Organic Chemistry Assignment Report
VerifiedAdded on 2022/09/13
|11
|795
|8
AI Summary
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Chemistry 1
CHEMISTRY
By Name
Course
Instructor
Institution
Location
Date
CHEMISTRY
By Name
Course
Instructor
Institution
Location
Date
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Chemistry 2
Q2
Part a
i). Ethane
Ethane + Oxygen → Carbon (IV) +water
2C2H6 (g)+7O2 (g) → 4O2(g)+6H2O(l)
ii. Ethene
Ethene + Oxygen → Carbon (IV) +water
C2H4 (g)+3O2 (g) → 2O2 (g)+2H2O (l)
iii. Hydrogen
Hydrogen + Oxygen → Water
2H2 (g) + O2(g)→ 2H2O (l)
Part b
2C2H6 (g) + 7O2 (g) → 4O2(g)+6H2O(l)
C+ H2+ O2
∆ Hc= ( 6 ×285.5 ) + ( 4 ×−393 ) + ( 2 ×−83.6 ) −1713−1572+167.2
∆ Hc=3117.8
Q2
Part a
i). Ethane
Ethane + Oxygen → Carbon (IV) +water
2C2H6 (g)+7O2 (g) → 4O2(g)+6H2O(l)
ii. Ethene
Ethene + Oxygen → Carbon (IV) +water
C2H4 (g)+3O2 (g) → 2O2 (g)+2H2O (l)
iii. Hydrogen
Hydrogen + Oxygen → Water
2H2 (g) + O2(g)→ 2H2O (l)
Part b
2C2H6 (g) + 7O2 (g) → 4O2(g)+6H2O(l)
C+ H2+ O2
∆ Hc= ( 6 ×285.5 ) + ( 4 ×−393 ) + ( 2 ×−83.6 ) −1713−1572+167.2
∆ Hc=3117.8
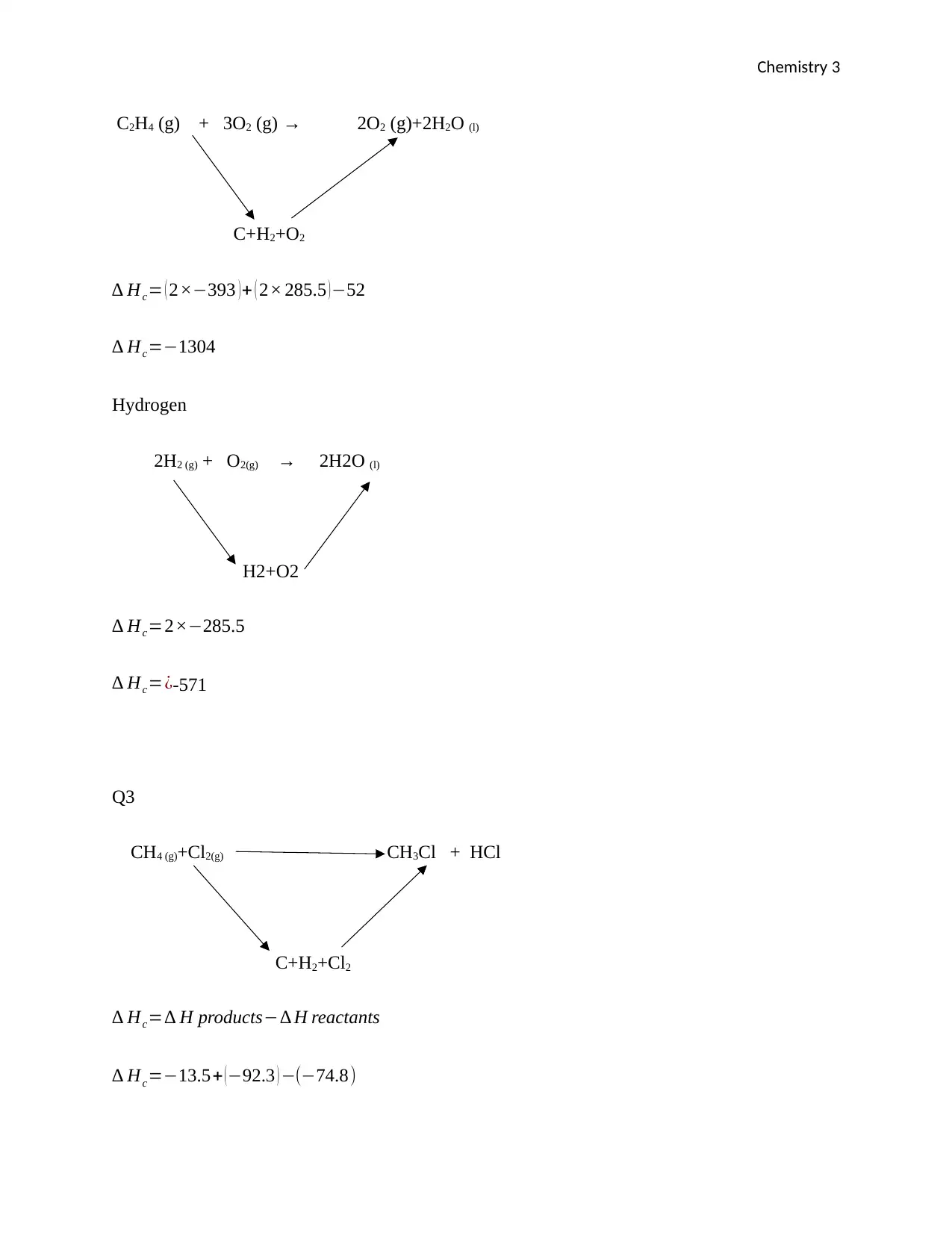
Chemistry 3
C2H4 (g) + 3O2 (g) → 2O2 (g)+2H2O (l)
C+H2+O2
∆ Hc= ( 2×−393 ) + ( 2× 285.5 ) −52
∆ Hc=−1304
Hydrogen
2H2 (g) + O2(g) → 2H2O (l)
H2+O2
∆ Hc=2×−285.5
∆ Hc=¿-571
Q3
CH4 (g)+Cl2(g) CH3Cl + HCl
C+H2+Cl2
∆ Hc=∆ H products−∆ H reactants
∆ Hc=−13.5+ ( −92.3 ) −(−74.8)
C2H4 (g) + 3O2 (g) → 2O2 (g)+2H2O (l)
C+H2+O2
∆ Hc= ( 2×−393 ) + ( 2× 285.5 ) −52
∆ Hc=−1304
Hydrogen
2H2 (g) + O2(g) → 2H2O (l)
H2+O2
∆ Hc=2×−285.5
∆ Hc=¿-571
Q3
CH4 (g)+Cl2(g) CH3Cl + HCl
C+H2+Cl2
∆ Hc=∆ H products−∆ H reactants
∆ Hc=−13.5+ ( −92.3 ) −(−74.8)
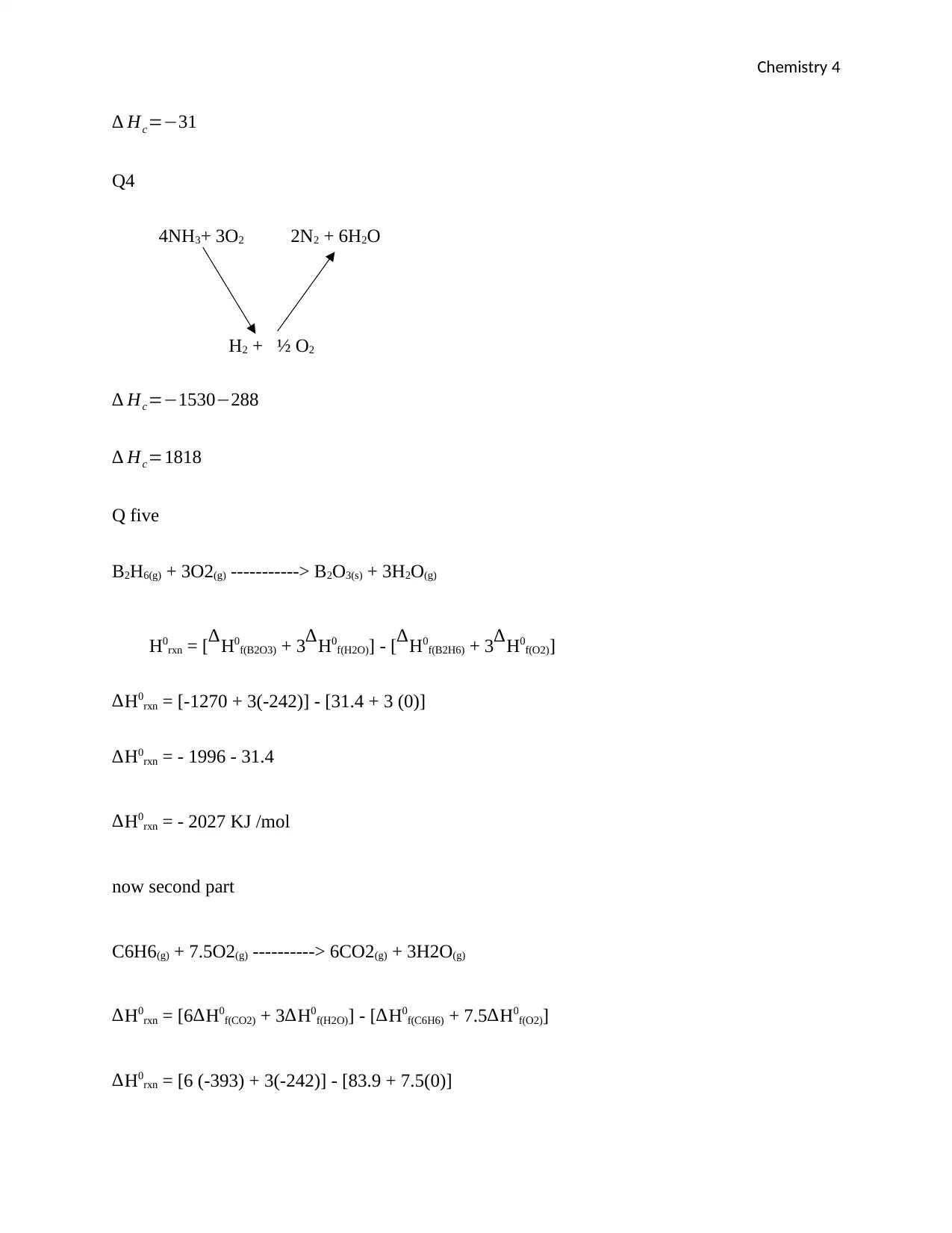
Chemistry 4
∆ Hc=−31
Q4
4NH3+ 3O2 2N2 + 6H2O
H2 + ½ O2
∆ Hc=−1530−288
∆ Hc=1818
Q five
B2H6(g) + 3O2(g) -----------> B2O3(s) + 3H2O(g)
H0rxn = [ ∆H0f(B2O3) + 3
∆H0f(H2O)] - [
∆H0f(B2H6) + 3 ∆H0f(O2)]
∆H0rxn = [-1270 + 3(-242)] - [31.4 + 3 (0)]
∆H0rxn = - 1996 - 31.4
∆H0rxn = - 2027 KJ /mol
now second part
C6H6(g) + 7.5O2(g) ----------> 6CO2(g) + 3H2O(g)
∆H0rxn = [6 ∆H0f(CO2) + 3 ∆H0f(H2O)] - [∆H0f(C6H6) + 7.5∆H0f(O2)]
∆H0rxn = [6 (-393) + 3(-242)] - [83.9 + 7.5(0)]
∆ Hc=−31
Q4
4NH3+ 3O2 2N2 + 6H2O
H2 + ½ O2
∆ Hc=−1530−288
∆ Hc=1818
Q five
B2H6(g) + 3O2(g) -----------> B2O3(s) + 3H2O(g)
H0rxn = [ ∆H0f(B2O3) + 3
∆H0f(H2O)] - [
∆H0f(B2H6) + 3 ∆H0f(O2)]
∆H0rxn = [-1270 + 3(-242)] - [31.4 + 3 (0)]
∆H0rxn = - 1996 - 31.4
∆H0rxn = - 2027 KJ /mol
now second part
C6H6(g) + 7.5O2(g) ----------> 6CO2(g) + 3H2O(g)
∆H0rxn = [6 ∆H0f(CO2) + 3 ∆H0f(H2O)] - [∆H0f(C6H6) + 7.5∆H0f(O2)]
∆H0rxn = [6 (-393) + 3(-242)] - [83.9 + 7.5(0)]
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Chemistry 5
∆H0rxn = [-2358 - 726] - [83.9]
∆H0rxn = - 3167.9 KJ/mol
Q6
C2H5OH + 3O2 2CO2 +3H2O ∆ H 1=1368
Reaction for formation of CO2
C+O2 CO2 ∆ H 1=−393.7
Reaction for formation of H2O is given below
H2+½O2 H2O ∆ H 1=−285.9
Reaction for formation of ethanol
2C+3H2+½O C2H5OH
2C+3H2+3/2 O2- C2H5OH-3O2 2CO2+3H2O-2CO2-3H2O
= (-393.2 ×2 ¿+ ( 3 × 285.9 ) −(−1368)
= -277.1kJ mol-1
The enthalpy of formation of ethanol is -277.1kJ mol-1
Q 7
Part a
CH4+Br CH3Br+HBr
∆ H f =∆ Hproduct−∆ Hreactant
∆H0rxn = [-2358 - 726] - [83.9]
∆H0rxn = - 3167.9 KJ/mol
Q6
C2H5OH + 3O2 2CO2 +3H2O ∆ H 1=1368
Reaction for formation of CO2
C+O2 CO2 ∆ H 1=−393.7
Reaction for formation of H2O is given below
H2+½O2 H2O ∆ H 1=−285.9
Reaction for formation of ethanol
2C+3H2+½O C2H5OH
2C+3H2+3/2 O2- C2H5OH-3O2 2CO2+3H2O-2CO2-3H2O
= (-393.2 ×2 ¿+ ( 3 × 285.9 ) −(−1368)
= -277.1kJ mol-1
The enthalpy of formation of ethanol is -277.1kJ mol-1
Q 7
Part a
CH4+Br CH3Br+HBr
∆ H f =∆ Hproduct−∆ Hreactant

Chemistry 6
∆ H = (3× 413 ¿+ ( 280 ) +366−193+(4 × 413)
∆ H =1239+280+366−193+1652
∆ H = 40kJmol-1
Part b
CH4 +F2 CH3F+HF
∆ H = ( 3 ×413 )+ 425+565− ( 4 × 413 ) +152
∆ H =1239+425+565−1652+152
∆ H =2229−1804
∆ H =425
Part c
∆ H =∆ Hproduct−∆ Hreactant
∆ H =366−1845
∆ H =−1479
Part d
C3H8+H2 C3H6
∆ H =611+347+ ( 6 × 413 )=3436
∆ H = ( 2× 347 ) + ( 8 × 413 ) +435=4433
∆ H =3436−4433=−997
∆ H = (3× 413 ¿+ ( 280 ) +366−193+(4 × 413)
∆ H =1239+280+366−193+1652
∆ H = 40kJmol-1
Part b
CH4 +F2 CH3F+HF
∆ H = ( 3 ×413 )+ 425+565− ( 4 × 413 ) +152
∆ H =1239+425+565−1652+152
∆ H =2229−1804
∆ H =425
Part c
∆ H =∆ Hproduct−∆ Hreactant
∆ H =366−1845
∆ H =−1479
Part d
C3H8+H2 C3H6
∆ H =611+347+ ( 6 × 413 )=3436
∆ H = ( 2× 347 ) + ( 8 × 413 ) +435=4433
∆ H =3436−4433=−997

Chemistry 7
∆ H =−997
Part e
C3H8+Br C3H6Br2 +H2
∆ H0 f = ( 2 ×347 )+ (6 × 413 )+ ( 2 ×280 )+870=4602
∆ H0 f = ( 2 ×347 )+ ( 8× 413 ) +(2 ×193)=4384
∆ H0 f =4602−4384
Part f
CH4+2O2 CO2+2H2O
∆ H0 f = ( 2 ×805 )+ (2 × 464 ×2 )− ( 4 × 413 )+(498 × 2)
∆ H0 f =1610+1856−1652+ 996
∆ H0 f =3466−2648
∆ H0 f =818
Part g
C2H4+H2 CH6
∆ H0 f =347+ ( 6 × 413 )−611+(4 × 413)
∆ H0 f =347+2478−611+1652
∆ H0 f =2825−2263
∆ H0 f =562
∆ H =−997
Part e
C3H8+Br C3H6Br2 +H2
∆ H0 f = ( 2 ×347 )+ (6 × 413 )+ ( 2 ×280 )+870=4602
∆ H0 f = ( 2 ×347 )+ ( 8× 413 ) +(2 ×193)=4384
∆ H0 f =4602−4384
Part f
CH4+2O2 CO2+2H2O
∆ H0 f = ( 2 ×805 )+ (2 × 464 ×2 )− ( 4 × 413 )+(498 × 2)
∆ H0 f =1610+1856−1652+ 996
∆ H0 f =3466−2648
∆ H0 f =818
Part g
C2H4+H2 CH6
∆ H0 f =347+ ( 6 × 413 )−611+(4 × 413)
∆ H0 f =347+2478−611+1652
∆ H0 f =2825−2263
∆ H0 f =562
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Chemistry 8
Part h
C2H6+3O2 2O2 + 2H2O
∆ H0 f = ( 2 ×805 )+ (2 × 464 ×2 )− ( 2× 347 ) + ( 6 × 413 ) +(3× 498)
∆ H0 f =3220+1850−694+2478+1494
∆ H0 f =5076−4666
∆ H0 f =410
Q 8
N2+3H2 2NH3
2x-945+(436) = 46
2 X
2 = 2299
2
x=1149.5
But there are 3 bonds of N-H
3x =1149.5
3 x
3 = 1149.5
3
X=383
Q9
-135.5+715+242
Part h
C2H6+3O2 2O2 + 2H2O
∆ H0 f = ( 2 ×805 )+ (2 × 464 ×2 )− ( 2× 347 ) + ( 6 × 413 ) +(3× 498)
∆ H0 f =3220+1850−694+2478+1494
∆ H0 f =5076−4666
∆ H0 f =410
Q 8
N2+3H2 2NH3
2x-945+(436) = 46
2 X
2 = 2299
2
x=1149.5
But there are 3 bonds of N-H
3x =1149.5
3 x
3 = 1149.5
3
X=383
Q9
-135.5+715+242

Chemistry 9
-135.5+957
= 821.5
Q 10
C4H10+I2 C4H9I+HI
X+ ( ( 3 ×347 )+ ( 9 ×413 )−4130+ ( 3× 347 ) +214=−52
X+1041+3717-4130+1041+214=−52
X+3717-4130+214 = −52
X-413+214= −52
X= −52+199=147
The bond between C-I is therefore 147
Q11
C4+O2 C2H5OH
347+ ( 5 × 435 ) +464+ X −611+ ( 4 × 413 ) =−277
2986-2263+X = −277
X+723= −277
X=−277−723
X= −1000
The enthalpy of the C-O bond is −1000
-135.5+957
= 821.5
Q 10
C4H10+I2 C4H9I+HI
X+ ( ( 3 ×347 )+ ( 9 ×413 )−4130+ ( 3× 347 ) +214=−52
X+1041+3717-4130+1041+214=−52
X+3717-4130+214 = −52
X-413+214= −52
X= −52+199=147
The bond between C-I is therefore 147
Q11
C4+O2 C2H5OH
347+ ( 5 × 435 ) +464+ X −611+ ( 4 × 413 ) =−277
2986-2263+X = −277
X+723= −277
X=−277−723
X= −1000
The enthalpy of the C-O bond is −1000

Chemistry 10
Q12
Q13
Q12
Q13
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

Chemistry 11
Reference.
Coley, J.S. and Hess, D.J., 2012. Green energy laws and Republican legislators in the United
States. Energy Policy, 48, pp.576-583.
Gücüyener, C., van den Bergh, J., Gascon, J., & Kapteijn, F. (2010). Ethane/ethene separation
turned on its head: selective ethane adsorption on the metal− organic Framework ZIF-7 through a
gate-opening mechanism. Journal of the American Chemical Society, 132(50), 17704-17706.
Jackman, L.M. and Sternhell, S., 2013. Application of Nuclear Magnetic Resonance
Spectroscopy in Organic Chemistry: International Series in Organic Chemistry. Elsevier.
Koutsoyiannis, D., 2010. HESS Opinions" A random walk on water". Hydrology and Earth
System Sciences, 14(3), pp.585-601.
Martinez-Garcia, J.C., Martinez-Garcia, J., Rzoska, S.J. and Hulliger, J., 2012. The new insight
into dynamic crossover in glass forming liquids from the apparent enthalpy analysis. The
Journal of chemical physics, 137(6), p.064501.
Mills, J. and White, R., 2012. Organic chemistry of museum objects. Routledge.
Schwarzenbach, R.P., Gschwend, P.M. and Imboden, D.M., 2016. Environmental organic
chemistry. John Wiley & Sons.
Shevelyova, M.P., Paulechka, Y.U., Kabo, G.J., Blokhin, A.V., Kabo, A.G. and Gubarevich,
T.M., 2013. Physicochemical properties of imidazolium-based ionic nanofluids: density, heat
capacity, and enthalpy of formation. The Journal of Physical Chemistry C, 117(9), pp.4782-
4790.
Reference.
Coley, J.S. and Hess, D.J., 2012. Green energy laws and Republican legislators in the United
States. Energy Policy, 48, pp.576-583.
Gücüyener, C., van den Bergh, J., Gascon, J., & Kapteijn, F. (2010). Ethane/ethene separation
turned on its head: selective ethane adsorption on the metal− organic Framework ZIF-7 through a
gate-opening mechanism. Journal of the American Chemical Society, 132(50), 17704-17706.
Jackman, L.M. and Sternhell, S., 2013. Application of Nuclear Magnetic Resonance
Spectroscopy in Organic Chemistry: International Series in Organic Chemistry. Elsevier.
Koutsoyiannis, D., 2010. HESS Opinions" A random walk on water". Hydrology and Earth
System Sciences, 14(3), pp.585-601.
Martinez-Garcia, J.C., Martinez-Garcia, J., Rzoska, S.J. and Hulliger, J., 2012. The new insight
into dynamic crossover in glass forming liquids from the apparent enthalpy analysis. The
Journal of chemical physics, 137(6), p.064501.
Mills, J. and White, R., 2012. Organic chemistry of museum objects. Routledge.
Schwarzenbach, R.P., Gschwend, P.M. and Imboden, D.M., 2016. Environmental organic
chemistry. John Wiley & Sons.
Shevelyova, M.P., Paulechka, Y.U., Kabo, G.J., Blokhin, A.V., Kabo, A.G. and Gubarevich,
T.M., 2013. Physicochemical properties of imidazolium-based ionic nanofluids: density, heat
capacity, and enthalpy of formation. The Journal of Physical Chemistry C, 117(9), pp.4782-
4790.
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.