Structural and Functional Assessment of Mycoplasma genitalium Protein Sequence
22 Pages4004 Words416 Views
Added on 2023-04-21
About This Document
This paper evaluates the sequence related to Mycoplasma genitalium through the utilization of various bioinformatics tools, methods, and databases. It analyzes the structural and functional aspects of the protein sequence and provides insights into its biological potential.
Structural and Functional Assessment of Mycoplasma genitalium Protein Sequence
Added on 2023-04-21
ShareRelated Documents
P a g e | 1
Abstract
The presented paper evaluated the sequence related to Mycoplasma genitalium through the
utilization of various bioinformatics tools, methods, and databases. BLAST-p, PSI-BLAST,
FASTA, PROSITE, Pfam, EBI, and SWISS PROT include some of the significant tools that
were utilized in the paper for retrieving the protein variant of M. genitalium and comparing
the same with other protein structures. The structural and functional assessment of the protein
structure assisted in predicting the consistency and engagement of M. genitalium protein in
various biological activities. The provided sequence of M. genitalium pertained to the
MG045 solute binding family. The retrieved protein structure was found to be related to the
significant processes including polyamines uptake and periplasmic binding. The results also
revealed the smallest structure of M. genitalium protein as compared to the structures of other
free-living organisms. The presented structure exhibited substantial homology with other
known protein sequences of the same organism. The multiple alignments of M. genitalium
protein sequence with a randomly selected sequence also revealed substantial similarity in
some of the selected base pairs. The secondary structures of M. genitalium revealed 12-23%
sequence identity. The structural assessment revealed the high capacity of M. genitalium
proteins to modify themselves in accordance with the cellular metabolic requirements. 83%-
85% scores revealed from the Ramachandran Plot demonstrated the structural consistency of
M. genitalium protein. However, a range of M. genitalium variants still requires a structural
and functional assessment to evaluate their biological potential. The present findings from the
existing bioinformatics tool despite their accuracy might not be generalized across the wider
genome pool of M. genitalium because of their limited scalability. However, the study has
substantially enhanced the insight regarding the biological structure and function of the
presented M. genitalium protein sequence.
Introduction
The genital mycoplasmas categorize Mycoplasma genitalium as a microbe that leads to the
development of sexually transmitted infections in the human population (Sethi, Singh,
Samanta, & Sharma, 2012). Evidence-based literature describes the flask-shaped morphology
of Mycoplasma genitalium based on its terminal orgallene (i.e. cell membrane protrusion).
This organelle is a virulence factor that facilitates the motility and adhesion of Mycoplasma
genitalium across the host cell line. The cytoskeleton of the terminal organelle of
Mycoplasma genitalium corresponds to 300nm and incorporates the wheel complex, rod, and
terminal button (Martinelli et al., 2016). The crystal structure of Mycoplasma genitalium
protein (MG491) is based on a resolution of 3-angstrom. The subunits of Mycoplasma
Abstract
The presented paper evaluated the sequence related to Mycoplasma genitalium through the
utilization of various bioinformatics tools, methods, and databases. BLAST-p, PSI-BLAST,
FASTA, PROSITE, Pfam, EBI, and SWISS PROT include some of the significant tools that
were utilized in the paper for retrieving the protein variant of M. genitalium and comparing
the same with other protein structures. The structural and functional assessment of the protein
structure assisted in predicting the consistency and engagement of M. genitalium protein in
various biological activities. The provided sequence of M. genitalium pertained to the
MG045 solute binding family. The retrieved protein structure was found to be related to the
significant processes including polyamines uptake and periplasmic binding. The results also
revealed the smallest structure of M. genitalium protein as compared to the structures of other
free-living organisms. The presented structure exhibited substantial homology with other
known protein sequences of the same organism. The multiple alignments of M. genitalium
protein sequence with a randomly selected sequence also revealed substantial similarity in
some of the selected base pairs. The secondary structures of M. genitalium revealed 12-23%
sequence identity. The structural assessment revealed the high capacity of M. genitalium
proteins to modify themselves in accordance with the cellular metabolic requirements. 83%-
85% scores revealed from the Ramachandran Plot demonstrated the structural consistency of
M. genitalium protein. However, a range of M. genitalium variants still requires a structural
and functional assessment to evaluate their biological potential. The present findings from the
existing bioinformatics tool despite their accuracy might not be generalized across the wider
genome pool of M. genitalium because of their limited scalability. However, the study has
substantially enhanced the insight regarding the biological structure and function of the
presented M. genitalium protein sequence.
Introduction
The genital mycoplasmas categorize Mycoplasma genitalium as a microbe that leads to the
development of sexually transmitted infections in the human population (Sethi, Singh,
Samanta, & Sharma, 2012). Evidence-based literature describes the flask-shaped morphology
of Mycoplasma genitalium based on its terminal orgallene (i.e. cell membrane protrusion).
This organelle is a virulence factor that facilitates the motility and adhesion of Mycoplasma
genitalium across the host cell line. The cytoskeleton of the terminal organelle of
Mycoplasma genitalium corresponds to 300nm and incorporates the wheel complex, rod, and
terminal button (Martinelli et al., 2016). The crystal structure of Mycoplasma genitalium
protein (MG491) is based on a resolution of 3-angstrom. The subunits of Mycoplasma

P a g e | 2
genitalium protein incorporate the 60-residue N-terminus that includes the flexible C-terminal
location based on 150 residues of a centralized 3-helix bundle. This location communicates
with a distantly located terminal organelle protein (i.e. MG200). The MG491 protein of
Mycoplasma genitalium contains symmetrically oriented dimer subunits that configure the
shape of a tetramer. The genome of Mycoplasma genitalium is based on 580kb genome with
a small structure (Iverson-Cabral, Astete, Cohen, Rocha, & Totten, 2006). The MgPar
sequences replicate repeatedly in Mycoplasma genitalium in relation to their shared ancestry
with the mgpB gene. MgPar and mgpB sequences undergo recombination episodes to
facilitate the configuration of MgPa proteins. The whole genome sequencing of Mycoplasma
genitalium by Fookes et al. (2017) revealed twenty-five locations that exhibited elevated SNP
density levels. These locations were based on the host interactions of MgPar loci. The
phylogenetic assessment of Mycoplasma genitalium revealed the existence of 2-distinct clads
with variable genetic structure. The SNP locations including parC and 23S rRNA (V-regions)
of Mycoplasma genitalium evidently contributed to its resistance against fluoroquinolone,
erythromycin, and azithromycin (Fookes et al., 2017). These SNPs were also found to be
consistent with minimum inhibitory concentration data of the respective microorganisms. The
proteome/complete genome assessment of Mycoplasma genitalium by Ma, et al. (2007)
revealed the recombination episodes through gene conversion/crossover between MgPar
sequences and MG192 expression location that resulted in several variations in the MG192
sequence.
The evidence-based findings reveal several sequence combinations/alliances that lead to the
significant structural/functional variability within the proteins of Mycoplasma genitalium. A
range of significant bioinformatics-based databases assists in evaluating the structure of
complex proteins. Some of the significant databases include UniProtKB, UniGene, GenBank,
and RefSeq (NCBI Reference Sequence Database) (Chen, Huang, & Wu, 2017). The
significant protein search tools include ‘BLAST’, ‘LinkOut’, ‘E-Utilities’, and ‘Batch
Entrez’. PROSITE assists in evaluating the functional patterns, locations, and domains of
significant proteins (PROSITE, 2019). Alternatively, the tools including Pfam, PRINTS,
ProDOM, Blocks, and MOTIF substantially assist in analyzing various protein sequences in
accordance with their multifactorial parameters (MOTIFS, 2019). The presented paper
effectively analyses the preselected protein sequence pertaining to the hypothetical protein
‘Mycoplasma genitalium’ across various protein sequence databases. The paper evaluates the
domain structure, likely function, appearance, comparative appearance (in other
genitalium protein incorporate the 60-residue N-terminus that includes the flexible C-terminal
location based on 150 residues of a centralized 3-helix bundle. This location communicates
with a distantly located terminal organelle protein (i.e. MG200). The MG491 protein of
Mycoplasma genitalium contains symmetrically oriented dimer subunits that configure the
shape of a tetramer. The genome of Mycoplasma genitalium is based on 580kb genome with
a small structure (Iverson-Cabral, Astete, Cohen, Rocha, & Totten, 2006). The MgPar
sequences replicate repeatedly in Mycoplasma genitalium in relation to their shared ancestry
with the mgpB gene. MgPar and mgpB sequences undergo recombination episodes to
facilitate the configuration of MgPa proteins. The whole genome sequencing of Mycoplasma
genitalium by Fookes et al. (2017) revealed twenty-five locations that exhibited elevated SNP
density levels. These locations were based on the host interactions of MgPar loci. The
phylogenetic assessment of Mycoplasma genitalium revealed the existence of 2-distinct clads
with variable genetic structure. The SNP locations including parC and 23S rRNA (V-regions)
of Mycoplasma genitalium evidently contributed to its resistance against fluoroquinolone,
erythromycin, and azithromycin (Fookes et al., 2017). These SNPs were also found to be
consistent with minimum inhibitory concentration data of the respective microorganisms. The
proteome/complete genome assessment of Mycoplasma genitalium by Ma, et al. (2007)
revealed the recombination episodes through gene conversion/crossover between MgPar
sequences and MG192 expression location that resulted in several variations in the MG192
sequence.
The evidence-based findings reveal several sequence combinations/alliances that lead to the
significant structural/functional variability within the proteins of Mycoplasma genitalium. A
range of significant bioinformatics-based databases assists in evaluating the structure of
complex proteins. Some of the significant databases include UniProtKB, UniGene, GenBank,
and RefSeq (NCBI Reference Sequence Database) (Chen, Huang, & Wu, 2017). The
significant protein search tools include ‘BLAST’, ‘LinkOut’, ‘E-Utilities’, and ‘Batch
Entrez’. PROSITE assists in evaluating the functional patterns, locations, and domains of
significant proteins (PROSITE, 2019). Alternatively, the tools including Pfam, PRINTS,
ProDOM, Blocks, and MOTIF substantially assist in analyzing various protein sequences in
accordance with their multifactorial parameters (MOTIFS, 2019). The presented paper
effectively analyses the preselected protein sequence pertaining to the hypothetical protein
‘Mycoplasma genitalium’ across various protein sequence databases. The paper evaluates the
domain structure, likely function, appearance, comparative appearance (in other

P a g e | 3
organisms/life kingdoms), sequence alignment patterns, structure of related sequences,
transmembrane segments, and secondary structure of the selected protein sequence.
Methods
The presented study effectively evaluated the following protein sequence pertaining to M.
genitalium through the utilization of bioinformatics tool, techniques, methods, and databases.
MKKQLKYCFF SLFVSLSSIL SSCGSTTFVL ANFESYISPL LLERVQEKHP
LTFLTYPSNE
KLINGFANNT YSVAVASTYA VSELIERDLL SPIDWSQFNL KKSSSSSDKV
NNASDAKDLF
IDSIKEISQQ TKDSKNNELL HWAVPYFLQN LVFVYRGEKI SELEQENVSW
TDVIKAIVKH
KDRFNDNRLV FIDDARTIFS LANIVNTNNN SADVNPKEDG IGYFTNVYES
FQRLGLTKSN
LDSIFVNSDS NIVINELASG RRQGGIVYNG DAVYAALGGD LRDELSEEQI
PDGNNFHIVQ
PKISPVALDL LVINKQQSNF QKEAHEIIFD LALDGADQTK EQLIKTDEEL
GTDDEDFYLK
GAMQNFSYVN YVSPLKVISD PSTGIVSSKK NNAEMKSKQM STDQMTSEKE
FDYYTETLKA
LLEKEDSAEL NENEKKLVET IKKAYTIEKD SSIRWNQLVE KPISPLQRSN
LSLSWLDFKL
HWW
The study utilized Blast-p, PSI-BLAST, FASTA, Expasy, PROSITE, Pfam, EBI, and
SwissProt to evaluate and coherently explore/analyze the protein sequence outcomes and
structures. The BLAST findings in retrieving the significant M. genitalium sequences along
with their alignments. The extraction of gaps and identities assisted in predicting the
structural configuration of the retrieved protein variants. BLAST also assisted in evaluating
the identical-proteins and the comparatively analyzing different protein structures for
predicting their associated lipoproteins and functional variations. FASTA tool assisted in
evaluating the structural variants of Mycoplasma genitalium and PROSITE evaluation helped
in predicting the lipids and their structural/functional variations. The SWISS model proved to
be a significant tool in exploring the secondary protein structures for their comparative
assessment. This selected bioinformatics tools provided a significant interface to explore and
organisms/life kingdoms), sequence alignment patterns, structure of related sequences,
transmembrane segments, and secondary structure of the selected protein sequence.
Methods
The presented study effectively evaluated the following protein sequence pertaining to M.
genitalium through the utilization of bioinformatics tool, techniques, methods, and databases.
MKKQLKYCFF SLFVSLSSIL SSCGSTTFVL ANFESYISPL LLERVQEKHP
LTFLTYPSNE
KLINGFANNT YSVAVASTYA VSELIERDLL SPIDWSQFNL KKSSSSSDKV
NNASDAKDLF
IDSIKEISQQ TKDSKNNELL HWAVPYFLQN LVFVYRGEKI SELEQENVSW
TDVIKAIVKH
KDRFNDNRLV FIDDARTIFS LANIVNTNNN SADVNPKEDG IGYFTNVYES
FQRLGLTKSN
LDSIFVNSDS NIVINELASG RRQGGIVYNG DAVYAALGGD LRDELSEEQI
PDGNNFHIVQ
PKISPVALDL LVINKQQSNF QKEAHEIIFD LALDGADQTK EQLIKTDEEL
GTDDEDFYLK
GAMQNFSYVN YVSPLKVISD PSTGIVSSKK NNAEMKSKQM STDQMTSEKE
FDYYTETLKA
LLEKEDSAEL NENEKKLVET IKKAYTIEKD SSIRWNQLVE KPISPLQRSN
LSLSWLDFKL
HWW
The study utilized Blast-p, PSI-BLAST, FASTA, Expasy, PROSITE, Pfam, EBI, and
SwissProt to evaluate and coherently explore/analyze the protein sequence outcomes and
structures. The BLAST findings in retrieving the significant M. genitalium sequences along
with their alignments. The extraction of gaps and identities assisted in predicting the
structural configuration of the retrieved protein variants. BLAST also assisted in evaluating
the identical-proteins and the comparatively analyzing different protein structures for
predicting their associated lipoproteins and functional variations. FASTA tool assisted in
evaluating the structural variants of Mycoplasma genitalium and PROSITE evaluation helped
in predicting the lipids and their structural/functional variations. The SWISS model proved to
be a significant tool in exploring the secondary protein structures for their comparative
assessment. This selected bioinformatics tools provided a significant interface to explore and

P a g e | 4
align the protein variants in the context of correlating their structural
similarities/dissimilarities with the predicted functional outcomes. In summary, the selected
tools provided a platform to predict, model, analyze, integrate, and visualize the provided M.
genitalium sequence with a substantial matching score.
Results and Discussion
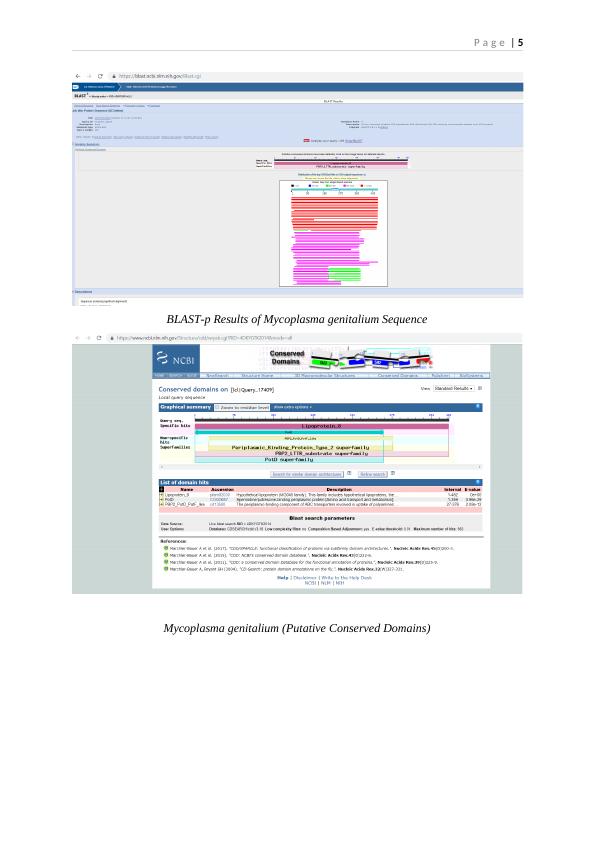
Sequence Analysis Through Blast-p and FASTA Algorithms (Structural and Functional
Assessment of the Conserved Putative Domains)
The analysis of the selected protein sequence through Blast-p tool revealed the occurrence of
putative conserved domains that included Lipoprotein_8, PotD, and PBP2_PotD_PotF_like
components (BLAST_Query_52229, 2019). Lipoprotein 8 revealed the hypothetical
lipoprotein that pertained to the solute binding family ‘MG045’. This finding suggests the
solute binding potential of Lipoprotein-8. PotD belonged to the periplasmic
Putrescine/Spermidine-binding protein associated with amino acid metabolism and transport.
The ABC transporters’ periplasmic-binding element was represented by
‘PBP2_PotD_PotF_like’ family that exhibited the capacity to facilitate polyamines' uptake.
This element included the periplasmic-binding fold (type II) (BLAST_Query_17409, 2019).
The family of this periplasmic substrate-binding conserved domain coordinates with
polyamine (ABC-type) transport processes in the form of elevated affinity primary receptors
that effectively regulate the intracellular levels of polyamine in various tumors and
proliferating cells. The polyamines including spermine, spermidine, and putrescine are the
natural polyamines that facilitate disease resistance, cellular proliferation/survival, and cell
growth. The natural polyamines also modulate the function of nucleic acids and other
negatively charged molecules. PBP2 family members represent periplasmic-binding (type II)
fold superfamily that contains the globular subdomains that connect with their individualized
ligands through the formation of a flexible hinge pattern. The binding of PBP2 ligands occurs
across the interdomain clefts while following a Venus flytrap pattern.
align the protein variants in the context of correlating their structural
similarities/dissimilarities with the predicted functional outcomes. In summary, the selected
tools provided a platform to predict, model, analyze, integrate, and visualize the provided M.
genitalium sequence with a substantial matching score.
Results and Discussion
Sequence Analysis Through Blast-p and FASTA Algorithms (Structural and Functional
Assessment of the Conserved Putative Domains)
The analysis of the selected protein sequence through Blast-p tool revealed the occurrence of
putative conserved domains that included Lipoprotein_8, PotD, and PBP2_PotD_PotF_like
components (BLAST_Query_52229, 2019). Lipoprotein 8 revealed the hypothetical
lipoprotein that pertained to the solute binding family ‘MG045’. This finding suggests the
solute binding potential of Lipoprotein-8. PotD belonged to the periplasmic
Putrescine/Spermidine-binding protein associated with amino acid metabolism and transport.
The ABC transporters’ periplasmic-binding element was represented by
‘PBP2_PotD_PotF_like’ family that exhibited the capacity to facilitate polyamines' uptake.
This element included the periplasmic-binding fold (type II) (BLAST_Query_17409, 2019).
The family of this periplasmic substrate-binding conserved domain coordinates with
polyamine (ABC-type) transport processes in the form of elevated affinity primary receptors
that effectively regulate the intracellular levels of polyamine in various tumors and
proliferating cells. The polyamines including spermine, spermidine, and putrescine are the
natural polyamines that facilitate disease resistance, cellular proliferation/survival, and cell
growth. The natural polyamines also modulate the function of nucleic acids and other
negatively charged molecules. PBP2 family members represent periplasmic-binding (type II)
fold superfamily that contains the globular subdomains that connect with their individualized
ligands through the formation of a flexible hinge pattern. The binding of PBP2 ligands occurs
across the interdomain clefts while following a Venus flytrap pattern.

P a g e | 5
BLAST-p Results of Mycoplasma genitalium Sequence
Mycoplasma genitalium (Putative Conserved Domains)
BLAST-p Results of Mycoplasma genitalium Sequence
Mycoplasma genitalium (Putative Conserved Domains)
P a g e | 6
M. genitalium (Basic Details)
Domain Structure Assessment Through FASTA revealed the following output
(FASTA_Query_I20190122-125408-0265-15530702-p2m, 2019).
M. genitalium (Basic Details)
Domain Structure Assessment Through FASTA revealed the following output
(FASTA_Query_I20190122-125408-0265-15530702-p2m, 2019).

End of preview
Want to access all the pages? Upload your documents or become a member.
