CSA742 Assignment: Adherent Cell Culture Passage Protocol & Analysis
VerifiedAdded on 2023/06/11
|11
|2690
|304
Report
AI Summary
This report provides a detailed protocol for passaging an adherent cell culture, explaining each step with reasons and relevant references. It covers the removal of the initial culture, washing the cells, adding a separation solution (like Trypsin or Express), incubating the cells, and observing detachment under a microscope. The report also discusses cell counting using a hemocytometer, highlighting the effectiveness of this method. Additionally, it describes the use of microtitre plates and multichannel pipettes, along with protein determination using the Biuret Assay method. Finally, it contrasts the passaging of adherent cells with the simpler process for suspension cells, emphasizing the advantages of spinner flasks for suspension cultures. The document includes data from protein tests and cell counting experiments, reflecting practical laboratory experiences.
1
UNIT:
NAME:
DATE:
UNIT:
NAME:
DATE:
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

2
Introduction
An Adherent Cell Culture is refers to a group of cells that are thriving inside a defined
culture. [1] Adherent cells bind to substrates in form of a monolayer. The form of culturing is
effective for a majority of cells such as those of primary and secondary culture. An appropriate
maintenance protocol requires the laboratory technician systematically passage the cells in vitro
from one culture to the other. The handling of the cells should ensure simple cell inspection by
the aid of a microscope. The disassociation of cells occurs in an enzymatic manner. Common
enzymes used in the passage protocol include: Typsin and Express. Laboratory technicians also
use mechanical means to separate the cells from each other. The major disadvantage of adherent
culture is the restriction in the growth rates of the cells. The surface area of the adherent substrate
is small hence limits the growth of the microorganisms. Therefore, the number of cells produced
at the end of the experiment reduced significantly due to the limitation. The microbiologists must
disinfect the vessel for the experiment before the onset of the experiment. Proper maintenance of
hygiene eliminates germs which interfere with the growth of the cells. An efficient research
method is essential in the process of passage. Examples of the methods include: cytology and
continuous harvest of the generated cells.
Protocol on How to Passage an Adherent Cell Culture
The first procedure involves the removal and throwing away of the culture used in the
experiment. [2] The researchers must get rid of the aiding culture to remain with the monolayer of
the cells. The removal ensures clarity in vision when the cells are under the microscopic
investigations. When the cells exist in an isolation form, they obtain enough nutrients to support
their growth. Furthermore, getting rid of the culture free the cells to easily adhere to the substrate
on the wall of the experimental vessel.
Introduction
An Adherent Cell Culture is refers to a group of cells that are thriving inside a defined
culture. [1] Adherent cells bind to substrates in form of a monolayer. The form of culturing is
effective for a majority of cells such as those of primary and secondary culture. An appropriate
maintenance protocol requires the laboratory technician systematically passage the cells in vitro
from one culture to the other. The handling of the cells should ensure simple cell inspection by
the aid of a microscope. The disassociation of cells occurs in an enzymatic manner. Common
enzymes used in the passage protocol include: Typsin and Express. Laboratory technicians also
use mechanical means to separate the cells from each other. The major disadvantage of adherent
culture is the restriction in the growth rates of the cells. The surface area of the adherent substrate
is small hence limits the growth of the microorganisms. Therefore, the number of cells produced
at the end of the experiment reduced significantly due to the limitation. The microbiologists must
disinfect the vessel for the experiment before the onset of the experiment. Proper maintenance of
hygiene eliminates germs which interfere with the growth of the cells. An efficient research
method is essential in the process of passage. Examples of the methods include: cytology and
continuous harvest of the generated cells.
Protocol on How to Passage an Adherent Cell Culture
The first procedure involves the removal and throwing away of the culture used in the
experiment. [2] The researchers must get rid of the aiding culture to remain with the monolayer of
the cells. The removal ensures clarity in vision when the cells are under the microscopic
investigations. When the cells exist in an isolation form, they obtain enough nutrients to support
their growth. Furthermore, getting rid of the culture free the cells to easily adhere to the substrate
on the wall of the experimental vessel.

3
The second protocol includes the cleaning of the cells. A solution of salt that is
chemically balanced is used to wash the adherent cells. The washing solution must not contain
traces of magnesium or calcium due to the ions' osmotic potentials. The researchers should then
add the solution used in washing to the opposite side of the vessel where the cells are attached.
Addition of the solution onto the opposite side prevents the interference of the cells. The
technician should then shake the vessel occasionally to ensure that the answer efficiently covers
the opposite side of the reaction vessel.
The next procedure of passage is to get rid of the solution used in washing the opposite
sides of the vessel. The solution contains Magnesium, Serum, and Calcium. [3] The three
components interfere with the activity of the reagents used in cell separation. The Cations have
high Valency, and osmotic pressure than the separation agent hence can stop the reaction.
The laboratory scientists should then add the separation solution to the section of the
vessel that contains the monolayer. The microbiologists should first warm the solution to get rid
of germs and to optimize the temperature for the reacting enzymes. The efficient agents of cell
separation include Express and Trypsin reagents. [4] The researchers should ensure that the
number of reagents used covers the entire monolayer. The lab technicians should use an
approximate 0.4 milliliters in ten square centimeters. The researchers should then shake the
vessel gently to ensure that the monolayer reacts efficiently with the reagent.
The incubation process follows the reaction between the monolayer and the cell
separation solution. The incubation should take three minutes and done under optimal enzymatic
temperatures. However, the conditions for the experiment depend on the type of cells and the
separation agents. Trypsin requires an extended time and a room temperature to effectively react
The second protocol includes the cleaning of the cells. A solution of salt that is
chemically balanced is used to wash the adherent cells. The washing solution must not contain
traces of magnesium or calcium due to the ions' osmotic potentials. The researchers should then
add the solution used in washing to the opposite side of the vessel where the cells are attached.
Addition of the solution onto the opposite side prevents the interference of the cells. The
technician should then shake the vessel occasionally to ensure that the answer efficiently covers
the opposite side of the reaction vessel.
The next procedure of passage is to get rid of the solution used in washing the opposite
sides of the vessel. The solution contains Magnesium, Serum, and Calcium. [3] The three
components interfere with the activity of the reagents used in cell separation. The Cations have
high Valency, and osmotic pressure than the separation agent hence can stop the reaction.
The laboratory scientists should then add the separation solution to the section of the
vessel that contains the monolayer. The microbiologists should first warm the solution to get rid
of germs and to optimize the temperature for the reacting enzymes. The efficient agents of cell
separation include Express and Trypsin reagents. [4] The researchers should ensure that the
number of reagents used covers the entire monolayer. The lab technicians should use an
approximate 0.4 milliliters in ten square centimeters. The researchers should then shake the
vessel gently to ensure that the monolayer reacts efficiently with the reagent.
The incubation process follows the reaction between the monolayer and the cell
separation solution. The incubation should take three minutes and done under optimal enzymatic
temperatures. However, the conditions for the experiment depend on the type of cells and the
separation agents. Trypsin requires an extended time and a room temperature to effectively react
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

4
with the cell layer during incubation. Moreover, there are cell lines which need less than one
minute in the incubation stage.
The researchers then use the microscope for cells observation. The viewing process
checks for cell detachment from the substrate on the sides of the vessel. The laboratory
technician should elevate the time for incubation in case the detachment does not exceed ninety
percent. The researchers should monitor the progress of cell disassociation at the intervals of
thirty seconds. The investigators can gently tap the container to increase the speed of
detachment.
The researchers should ensure that the percentage of cell detachment is equal or more
than ninety percent before the next step. The investigators should then drain the cells from the
reaction vessel by tilting the container. The tilting process should go on until all the cells have
detached from the sides of the container. The researchers should then transfer the solution of the
separated cells to the medium of growth. The volume of the transferred cells should be twice that
of the separation reagent. The next step involves pipetting the growth medium onto the
monolayer. The procedure should take up to two minutes.
Cell transfer to a conical flask is the next procedure. The researcher should transfer the
vial containing the detached cells into the centrifuge. The centrifugation process should last for
fifteen minutes at 250g. [5] However, the time and speed of centrifugation depend on the type of
cell under investigation.
The cells should again be suspended on an appropriate growth medium. The researcher
must warm the medium before use to optimize temperature for enzyme activity. The volume of
the medium should be insignificant to aid in cell growth and development efficiently. The
with the cell layer during incubation. Moreover, there are cell lines which need less than one
minute in the incubation stage.
The researchers then use the microscope for cells observation. The viewing process
checks for cell detachment from the substrate on the sides of the vessel. The laboratory
technician should elevate the time for incubation in case the detachment does not exceed ninety
percent. The researchers should monitor the progress of cell disassociation at the intervals of
thirty seconds. The investigators can gently tap the container to increase the speed of
detachment.
The researchers should ensure that the percentage of cell detachment is equal or more
than ninety percent before the next step. The investigators should then drain the cells from the
reaction vessel by tilting the container. The tilting process should go on until all the cells have
detached from the sides of the container. The researchers should then transfer the solution of the
separated cells to the medium of growth. The volume of the transferred cells should be twice that
of the separation reagent. The next step involves pipetting the growth medium onto the
monolayer. The procedure should take up to two minutes.
Cell transfer to a conical flask is the next procedure. The researcher should transfer the
vial containing the detached cells into the centrifuge. The centrifugation process should last for
fifteen minutes at 250g. [5] However, the time and speed of centrifugation depend on the type of
cell under investigation.
The cells should again be suspended on an appropriate growth medium. The researcher
must warm the medium before use to optimize temperature for enzyme activity. The volume of
the medium should be insignificant to aid in cell growth and development efficiently. The
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
5
sample is now ready hence the technician can remove a small section for the process of cell
enumeration.
The researchers should use hemacytometer to enumerate the cells. Other counting
equipment include trypan blue and cell counter. [5] The counting reveals the number of cells that
have undergone a successful detachment. The improvement of the concentration of cells involves
the addition of growth medium. The number of cells gradually increases after the recount due to
the increased growth rates. The researchers should then dilute the suspensions to appropriate
densities. Afterward, the lab technicians should pipette the required amount of cells into another
container that has a culture. The researcher should then incubate the cells for two minutes.
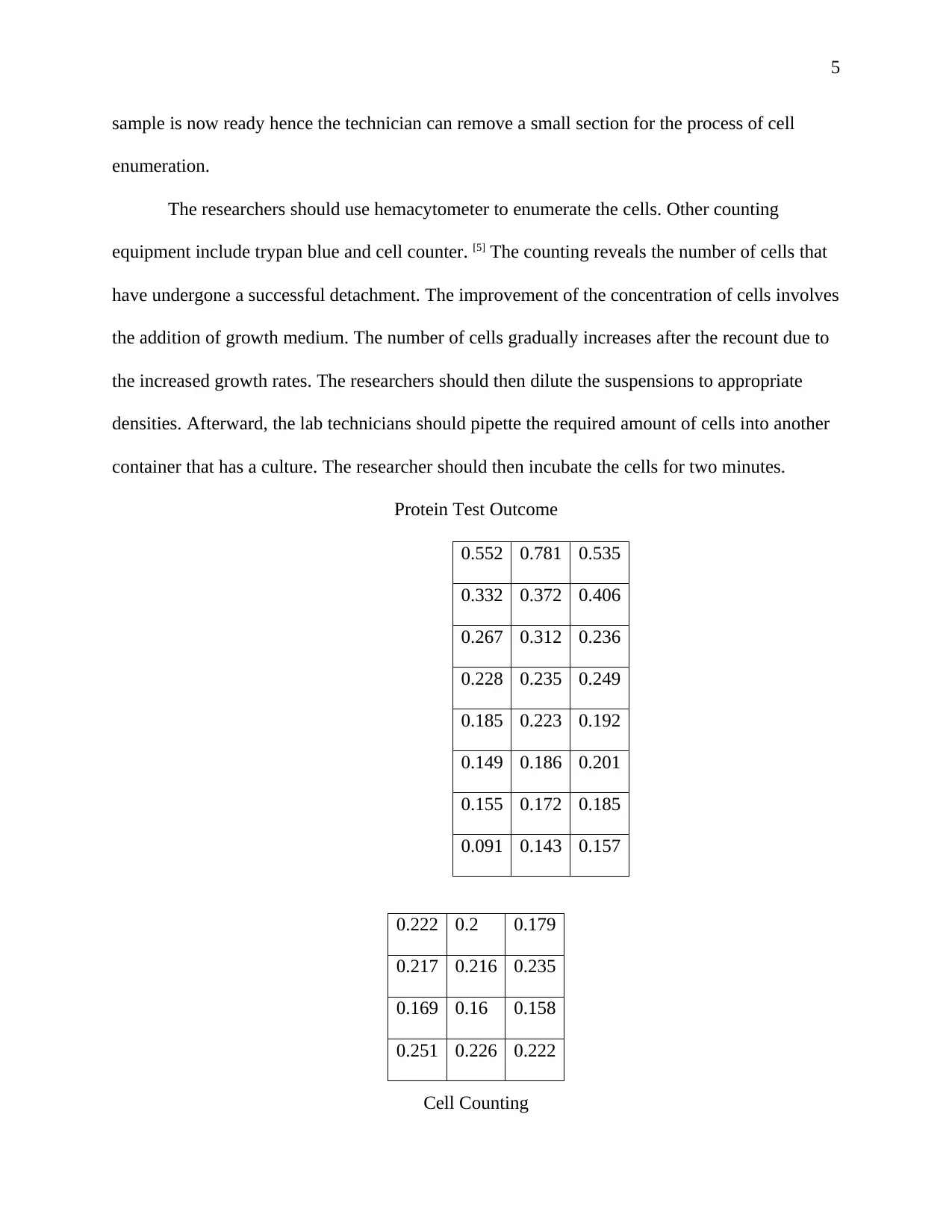
Protein Test Outcome
0.552 0.781 0.535
0.332 0.372 0.406
0.267 0.312 0.236
0.228 0.235 0.249
0.185 0.223 0.192
0.149 0.186 0.201
0.155 0.172 0.185
0.091 0.143 0.157
Cell Counting
0.222 0.2 0.179
0.217 0.216 0.235
0.169 0.16 0.158
0.251 0.226 0.222
sample is now ready hence the technician can remove a small section for the process of cell
enumeration.
The researchers should use hemacytometer to enumerate the cells. Other counting
equipment include trypan blue and cell counter. [5] The counting reveals the number of cells that
have undergone a successful detachment. The improvement of the concentration of cells involves
the addition of growth medium. The number of cells gradually increases after the recount due to
the increased growth rates. The researchers should then dilute the suspensions to appropriate
densities. Afterward, the lab technicians should pipette the required amount of cells into another
container that has a culture. The researcher should then incubate the cells for two minutes.
Protein Test Outcome
0.552 0.781 0.535
0.332 0.372 0.406
0.267 0.312 0.236
0.228 0.235 0.249
0.185 0.223 0.192
0.149 0.186 0.201
0.155 0.172 0.185
0.091 0.143 0.157
Cell Counting
0.222 0.2 0.179
0.217 0.216 0.235
0.169 0.16 0.158
0.251 0.226 0.222

6
Members of the group counted the detached cells using a hemocytometer. The lesson
from experience is that hemocytometer is the most efficient method of counting cells. The
procedure provides an elaborate way of enumerating viable cells. [6] The group finding concurred
with individual results during the enumeration process. The group members obtained the
expected results hence the quality of data was top notch. The group prepared the tool before
starting the process. The first step of using the equipment is by cleaning it with an appropriate
alcohol. The second step involves smearing the coverslip with moisture before fixing it onto the
counting equipment.
The researcher should swirl the vessel containing the cells to ensure even distribution
before placement onto the hemocytometer. The investigator should then use a pipette to transfer
the sample to be counted into the Eppendorf flask. The researcher should add Typhan to the
contents of the vial and gently stir the solution. Afterward, the investigator should transfer 100
microlitres of the answer from the Eppendorf to the enumeration machine. The hemocytometer
has a counting segment where capillary movement facilitates the enumeration process. The next
process involves focusing the microscope on the hemocytometer. The group then used the tally
method to count both the dead and live cells. Live cells do not have the stains whereas; the dead
cells are stained in the blue color of trypsin.
Microtitre plates and Multichannel pipettes.
The microtitre plates and the pipettes were useful due to the small size of the samples.
The dish is flat and has numerous wells that serve as test tubes. Every well in the plate can
contain many nanometers of the cell sample. The wells are square or circular in orientation.
However, the group used dishes that had circular shapes. The group members used caps made of
silicon to close the wells after the experiment. Multichannel pipettes make the uptake of samples
Members of the group counted the detached cells using a hemocytometer. The lesson
from experience is that hemocytometer is the most efficient method of counting cells. The
procedure provides an elaborate way of enumerating viable cells. [6] The group finding concurred
with individual results during the enumeration process. The group members obtained the
expected results hence the quality of data was top notch. The group prepared the tool before
starting the process. The first step of using the equipment is by cleaning it with an appropriate
alcohol. The second step involves smearing the coverslip with moisture before fixing it onto the
counting equipment.
The researcher should swirl the vessel containing the cells to ensure even distribution
before placement onto the hemocytometer. The investigator should then use a pipette to transfer
the sample to be counted into the Eppendorf flask. The researcher should add Typhan to the
contents of the vial and gently stir the solution. Afterward, the investigator should transfer 100
microlitres of the answer from the Eppendorf to the enumeration machine. The hemocytometer
has a counting segment where capillary movement facilitates the enumeration process. The next
process involves focusing the microscope on the hemocytometer. The group then used the tally
method to count both the dead and live cells. Live cells do not have the stains whereas; the dead
cells are stained in the blue color of trypsin.
Microtitre plates and Multichannel pipettes.
The microtitre plates and the pipettes were useful due to the small size of the samples.
The dish is flat and has numerous wells that serve as test tubes. Every well in the plate can
contain many nanometers of the cell sample. The wells are square or circular in orientation.
However, the group used dishes that had circular shapes. The group members used caps made of
silicon to close the wells after the experiment. Multichannel pipettes make the uptake of samples
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

7
to be a natural process. The manufactures autoclave them; hence they arrive in the laboratory in a
sterile state. [7] The pipettes can tolerate dangerous chemical compositions thus has an endless
range of application. The microchannel pipettes are light in weight and have from eight to
sixteen working channels. The laboratory apparatus has an efficient force of ejection and a
plunger located at the lowest level. The two qualities make it provide the users with a working
comfort. The Ultra Violet rays cannot affect the pipettes hence maintaining the chemical and
physical characteristics of the samples under investigation.
Protein Determination
The most accurate procedure of protein determination that the group applied is the Biuret
Assay method. The Lowry principle of protein determination is identical to the Biuret test. [8] The
only fundamental difference between the two is the incubation period. The Biuret test requires
twenty minutes for incubation whereas the other method requires less than fifteen minutes
completing the same process. The principle of the determination states that protein components
that have more than two bonds yield an elaborate compound in a solution of copper. However,
the investigators must create conditions of alkaline to facilitate the process.
The equipment required for detection include glass and a light spectrophotometer. The
reagents that the group used are tartrate, Potassium iodide, and copper sulfate. The investigators
should then dissolve the reagents in sodium hydroxide and top up the solution with distilled
water up to the 100cm mark. The laboratory technicians should get rid of any precipitate formed
in the initial stages of protein determination. The spectrophotometer needs ten minutes of
warming before the application. A tube of reference is necessary and should contain a significant
amount of buffer. The amount of sample from each essay should be 0.5 milliliters. The
researchers should add 10millilitres of Biuret solution to every tube that contains the protein
to be a natural process. The manufactures autoclave them; hence they arrive in the laboratory in a
sterile state. [7] The pipettes can tolerate dangerous chemical compositions thus has an endless
range of application. The microchannel pipettes are light in weight and have from eight to
sixteen working channels. The laboratory apparatus has an efficient force of ejection and a
plunger located at the lowest level. The two qualities make it provide the users with a working
comfort. The Ultra Violet rays cannot affect the pipettes hence maintaining the chemical and
physical characteristics of the samples under investigation.
Protein Determination
The most accurate procedure of protein determination that the group applied is the Biuret
Assay method. The Lowry principle of protein determination is identical to the Biuret test. [8] The
only fundamental difference between the two is the incubation period. The Biuret test requires
twenty minutes for incubation whereas the other method requires less than fifteen minutes
completing the same process. The principle of the determination states that protein components
that have more than two bonds yield an elaborate compound in a solution of copper. However,
the investigators must create conditions of alkaline to facilitate the process.
The equipment required for detection include glass and a light spectrophotometer. The
reagents that the group used are tartrate, Potassium iodide, and copper sulfate. The investigators
should then dissolve the reagents in sodium hydroxide and top up the solution with distilled
water up to the 100cm mark. The laboratory technicians should get rid of any precipitate formed
in the initial stages of protein determination. The spectrophotometer needs ten minutes of
warming before the application. A tube of reference is necessary and should contain a significant
amount of buffer. The amount of sample from each essay should be 0.5 milliliters. The
researchers should add 10millilitres of Biuret solution to every tube that contains the protein
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

8
sample. The next step is to vortex the tubes and allows them to stand for twenty minutes. Finally,
the technicians should take the readings at 600 nanometers.
Passaging of Suspension Cells
The process of Passaging Suspension cells is more straightforward than dealing with
adherent cells. The suspension cells are already in the culture medium as opposed to adherent
cells which are attached to the substrate. The process of passing suspended cultures is efficient
and rapid compared to the adherent cultures. [9] The first procedure involves maintaining the cells
for up to three days before the confluence stage. The cells require current act provision of
nutrients to aid in their growth and development. Sterile flasks are necessary for the suspension
of the cultures. However, the suspension cultures can also survive in non-sterile environments. A
majority of laboratory technicians prefer spinner to shaker flasks. The prior container enhances
gaseous exchange. Moreover, the spinner flasks create room for the culturing of a high number
of cells at any given time. [10] The bowls are either medium or vertical in orientation. The group
members used the upright vials due to the aeration qualities hence leading to accurate data.
Conclusion
An adherent group of cells is those that stick onto the surface of the reaction vessel. The
first procedure of passing the adherent cells is the removal and throwing away of the culture used
in the experiment. The cleaning of the cells is the next step as the washing process eliminates
cations such as magnesium. The researchers should get rid of the washing solution after the
completion of the cleaning process. The next step involves passing the used solution on the
opposite side of the adherent cells. The researcher then adds a dissociation solution onto the
monolayer of cells bound to the substrate.
sample. The next step is to vortex the tubes and allows them to stand for twenty minutes. Finally,
the technicians should take the readings at 600 nanometers.
Passaging of Suspension Cells
The process of Passaging Suspension cells is more straightforward than dealing with
adherent cells. The suspension cells are already in the culture medium as opposed to adherent
cells which are attached to the substrate. The process of passing suspended cultures is efficient
and rapid compared to the adherent cultures. [9] The first procedure involves maintaining the cells
for up to three days before the confluence stage. The cells require current act provision of
nutrients to aid in their growth and development. Sterile flasks are necessary for the suspension
of the cultures. However, the suspension cultures can also survive in non-sterile environments. A
majority of laboratory technicians prefer spinner to shaker flasks. The prior container enhances
gaseous exchange. Moreover, the spinner flasks create room for the culturing of a high number
of cells at any given time. [10] The bowls are either medium or vertical in orientation. The group
members used the upright vials due to the aeration qualities hence leading to accurate data.
Conclusion
An adherent group of cells is those that stick onto the surface of the reaction vessel. The
first procedure of passing the adherent cells is the removal and throwing away of the culture used
in the experiment. The cleaning of the cells is the next step as the washing process eliminates
cations such as magnesium. The researchers should get rid of the washing solution after the
completion of the cleaning process. The next step involves passing the used solution on the
opposite side of the adherent cells. The researcher then adds a dissociation solution onto the
monolayer of cells bound to the substrate.

9
The incubation process follows the addition of the separation reagents. The process
should take up to three minutes and carry out under optimal temperatures. The researchers then
use a microscope to observe the cells. The dead cells strain with Tryphan and hence appear
bluish under the microscope. The live cells are stainless since they do not react with the reagent.
The groups used hemocytometer to count both viable and dead cells. The microtitre plate and the
microchannel pipettes make work easy due to their rapid functioning. The group used Biuret test
to determine the presence of proteins in the assay. Passaging of suspension cells is faster than
adherent cells.
The incubation process follows the addition of the separation reagents. The process
should take up to three minutes and carry out under optimal temperatures. The researchers then
use a microscope to observe the cells. The dead cells strain with Tryphan and hence appear
bluish under the microscope. The live cells are stainless since they do not react with the reagent.
The groups used hemocytometer to count both viable and dead cells. The microtitre plate and the
microchannel pipettes make work easy due to their rapid functioning. The group used Biuret test
to determine the presence of proteins in the assay. Passaging of suspension cells is faster than
adherent cells.
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

10
References
1. Bigildeev A, Sats N, Shipounova I, Petinati N, Surin V, Cornils K, Riecken K, Aranyossy
T, Fehse B, Drize N. Fibroblastic colony forming units (CFU-F) within adherent cell
layer from long-term bone marrow cultures correspond to the progeny of distinct
mesenchymal precursor cells. Experimental Hematology. 2015 Sep 1;43(9): S53.
2. Phelan K, May KM. Basic techniques in mammalian cell tissue culture. Current protocols
in cell biology. 2015 Mar;66(1):1-.
3. Freshney RI. The culture of animal cells: a manual of basic technique and specialized
applications. John Wiley & Sons; 2015 Dec 23.
4. Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell
preparation protocols. BioMed research international. 2014;2014.
5. Rahman M, Reyner K, Deleyrolle L, Millette S, Azari H, Day BW, Stringer BW, Boyd
AW, Johns TG, Blot V, Duggal R. Neurosphere and adherent culture conditions are
equivalent for malignant glioma stem cell lines. Anatomy & cell biology. 2015 Mar
1;48(1):25-35.
6. Grishagin IV. Automatic cell counting with ImageJ. Analytical biochemistry. 2015 Mar
15;473:63-5.
7. Kasama T, Kaji N, Tokeshi M, Baba Y. Fabrication and Evaluation of Microfluidic
Immunoassay Devices with Antibody-Immobilized Microbeads Retained in Porous
Hydrogel Micropillars. InMicrochip Diagnostics 2017 (pp. 49-56). Humana Press, New
York, NY.
8. Janairo G, Linley MS, Yap L, Llanos-Lazaro N, Robles J. Determination of the
sensitivity range of biuret test for undergraduate biochemistry experiments.
References
1. Bigildeev A, Sats N, Shipounova I, Petinati N, Surin V, Cornils K, Riecken K, Aranyossy
T, Fehse B, Drize N. Fibroblastic colony forming units (CFU-F) within adherent cell
layer from long-term bone marrow cultures correspond to the progeny of distinct
mesenchymal precursor cells. Experimental Hematology. 2015 Sep 1;43(9): S53.
2. Phelan K, May KM. Basic techniques in mammalian cell tissue culture. Current protocols
in cell biology. 2015 Mar;66(1):1-.
3. Freshney RI. The culture of animal cells: a manual of basic technique and specialized
applications. John Wiley & Sons; 2015 Dec 23.
4. Ikebe C, Suzuki K. Mesenchymal stem cells for regenerative therapy: optimization of cell
preparation protocols. BioMed research international. 2014;2014.
5. Rahman M, Reyner K, Deleyrolle L, Millette S, Azari H, Day BW, Stringer BW, Boyd
AW, Johns TG, Blot V, Duggal R. Neurosphere and adherent culture conditions are
equivalent for malignant glioma stem cell lines. Anatomy & cell biology. 2015 Mar
1;48(1):25-35.
6. Grishagin IV. Automatic cell counting with ImageJ. Analytical biochemistry. 2015 Mar
15;473:63-5.
7. Kasama T, Kaji N, Tokeshi M, Baba Y. Fabrication and Evaluation of Microfluidic
Immunoassay Devices with Antibody-Immobilized Microbeads Retained in Porous
Hydrogel Micropillars. InMicrochip Diagnostics 2017 (pp. 49-56). Humana Press, New
York, NY.
8. Janairo G, Linley MS, Yap L, Llanos-Lazaro N, Robles J. Determination of the
sensitivity range of biuret test for undergraduate biochemistry experiments.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

11
9. Cotter CA, Earl PL, Wyatt LS, Moss B. Preparation of cell cultures and vaccinia virus
stocks. Current protocols in molecular biology. 2017 Jan; 117 (1):16-.
10. Mollard R. Culture, cryobanking and passaging of karyotypically validated native
Australian amphibian cells. Cryobiology. 2018 Apr 1; 81:201-5.
9. Cotter CA, Earl PL, Wyatt LS, Moss B. Preparation of cell cultures and vaccinia virus
stocks. Current protocols in molecular biology. 2017 Jan; 117 (1):16-.
10. Mollard R. Culture, cryobanking and passaging of karyotypically validated native
Australian amphibian cells. Cryobiology. 2018 Apr 1; 81:201-5.
1 out of 11
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2025 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.