Report on Expansion Processes of a Perfect Gas Experiment Analysis
VerifiedAdded on 2023/06/11
|7
|1548
|293
Practical Assignment
AI Summary
This document presents an experimental analysis of the expansion processes of a perfect gas, focusing on familiarizing students with fundamental thermodynamic principles. The experiment aims to understand the first and second laws of thermodynamics and the P-V-T relationships relevant to industrial applications. The methodology involves measuring heat capacity and volume ratios, using TH5-B equipment to record atmospheric pressure, and employing valves to control air pressure within a closed system. The experiment includes two tests: one determining the heat capacity ratio through pressure measurements and the other calculating the volume ratio using the ideal gas equation. Results are presented in tables and graphs, showing the relationship between heat capacity ratio and average pressure, as well as volume ratio and final pressure. The discussion interprets the data, addresses potential errors, and explains why the initial expansion process is considered adiabatic. The conclusion states that the experiment successfully achieved its objectives, demonstrating the relationships between volume, temperature, and pressure in gases.

Expansion Processes of a Perfect Gas
Introdction:
The paper has aim in the expansion of the perfect gas experiment processes that will
familiarize the student about the fundamental processes of thermodynamic. In overall, it is
important to understand the thermodynamics first and second law and the relationship of
P-V-T for the industry of thermodynamics applications. The report has provided an outcome
with description of the methodology that has been used for the experiment. The paper will
then provide with the experimental result and some question will be discussed with final
conclusion.
Description of the methodology:
The methodology will describe the capacity of heat and experiments the volume ratio that
can measure and then proof about (Patm ¿ with the use of barometer which is in (101.325
KN /m2 ¿ and another use is the temperature of 31.3℃. In the experiment TH5-B equipment
will be used to measure and keep the record of Patm with the use of barometer. There are
two close valves which include V1, V2 and one open valve namely V4. It will approximately
pressed the atmospheric pressure that are allowed in the space for 30 KN /m2. It then close
the V4 and the air pump. Throughout the process the remaining air temperature will
change in the room just after the valve is closed. This will give the reading of pressure Ps
value from most of the computer were the pressure is steady inside the room. There are air
of small percentage that allow to get air away from the container when the valve V1 that is
closed is quickly open at the record time Pi . The final pressure Pf has to read with the
container whose contents are return to the temperature of ambient. Thus, the ratio of heat
capacity is determine that are same with the volume ratio that will be determine. However,
the content of the pressures is steady that record the pressurePs reading. It is important to
make sure that the V5 valve is completely closed and then it will open the valve V6 that is
isolating and then the valve V5 has to open again for very little were the air are let to escape
through a large container into a smaller container. It will slowly reduce the valve V5 taking
into consideration that there is no temperature change.
Fahad Aljehani 17416232
Introdction:
The paper has aim in the expansion of the perfect gas experiment processes that will
familiarize the student about the fundamental processes of thermodynamic. In overall, it is
important to understand the thermodynamics first and second law and the relationship of
P-V-T for the industry of thermodynamics applications. The report has provided an outcome
with description of the methodology that has been used for the experiment. The paper will
then provide with the experimental result and some question will be discussed with final
conclusion.
Description of the methodology:
The methodology will describe the capacity of heat and experiments the volume ratio that
can measure and then proof about (Patm ¿ with the use of barometer which is in (101.325
KN /m2 ¿ and another use is the temperature of 31.3℃. In the experiment TH5-B equipment
will be used to measure and keep the record of Patm with the use of barometer. There are
two close valves which include V1, V2 and one open valve namely V4. It will approximately
pressed the atmospheric pressure that are allowed in the space for 30 KN /m2. It then close
the V4 and the air pump. Throughout the process the remaining air temperature will
change in the room just after the valve is closed. This will give the reading of pressure Ps
value from most of the computer were the pressure is steady inside the room. There are air
of small percentage that allow to get air away from the container when the valve V1 that is
closed is quickly open at the record time Pi . The final pressure Pf has to read with the
container whose contents are return to the temperature of ambient. Thus, the ratio of heat
capacity is determine that are same with the volume ratio that will be determine. However,
the content of the pressures is steady that record the pressurePs reading. It is important to
make sure that the V5 valve is completely closed and then it will open the valve V6 that is
isolating and then the valve V5 has to open again for very little were the air are let to escape
through a large container into a smaller container. It will slowly reduce the valve V5 taking
into consideration that there is no temperature change.
Fahad Aljehani 17416232
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
Results:
Test A – Heat Capacity ratio:
To c calculate the Absolute Pressure in heat capacity ratio can determine by:
PAP=Patm+ Ps
The heat capacity ratio in the two- step process can determine by:
Cp
Cv = ln Ps−ln Pi
ln Ps−ln Pf
To calculate the error in heat capacity ratio can determined by:
Error=
C p
Cv
−Exact heat capacity
exact heat capacity ∗100
To c calculate the Average Pressure in heat capacity ratio can determine by:
Average Pressure= Ps +Pf
2
Table 1
kPa % kPa kPa
Runs Ps Pi Pf Cp/Cv Exact heat
capacity
theoretical
Error Absolute
Pressures
Average
Pressures
1 29.77 19.6 22.7 1.54156 1.401 10.11143 131.095 26.235
2 22.7 17.36 19.07 1.53916 1.401 9.86152 124.025 20.885
3 19.07 16.23 17.17 1.53645 1.401 9.66809 120.395 18.12
4 17.17 14.97 15.85 1.71406 1.401 22.43285 118.495 16.51
5 15.85 18.37 21.04 0.52090 1.401 62.81941 131.025 18.445
6 21.04 16.11 17.78 1.58588 1.401 13.27714 122.365 19.41
7 17.78 6.26 10.67 2.06315 1.401 47.26266 113.105 14.225
Fahad Aljehani 17416232
Test A – Heat Capacity ratio:
To c calculate the Absolute Pressure in heat capacity ratio can determine by:
PAP=Patm+ Ps
The heat capacity ratio in the two- step process can determine by:
Cp
Cv = ln Ps−ln Pi
ln Ps−ln Pf
To calculate the error in heat capacity ratio can determined by:
Error=
C p
Cv
−Exact heat capacity
exact heat capacity ∗100
To c calculate the Average Pressure in heat capacity ratio can determine by:
Average Pressure= Ps +Pf
2
Table 1
kPa % kPa kPa
Runs Ps Pi Pf Cp/Cv Exact heat
capacity
theoretical
Error Absolute
Pressures
Average
Pressures
1 29.77 19.6 22.7 1.54156 1.401 10.11143 131.095 26.235
2 22.7 17.36 19.07 1.53916 1.401 9.86152 124.025 20.885
3 19.07 16.23 17.17 1.53645 1.401 9.66809 120.395 18.12
4 17.17 14.97 15.85 1.71406 1.401 22.43285 118.495 16.51
5 15.85 18.37 21.04 0.52090 1.401 62.81941 131.025 18.445
6 21.04 16.11 17.78 1.58588 1.401 13.27714 122.365 19.41
7 17.78 6.26 10.67 2.06315 1.401 47.26266 113.105 14.225
Fahad Aljehani 17416232
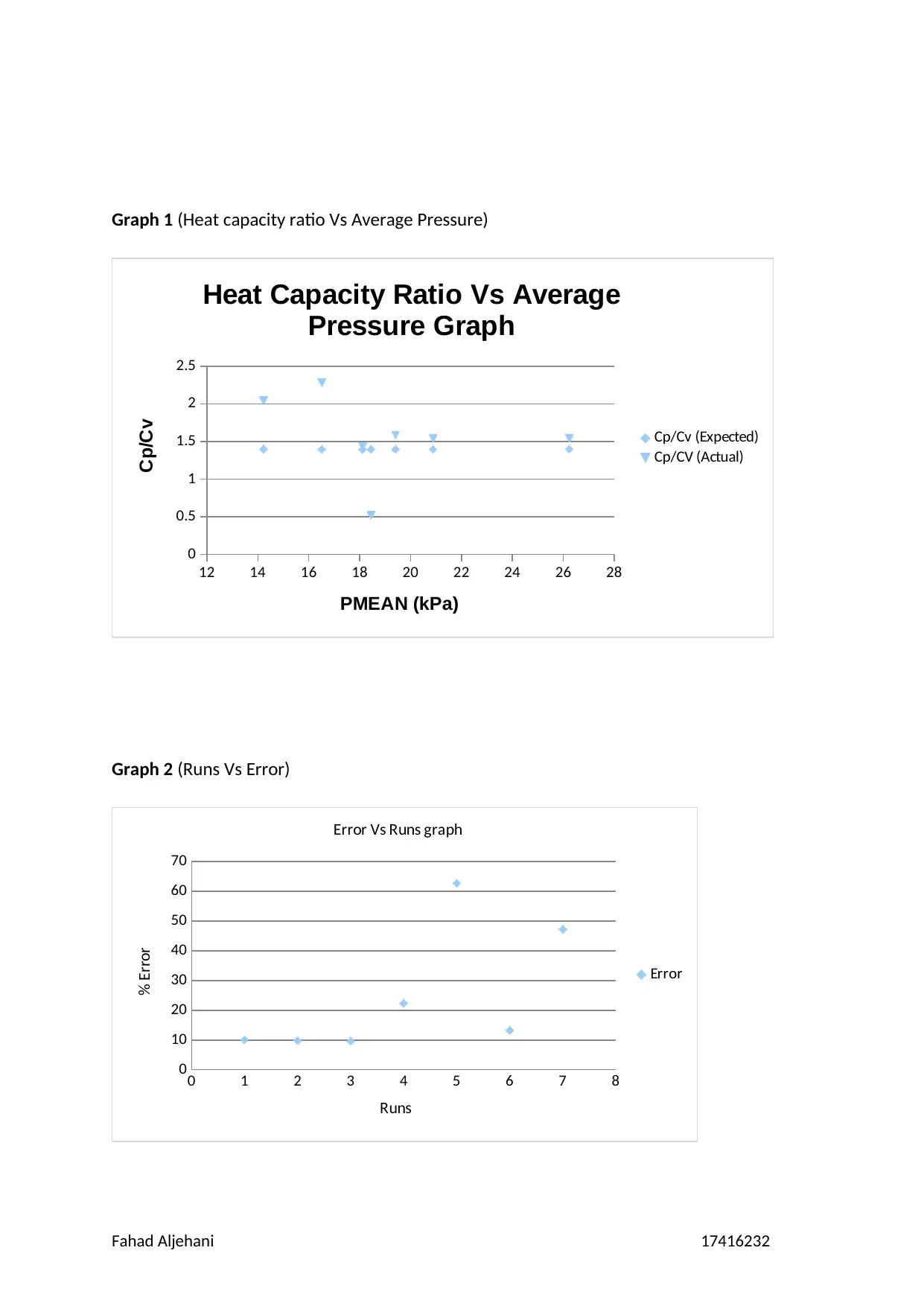
Graph 1 (Heat capacity ratio Vs Average Pressure)
12 14 16 18 20 22 24 26 28
0
0.5
1
1.5
2
2.5
Heat Capacity Ratio Vs Average
Pressure Graph
Cp/Cv (Expected)
Cp/CV (Actual)
PMEAN (kPa)
Cp/Cv
Graph 2 (Runs Vs Error)
0 1 2 3 4 5 6 7 8
0
10
20
30
40
50
60
70
Error Vs Runs graph
Error
Runs
% Error
Fahad Aljehani 17416232
12 14 16 18 20 22 24 26 28
0
0.5
1
1.5
2
2.5
Heat Capacity Ratio Vs Average
Pressure Graph
Cp/Cv (Expected)
Cp/CV (Actual)
PMEAN (kPa)
Cp/Cv
Graph 2 (Runs Vs Error)
0 1 2 3 4 5 6 7 8
0
10
20
30
40
50
60
70
Error Vs Runs graph
Error
Runs
% Error
Fahad Aljehani 17416232
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
Test B – Ratio of Volume:
Ideal gas equation of state, the volume ratio of the vessels can determine by:
V 1
V 2
= P2 s −Pf
Pf −P1 s
To calculate the error in heat capacity ratio can determined by:
Error=
V 1
V 2
−Therotical volume ratio
Therotical volume ratio ∗100
Table 2
℃ kPa %
Runs T P atm P1s P2s Pf V1/V2 Error Ratio of
volume
theoretical
1 31.1 101.325 24.92 21.20 22.83 0.77990 68.3160 2.4615
2 31.1 101.325 29.53 20.74 27.12 2.64730 7.5482 2.4615
3 31.5 101.325 29.52 6.62 22.06 2.06970 15.9171 2.4615
4 31.8 101.325 28.77 9.75 22.4 1.98587 19.3227 2.4615
5 31.7 101.325 29.23 9.33 23.32 2.36717 3.8322 2.4615
6 31.8 101.325 30.15 9.58 23.4 2.04740 16.8230 2.4615
Graph 3 ( Volume ratio VS Final Pressure)
Fahad Aljehani 17416232
Ideal gas equation of state, the volume ratio of the vessels can determine by:
V 1
V 2
= P2 s −Pf
Pf −P1 s
To calculate the error in heat capacity ratio can determined by:
Error=
V 1
V 2
−Therotical volume ratio
Therotical volume ratio ∗100
Table 2
℃ kPa %
Runs T P atm P1s P2s Pf V1/V2 Error Ratio of
volume
theoretical
1 31.1 101.325 24.92 21.20 22.83 0.77990 68.3160 2.4615
2 31.1 101.325 29.53 20.74 27.12 2.64730 7.5482 2.4615
3 31.5 101.325 29.52 6.62 22.06 2.06970 15.9171 2.4615
4 31.8 101.325 28.77 9.75 22.4 1.98587 19.3227 2.4615
5 31.7 101.325 29.23 9.33 23.32 2.36717 3.8322 2.4615
6 31.8 101.325 30.15 9.58 23.4 2.04740 16.8230 2.4615
Graph 3 ( Volume ratio VS Final Pressure)
Fahad Aljehani 17416232
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
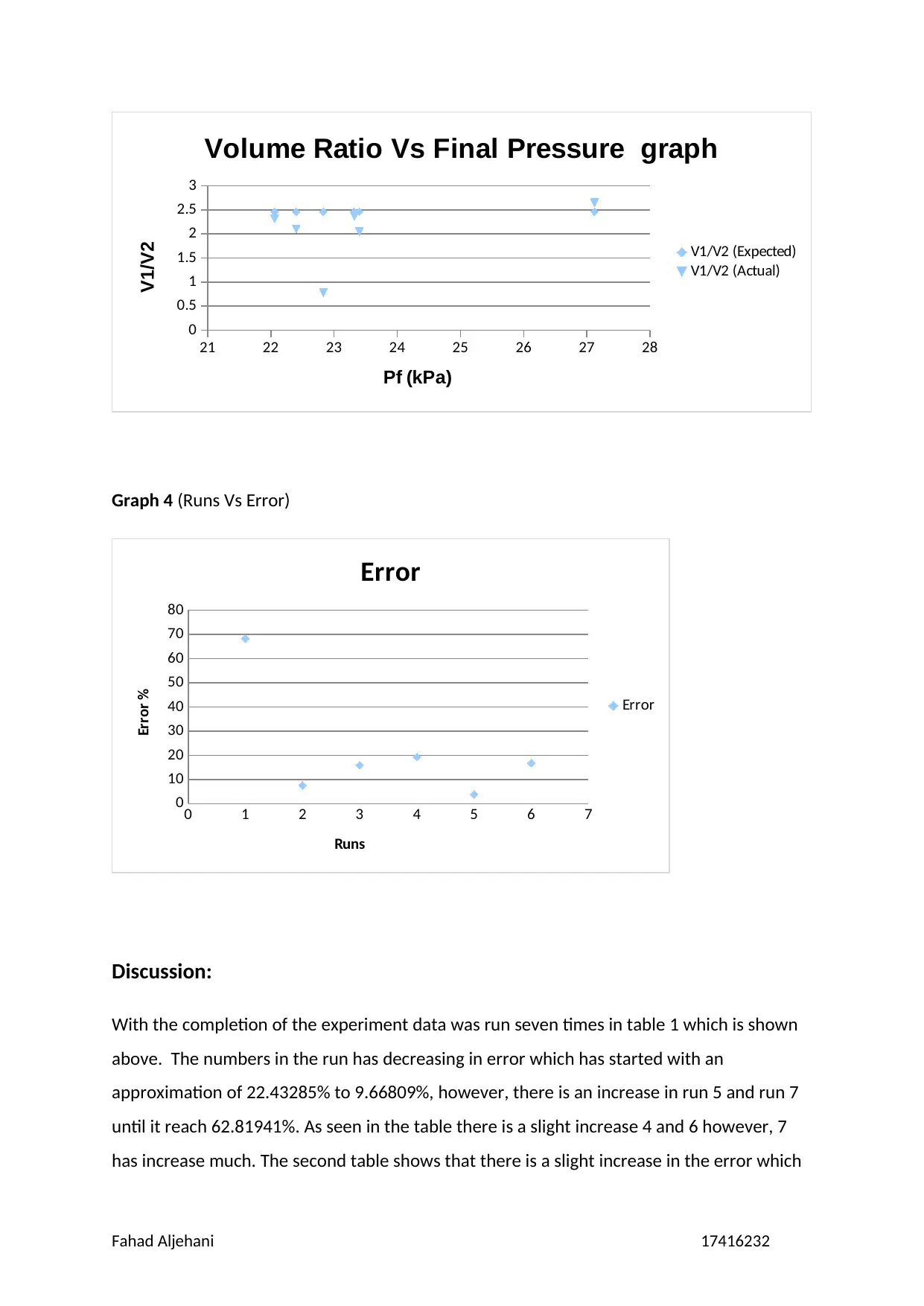
21 22 23 24 25 26 27 28
0
0.5
1
1.5
2
2.5
3
Volume Ratio Vs Final Pressure graph
V1/V2 (Expected)
V1/V2 (Actual)
Pf (kPa)
V1/V2
Graph 4 (Runs Vs Error)
0 1 2 3 4 5 6 7
0
10
20
30
40
50
60
70
80
Error
Error
Runs
Error %
Discussion:
With the completion of the experiment data was run seven times in table 1 which is shown
above. The numbers in the run has decreasing in error which has started with an
approximation of 22.43285% to 9.66809%, however, there is an increase in run 5 and run 7
until it reach 62.81941%. As seen in the table there is a slight increase 4 and 6 however, 7
has increase much. The second table shows that there is a slight increase in the error which
Fahad Aljehani 17416232
0
0.5
1
1.5
2
2.5
3
Volume Ratio Vs Final Pressure graph
V1/V2 (Expected)
V1/V2 (Actual)
Pf (kPa)
V1/V2
Graph 4 (Runs Vs Error)
0 1 2 3 4 5 6 7
0
10
20
30
40
50
60
70
80
Error
Error
Runs
Error %
Discussion:
With the completion of the experiment data was run seven times in table 1 which is shown
above. The numbers in the run has decreasing in error which has started with an
approximation of 22.43285% to 9.66809%, however, there is an increase in run 5 and run 7
until it reach 62.81941%. As seen in the table there is a slight increase 4 and 6 however, 7
has increase much. The second table shows that there is a slight increase in the error which
Fahad Aljehani 17416232

is from 3.8322% to 19.3227%, however, if run 1 is considered then it has reached to
68.3160%. Moreover, the pressure that is absolute and average could be calculated that can
give an accurate result. The experiment has a result that has a familiar procedure and also
has accurate lab measurement. The theoretical experiment shoes the exact ratio of heat
capacity that varies in between 1.399 and 1.401 which range the temperature from -40℃
to 50 ℃, and has the theoretical ratio of volume of 2.4615. Thus, with this it has indicated
that there is a consistent run of the experiment across all the run. The data of the heating
capacities can use the ratio of dependable sign.
Why can the initial expansion process be considered as adiabatic?
Adiabatic is considered for the initial expansion process because there is no transfer of heat
occurs in between the atmosphere and the system. The change in temperature and
pressure are connected to each other, however due to low pressure it go through valve V1
in a fast manner that there is a negligible change in temperature. Thus, for the mention
reason, the initial process of expansion was considered to be adiabatic.
How well the results do obtained compare to the expected result? Give possible reasons
for any difference.
Each of the run has different result and is similar to other group. Different run number give
mostly accurate result that probably fix most possible error. As seen in table 1, the Cp/ Cv has
run 7 as higher value that is 2.06315 kPa and the smallest value for run 5 is 0.52090 kPa.
However, the result of the rest are closest to value that are actual for Cp/ Cv. Throughout the
experiment there is a possibility of human errors which are reading display and the
condition of the surrounding which give a different percentage error.
Comment on how the rate of change affects the temperature of the air inside the
vessels?
The computer was set up with the pressure of transient response that take and keep the
record of reading in each second. There is an unstable readings of the pressure which drops
slowly until there is a consistency in the system to reach the equilibrium. Due the situation
Fahad Aljehani 17416232
68.3160%. Moreover, the pressure that is absolute and average could be calculated that can
give an accurate result. The experiment has a result that has a familiar procedure and also
has accurate lab measurement. The theoretical experiment shoes the exact ratio of heat
capacity that varies in between 1.399 and 1.401 which range the temperature from -40℃
to 50 ℃, and has the theoretical ratio of volume of 2.4615. Thus, with this it has indicated
that there is a consistent run of the experiment across all the run. The data of the heating
capacities can use the ratio of dependable sign.
Why can the initial expansion process be considered as adiabatic?
Adiabatic is considered for the initial expansion process because there is no transfer of heat
occurs in between the atmosphere and the system. The change in temperature and
pressure are connected to each other, however due to low pressure it go through valve V1
in a fast manner that there is a negligible change in temperature. Thus, for the mention
reason, the initial process of expansion was considered to be adiabatic.
How well the results do obtained compare to the expected result? Give possible reasons
for any difference.
Each of the run has different result and is similar to other group. Different run number give
mostly accurate result that probably fix most possible error. As seen in table 1, the Cp/ Cv has
run 7 as higher value that is 2.06315 kPa and the smallest value for run 5 is 0.52090 kPa.
However, the result of the rest are closest to value that are actual for Cp/ Cv. Throughout the
experiment there is a possibility of human errors which are reading display and the
condition of the surrounding which give a different percentage error.
Comment on how the rate of change affects the temperature of the air inside the
vessels?
The computer was set up with the pressure of transient response that take and keep the
record of reading in each second. There is an unstable readings of the pressure which drops
slowly until there is a consistency in the system to reach the equilibrium. Due the situation
Fahad Aljehani 17416232
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

being ambient there is no much change in temperature over time. The sensor of the
pressure is more unstable than the sensor of the temperature through which the pumped in
and pumped out air being increase for the pressure chamber. It slows the time that drop
over in order to reach equilibrium. The whole experiment shows lot of drop of pressure than
followed by temperature, thus neglecting the change in temperature over time.
Conclusion:
Thus, it could be concluded that objective has been achieve through experiment. The study
establishes that the ideal gas quantity has limited laws. However, the experiment shows
that, gas has shown the relationship between volume, temperature and pressure. The ratio
of gas volume expresses the gas growth and dynamics compression. The ratio of heat
capacity gives the heat amount by the process of gas growth.
Fahad Aljehani 17416232
pressure is more unstable than the sensor of the temperature through which the pumped in
and pumped out air being increase for the pressure chamber. It slows the time that drop
over in order to reach equilibrium. The whole experiment shows lot of drop of pressure than
followed by temperature, thus neglecting the change in temperature over time.
Conclusion:
Thus, it could be concluded that objective has been achieve through experiment. The study
establishes that the ideal gas quantity has limited laws. However, the experiment shows
that, gas has shown the relationship between volume, temperature and pressure. The ratio
of gas volume expresses the gas growth and dynamics compression. The ratio of heat
capacity gives the heat amount by the process of gas growth.
Fahad Aljehani 17416232
1 out of 7
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.

