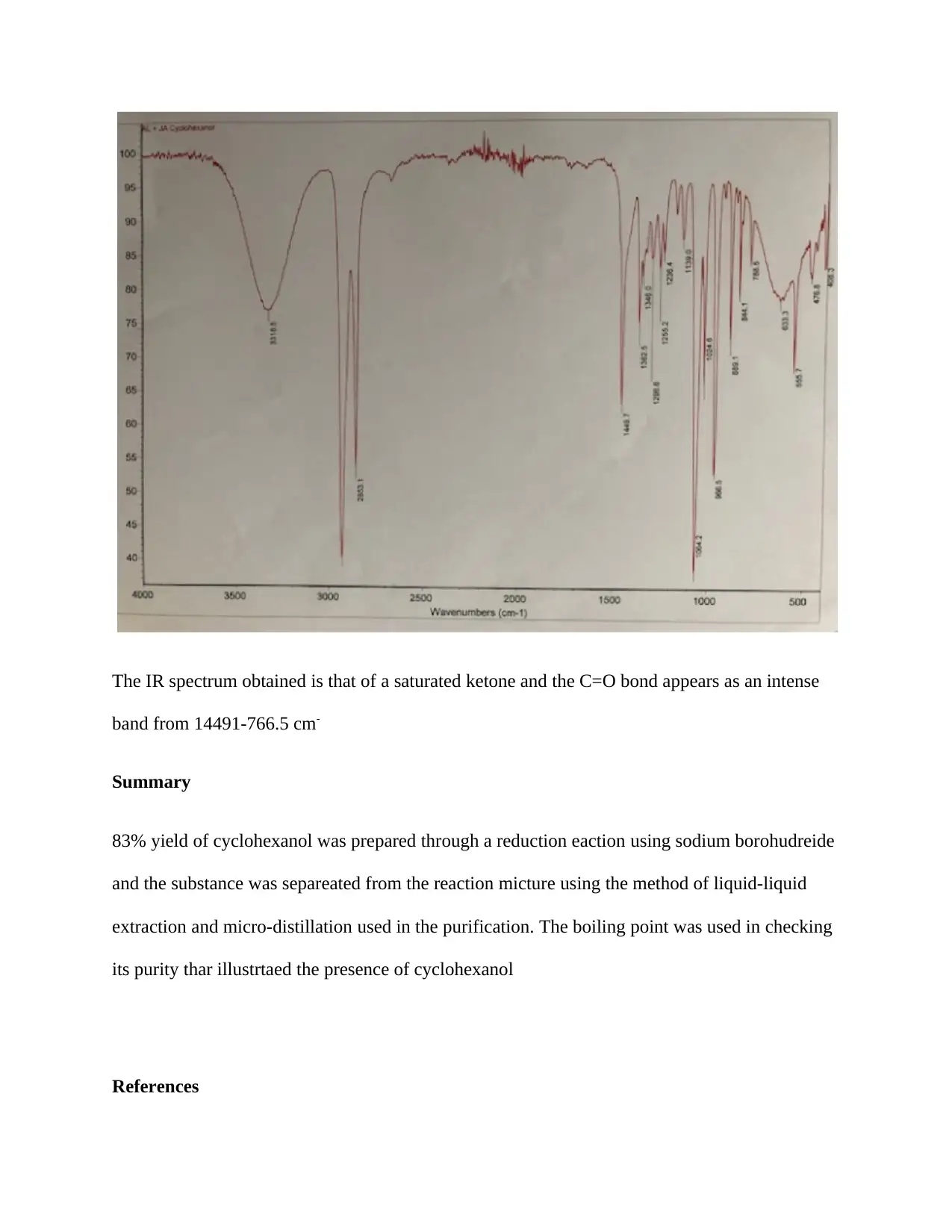
The IR spectrum obtained is that of a saturated ketone and the C=O bond appears as an intense
band from 14491-766.5 cm-
Summary
83% yield of cyclohexanol was prepared through a reduction eaction using sodium borohudreide
and the substance was separeated from the reaction micture using the method of liquid-liquid
extraction and micro-distillation used in the purification. The boiling point was used in checking
its purity thar illustrtaed the presence of cyclohexanol
References
band from 14491-766.5 cm-
Summary
83% yield of cyclohexanol was prepared through a reduction eaction using sodium borohudreide
and the substance was separeated from the reaction micture using the method of liquid-liquid
extraction and micro-distillation used in the purification. The boiling point was used in checking
its purity thar illustrtaed the presence of cyclohexanol
References
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chua, C. K., & Pumera, M. (2014). Chemical reduction of graphene oxide: a synthetic chemistry
viewpoint. Chemical Society Reviews, 43(1), 291-312
Park, B. S., & Wagner, P. (2017). Photoinduced hydrogen atom abstraction by carbonyl
compounds. In Organic photochemistry (pp. 227-366). Routledge
Scott, D. J., Fuchter, M. J., & Ashley, A. E. (2014). Nonmetal catalyzed hydrogenation of
carbonyl compounds. Journal of the American Chemical Society, 136(45), 15813-15816
Taheri, A., & Berben, L. A. (2015). Tailoring electrocatalysts for selective CO2 or H+ reduction:
Iron carbonyl clusters as a case study. Inorganic chemistry, 55(2), 378-385
viewpoint. Chemical Society Reviews, 43(1), 291-312
Park, B. S., & Wagner, P. (2017). Photoinduced hydrogen atom abstraction by carbonyl
compounds. In Organic photochemistry (pp. 227-366). Routledge
Scott, D. J., Fuchter, M. J., & Ashley, A. E. (2014). Nonmetal catalyzed hydrogenation of
carbonyl compounds. Journal of the American Chemical Society, 136(45), 15813-15816
Taheri, A., & Berben, L. A. (2015). Tailoring electrocatalysts for selective CO2 or H+ reduction:
Iron carbonyl clusters as a case study. Inorganic chemistry, 55(2), 378-385
1 out of 8
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.

