Effects of Resistance Training on Type 2 Diabetes: A Review
VerifiedAdded on 2022/12/20
|10
|10604
|86
Report
AI Summary
This report reviews the current understanding of resistance training (RT) as a strategy for managing type 2 diabetes (T2D). The prevalence of T2D is increasing, necessitating effective interventions. RT, through its effects on skeletal muscle, is a promising approach. The review focuses on RT's impact on glycemic control, substrate metabolism, and the molecular mechanisms involved, especially in skeletal muscle and adipose tissue. It highlights the role of muscle mass, fiber type (Type IIx and IIa), and mitochondrial function in mediating the benefits of RT. The document also discusses the interplay between RT and antidiabetic medications. The review identifies gaps in current research, especially concerning mitochondrial adaptations in skeletal muscle and adipose tissue, and suggests future research directions, including investigations into the mechanisms driving RT-mitigated metabolic adaptations and their link to improvements in glycemic control and other cardiovascular risk factors. The authors emphasize that understanding the molecular determinants of individual training responses in T2D is crucial for advancing the field.

R EV I E W Open Access
Resistance training to improve type 2
diabetes:working toward a prescription for
the future
Dominik H.Pesta1,2,3,4*
, Renata L.S.Goncalves5
, Anila K.Madiraju6
, Barbara Strasser7† and Lauren M.Sparks8,9*†
Abstract
The prevalence of type 2 diabetes (T2D) is rapidly increasing,and effective strategies to manage and prevent this
disease are urgently needed.Resistance training (RT) promotes health benefits through increased skeletalmuscle
mass and qualitative adaptations,such as enhanced glucose transport and mitochondrialoxidative capacity.In
particular,mitochondrialadaptations triggered by RT provide evidence for this type of exercise as a feasible life
recommendation to combat T2D,a disease typically characterized by altered muscle mitochondrialfunction.
Recently,the synergistic and antagonistic effects of combined training and Metformin use have come into qu
and warrant more in-depth prospective investigations.In the future,clinicalintervention studies should elucidate
the mechanisms driving RT-mitigated mitochondrialadaptations in muscle and their link to improvements in
glycemic control,cholesterolmetabolism and other cardiovascular disease risk factors in individuals with T2D.
Keywords:Resistance training,Type 2 diabetes,Skeletalmuscle,Mitochondrialfunction
Background
The significance of resistance training for individuals with
type 2 diabetes:moving beyond what we already know
The prevalence of type 2 diabetes (T2D) continues to in-
crease.Within the next 20 years,the number ofpeople
affected by this disease is expected to reach almost 600
million worldwide [1].T2D is accompanied by a host of
risk factorsincludingdyslipidemia,hypertension and
cardiovascular disease [2],thus putting a severe burden
on our globalhealth care systems.Apartfrom medica-
tion,chronic exercise (i.e.systematic training performed
repeatedly)is a proven prevention and treatmentstrat-
egy for individuals with pre-diabetes and T2D [3–5].Re-
cent reviews and meta-analyses,including the 2016 joint
position statementon physicalactivity and T2D from
the American Diabetes Association [6],have highlighted
the beneficial effects of chronic endurance training (ET),
resistance training (RT) and/or combined (ET + RT) inter-
ventions for ameliorating insulin sensitivity and glycemic
controlin individuals with T2D [7,8].Chronic ET alone
has a well-established role in enhancing insulin sensitivity
via glucose uptake and disposal(reviewed in [9]) and in
increasing musclemitochondrialdensityand oxidative
capacity [10].A limited number ofstudies have demon-
strated that chronic RT alone enhances glycemic control
[11,12]and muscle substrate metabolism in individuals
with T2D [13],yet the underlying mechanisms inducing
these health benefits,particularly those related to muscle
mitochondrial function, remain to be elucidated.
The presentreview focuses on the effects ofchronic
RT on glycemic control,substrate metabolism and the
molecular mechanisms that may influence these adapta-
tions in individuals with T2D.We place a specialem-
phasis on skeletalmuscle,the interaction between anti-
diabetic medication use and training stimulus,and in-
corporateadiposetissueas anothersignificanttarget
organ forRT-mediated metabolic adaptationsin T2D.
Since little is known aboutthe independenteffects of
chronic RT on mitochondrialadaptationsin skeletal
muscle and adipose tissue ofindividuals with T2D,we
identify gaps in the current literatureand raise
* Correspondence:Dominik.Pesta@ddz.uni-duesseldorf.de;
Lauren.Sparks@flhosp.org
†Equalcontributors
1Department of Sport Science,MedicalSection,University of Innsbruck,
Fürstenweg 185,Innsbruck,Austria
8TranslationalResearch Institute for Metabolism and Diabetes,Florida
Hospital,301 E.Princeton Street,Orlando,FL 32804,USA
Fulllist of author information is available at the end of the article
© The Author(s).2017 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0
InternationalLicense (http://creativecommons.org/licenses/by/4.0/),which permits unrestricted use,distribution,and
reproduction in any medium,provided you give appropriate credit to the originalauthor(s) and the source,provide a link to
the Creative Commons license,and indicate if changes were made.The Creative Commons Public Domain Dedication waiver
(http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article,unless otherwise stated.
Pesta et al.Nutrition & Metabolism (2017) 14:24
DOI10.1186/s12986-017-0173-7
Resistance training to improve type 2
diabetes:working toward a prescription for
the future
Dominik H.Pesta1,2,3,4*
, Renata L.S.Goncalves5
, Anila K.Madiraju6
, Barbara Strasser7† and Lauren M.Sparks8,9*†
Abstract
The prevalence of type 2 diabetes (T2D) is rapidly increasing,and effective strategies to manage and prevent this
disease are urgently needed.Resistance training (RT) promotes health benefits through increased skeletalmuscle
mass and qualitative adaptations,such as enhanced glucose transport and mitochondrialoxidative capacity.In
particular,mitochondrialadaptations triggered by RT provide evidence for this type of exercise as a feasible life
recommendation to combat T2D,a disease typically characterized by altered muscle mitochondrialfunction.
Recently,the synergistic and antagonistic effects of combined training and Metformin use have come into qu
and warrant more in-depth prospective investigations.In the future,clinicalintervention studies should elucidate
the mechanisms driving RT-mitigated mitochondrialadaptations in muscle and their link to improvements in
glycemic control,cholesterolmetabolism and other cardiovascular disease risk factors in individuals with T2D.
Keywords:Resistance training,Type 2 diabetes,Skeletalmuscle,Mitochondrialfunction
Background
The significance of resistance training for individuals with
type 2 diabetes:moving beyond what we already know
The prevalence of type 2 diabetes (T2D) continues to in-
crease.Within the next 20 years,the number ofpeople
affected by this disease is expected to reach almost 600
million worldwide [1].T2D is accompanied by a host of
risk factorsincludingdyslipidemia,hypertension and
cardiovascular disease [2],thus putting a severe burden
on our globalhealth care systems.Apartfrom medica-
tion,chronic exercise (i.e.systematic training performed
repeatedly)is a proven prevention and treatmentstrat-
egy for individuals with pre-diabetes and T2D [3–5].Re-
cent reviews and meta-analyses,including the 2016 joint
position statementon physicalactivity and T2D from
the American Diabetes Association [6],have highlighted
the beneficial effects of chronic endurance training (ET),
resistance training (RT) and/or combined (ET + RT) inter-
ventions for ameliorating insulin sensitivity and glycemic
controlin individuals with T2D [7,8].Chronic ET alone
has a well-established role in enhancing insulin sensitivity
via glucose uptake and disposal(reviewed in [9]) and in
increasing musclemitochondrialdensityand oxidative
capacity [10].A limited number ofstudies have demon-
strated that chronic RT alone enhances glycemic control
[11,12]and muscle substrate metabolism in individuals
with T2D [13],yet the underlying mechanisms inducing
these health benefits,particularly those related to muscle
mitochondrial function, remain to be elucidated.
The presentreview focuses on the effects ofchronic
RT on glycemic control,substrate metabolism and the
molecular mechanisms that may influence these adapta-
tions in individuals with T2D.We place a specialem-
phasis on skeletalmuscle,the interaction between anti-
diabetic medication use and training stimulus,and in-
corporateadiposetissueas anothersignificanttarget
organ forRT-mediated metabolic adaptationsin T2D.
Since little is known aboutthe independenteffects of
chronic RT on mitochondrialadaptationsin skeletal
muscle and adipose tissue ofindividuals with T2D,we
identify gaps in the current literatureand raise
* Correspondence:Dominik.Pesta@ddz.uni-duesseldorf.de;
Lauren.Sparks@flhosp.org
†Equalcontributors
1Department of Sport Science,MedicalSection,University of Innsbruck,
Fürstenweg 185,Innsbruck,Austria
8TranslationalResearch Institute for Metabolism and Diabetes,Florida
Hospital,301 E.Princeton Street,Orlando,FL 32804,USA
Fulllist of author information is available at the end of the article
© The Author(s).2017 Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0
InternationalLicense (http://creativecommons.org/licenses/by/4.0/),which permits unrestricted use,distribution,and
reproduction in any medium,provided you give appropriate credit to the originalauthor(s) and the source,provide a link to
the Creative Commons license,and indicate if changes were made.The Creative Commons Public Domain Dedication waiver
(http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article,unless otherwise stated.
Pesta et al.Nutrition & Metabolism (2017) 14:24
DOI10.1186/s12986-017-0173-7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

important questions that future RT-focused trials in in-
dividualswith T2D will hopefullyaddress.Improving
our understandingof the mechanismsunderpinning
chronic RT-mitigated metabolic adaptions in T2D will
move the scientific community (researchersand clini-
cians alike)beyond whatwe already know and toward
future investigations focused on molecular determinants
of the individual training responses in T2D.
Chronic resistance training effects on muscle
mass, fiber type and glycemic control
Resistance training-induced gains in muscle mass are not
solely responsible for improved muscle substrate metab-
olism in type 2 diabetes
Skeletalmuscleis responsiblefor ~80% of insulin-
mediated glucose uptake in the postprandialstate [14],
and uptake is markedly blunted in individuals with T2D
[15].In fact,when compared with lean healthy individ-
uals,skeletalmuscle ofindividuals with T2D exhibits a
decreased capacity to oxidize both glucose and fat [16].
Chronic RT increases muscle mass and strength,largely
due to induction of muscle hypertrophy and neuromus-
cular remodeling [17] through the phosphatidylinositol 3
kinase (PI3K)-Akt - mammalian targetof rapamycin
(mTOR) pathway [18] (Fig.1).These gains are typically
associated with and often surmised to underlie improve-
ments in muscle substrate (glucose and fat) metabolism
even in the absence ofdirectevidence.Recentreports
have shown that in addition to increasing strength [12],
9 months ofRT enhanced oxidation ofboth fatty acid-
and glycolytic-derived substrates in skeletal muscle of in-
dividuals with T2D [13].The 1.4 kg increase in muscle
mass observed was interpreted to be the rootcause of
these metabolic adaptations,yet many other factors such
as improvements in insulin signaling could be respon-
sible for the RT-induced improvements in substrate me-
tabolism and glycemic control[19].At the molecular
level,calmodulin-dependent protein kinase (CaMK) II,a
criticalsensorfor intracellularcalcium signalingand
muscle remodeling,is activated in an exercise intensity-
dependentmanner.CaMK II phosphorylates transcrip-
tion factors such as histone deacetylases (HDACs) [20],
which upon phosphorylation are exported from the nu-
cleus leading to activation oftranscription factors such
as myocyteenhancing factor(MEF) 2 and its target
genes[e.g.,peroxisome proliferator-activated receptor-
gammacoactivator1 alpha (PGC-1α),glucosetrans-
porter protein 4 (GLUT4),thereby improving glycemic
control[21] (Fig.1). Of note,a recentreview assessing
the differentcharacteristicsof ET, RT and combined
training interventionsand theirassociationswith gly-
cemic controlamong individualswith T2D concluded
that increasing the number ofexercise sessions (by vol-
ume and frequency) showed greater benefits than either
mode orintensity ofthe training itself[22].Unfortu-
nately,data regarding the effectsof varied intensities
and durations of RT on muscle mass are limited.To this
point,when expressed per kilogram of body weight,glu-
cose disposalrates are ~40–45% higher in weightlifter-
s—individuals characterized by large amounts of muscle
mass—compared to untrained individuals [23];however,
when normalized to muscle mass,glucose disposalrates
no longer differ between weightliftersand untrained
controls.These results underline the importance ofun-
derstandingthat chronic RT can have separatebut
equally important impacts on skeletal muscle in terms of
strength and substrateutilization,and that while in-
creased muscle mass can contribute to enhanced whole-
body glucose-disposalrates,it does not necessarily sug-
gest that the exercise regimen altered the inherent cap-
acity of muscle to more effectively metabolize substrate.
Metabolic adaptations within skeletal muscle in response
to resistance training:how much does fiber type matter?
Type IIx fibers are described as having a glycolytic pheno-
type,relying on glucose more than fat as a fuel,and facili-
tatingshort-duration anaerobicactivities.It has been
shown that Type IIx fibers are present in a higher propor-
tion in individualswith T2D [4]. Hyperinsulinemia—a
hallmark feature of insulin resistance and T2D—can shift
muscle fiber type toward a glycolytic phenotype by in-
creasing Type IIx myosin heavy chain gene expression [5].
Physical inactivity and immobilization have similar effects
on fiber type shift [21].Interestingly,first-degree relatives
of individuals with T2D have a ~30% higher proportion of
Type IIx fibers than individuals without a family history of
T2D. Type IIx fiber content was also negatively associated
with glucose disposalrates in these same individuals [6].
Paradoxically, elite strength and speed athletes have a high
proportionof Type IIx fibers and are metabolically
healthy,yetthe high proportion ofType IIx fibers ob-
served in individuals with T2D is concomitant with overall
blunted substrate oxidation and appears to be less advan-
tageous for these individuals.It is tempting to speculate
that the high number of Type IIx fibers in individuals with
T2D could be “trained” to utilize fuelmore effectively as
observed in strength-based athletes.Four to six weeks of
moderate intensity RT (at40–50% ofthe one-repetition
maximum,1RM) markedly increased skeletal muscle glu-
cose uptakein non-obeseindividualswith T2D [24],
which was largely attributed to a shift in fiber type toward
Type IIa fibers.Single fiber analysis revealed that Type IIa
fibers were the ones with the highest glucose uptake and
GLUT4 content among the Type II fiber population [25,
26].Type IIa fibers also had a higher capillary density and
showed a greater insulin response than Type IIx fibers
[27].Although Type IIa fibersexhibited more marked
glycogen depletion during an exercise boutand faster
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 2 of 10
dividualswith T2D will hopefullyaddress.Improving
our understandingof the mechanismsunderpinning
chronic RT-mitigated metabolic adaptions in T2D will
move the scientific community (researchersand clini-
cians alike)beyond whatwe already know and toward
future investigations focused on molecular determinants
of the individual training responses in T2D.
Chronic resistance training effects on muscle
mass, fiber type and glycemic control
Resistance training-induced gains in muscle mass are not
solely responsible for improved muscle substrate metab-
olism in type 2 diabetes
Skeletalmuscleis responsiblefor ~80% of insulin-
mediated glucose uptake in the postprandialstate [14],
and uptake is markedly blunted in individuals with T2D
[15].In fact,when compared with lean healthy individ-
uals,skeletalmuscle ofindividuals with T2D exhibits a
decreased capacity to oxidize both glucose and fat [16].
Chronic RT increases muscle mass and strength,largely
due to induction of muscle hypertrophy and neuromus-
cular remodeling [17] through the phosphatidylinositol 3
kinase (PI3K)-Akt - mammalian targetof rapamycin
(mTOR) pathway [18] (Fig.1).These gains are typically
associated with and often surmised to underlie improve-
ments in muscle substrate (glucose and fat) metabolism
even in the absence ofdirectevidence.Recentreports
have shown that in addition to increasing strength [12],
9 months ofRT enhanced oxidation ofboth fatty acid-
and glycolytic-derived substrates in skeletal muscle of in-
dividuals with T2D [13].The 1.4 kg increase in muscle
mass observed was interpreted to be the rootcause of
these metabolic adaptations,yet many other factors such
as improvements in insulin signaling could be respon-
sible for the RT-induced improvements in substrate me-
tabolism and glycemic control[19].At the molecular
level,calmodulin-dependent protein kinase (CaMK) II,a
criticalsensorfor intracellularcalcium signalingand
muscle remodeling,is activated in an exercise intensity-
dependentmanner.CaMK II phosphorylates transcrip-
tion factors such as histone deacetylases (HDACs) [20],
which upon phosphorylation are exported from the nu-
cleus leading to activation oftranscription factors such
as myocyteenhancing factor(MEF) 2 and its target
genes[e.g.,peroxisome proliferator-activated receptor-
gammacoactivator1 alpha (PGC-1α),glucosetrans-
porter protein 4 (GLUT4),thereby improving glycemic
control[21] (Fig.1). Of note,a recentreview assessing
the differentcharacteristicsof ET, RT and combined
training interventionsand theirassociationswith gly-
cemic controlamong individualswith T2D concluded
that increasing the number ofexercise sessions (by vol-
ume and frequency) showed greater benefits than either
mode orintensity ofthe training itself[22].Unfortu-
nately,data regarding the effectsof varied intensities
and durations of RT on muscle mass are limited.To this
point,when expressed per kilogram of body weight,glu-
cose disposalrates are ~40–45% higher in weightlifter-
s—individuals characterized by large amounts of muscle
mass—compared to untrained individuals [23];however,
when normalized to muscle mass,glucose disposalrates
no longer differ between weightliftersand untrained
controls.These results underline the importance ofun-
derstandingthat chronic RT can have separatebut
equally important impacts on skeletal muscle in terms of
strength and substrateutilization,and that while in-
creased muscle mass can contribute to enhanced whole-
body glucose-disposalrates,it does not necessarily sug-
gest that the exercise regimen altered the inherent cap-
acity of muscle to more effectively metabolize substrate.
Metabolic adaptations within skeletal muscle in response
to resistance training:how much does fiber type matter?
Type IIx fibers are described as having a glycolytic pheno-
type,relying on glucose more than fat as a fuel,and facili-
tatingshort-duration anaerobicactivities.It has been
shown that Type IIx fibers are present in a higher propor-
tion in individualswith T2D [4]. Hyperinsulinemia—a
hallmark feature of insulin resistance and T2D—can shift
muscle fiber type toward a glycolytic phenotype by in-
creasing Type IIx myosin heavy chain gene expression [5].
Physical inactivity and immobilization have similar effects
on fiber type shift [21].Interestingly,first-degree relatives
of individuals with T2D have a ~30% higher proportion of
Type IIx fibers than individuals without a family history of
T2D. Type IIx fiber content was also negatively associated
with glucose disposalrates in these same individuals [6].
Paradoxically, elite strength and speed athletes have a high
proportionof Type IIx fibers and are metabolically
healthy,yetthe high proportion ofType IIx fibers ob-
served in individuals with T2D is concomitant with overall
blunted substrate oxidation and appears to be less advan-
tageous for these individuals.It is tempting to speculate
that the high number of Type IIx fibers in individuals with
T2D could be “trained” to utilize fuelmore effectively as
observed in strength-based athletes.Four to six weeks of
moderate intensity RT (at40–50% ofthe one-repetition
maximum,1RM) markedly increased skeletal muscle glu-
cose uptakein non-obeseindividualswith T2D [24],
which was largely attributed to a shift in fiber type toward
Type IIa fibers.Single fiber analysis revealed that Type IIa
fibers were the ones with the highest glucose uptake and
GLUT4 content among the Type II fiber population [25,
26].Type IIa fibers also had a higher capillary density and
showed a greater insulin response than Type IIx fibers
[27].Although Type IIa fibersexhibited more marked
glycogen depletion during an exercise boutand faster
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 2 of 10
glycogen re-synthesis following the exercise bout [28],it
remains to be determined whether altering fiber type dis-
tribution benefits individuals with T2D in this respect. It is
entirely possible that fiber type composition is irrelevant if
the cellular metabolic machinery (e.g.,glucose transport,
mitochondria, etc.) is dysfunctional. In other words, quan-
tity doesnot necessarily equalquality.Challenging the
idea that switching fiber type confers a metabolic advan-
tage,two independent studies demonstrated that chronic
RT-driven improvementsin insulin responsivenessand
high-density lipoprotein (HDL) levels in individuals with
T2D occurred without any changes in fiber type compos-
ition [29, 30], a phenomenon routinelyobservedin
healthyindividuals[31]. Metabolicadaptationswithin
muscle can therefore occur independently of a change in
muscle fiber type composition. It is important to note that
directcomparisons ofthe effectsof differentdurations
and intensities of RT on fiber type composition are virtu-
ally absent from the literature.
Chronic resistance training and mitochondrial
fitness
Resistance training effects on muscle mitochondrial
function in individuals with type 2 diabetes:how much
do we really know?
Perturbations in mitochondrialoxidative capacity play a
major role in the development and progression ofinsu-
lin resistance and T2D [32].As few as 3 days of high-fat
feeding are sufficient to induce insulin resistance and re-
duce muscle mitochondrialoxidative phosphorylation at
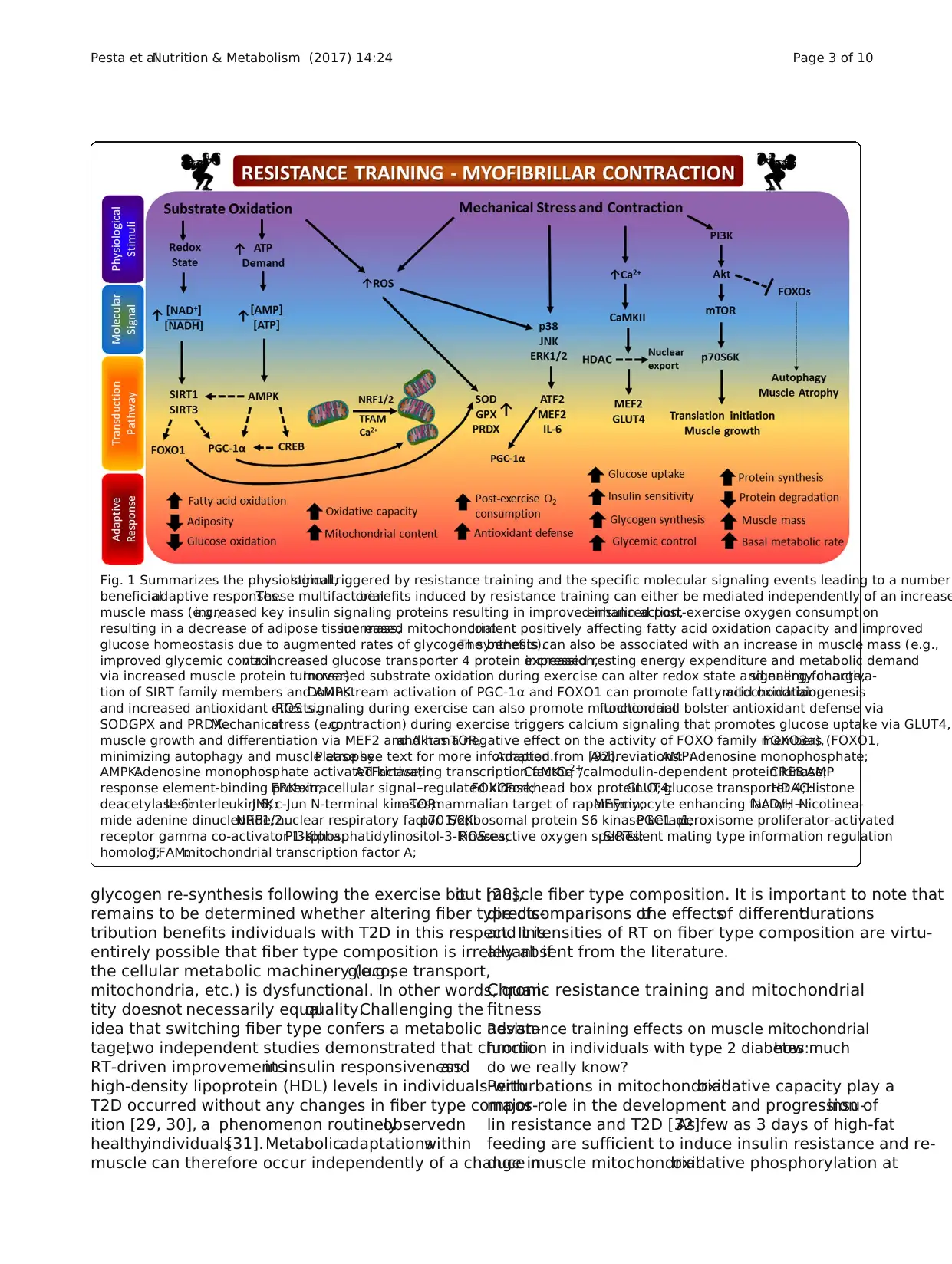
Fig. 1 Summarizes the physiologicalstimuli,triggered by resistance training and the specific molecular signaling events leading to a number
beneficialadaptive responses.These multifactorialbenefits induced by resistance training can either be mediated independently of an increase
muscle mass (e.g.,increased key insulin signaling proteins resulting in improved insulin action,enhanced post-exercise oxygen consumption
resulting in a decrease of adipose tissue mass,increased mitochondrialcontent positively affecting fatty acid oxidation capacity and improved
glucose homeostasis due to augmented rates of glycogen synthesis).The benefits can also be associated with an increase in muscle mass (e.g.,
improved glycemic controlvia increased glucose transporter 4 protein expression,increased resting energy expenditure and metabolic demand
via increased muscle protein turnover).Increased substrate oxidation during exercise can alter redox state and energy charge,signaling for activa-
tion of SIRT family members and AMPK.Downstream activation of PGC-1α and FOXO1 can promote fatty acid oxidation,mitochondrialbiogenesis
and increased antioxidant effects.ROS signaling during exercise can also promote mitochondrialfunction and bolster antioxidant defense via
SOD,GPX and PRDX.Mechanicalstress (e.g.,contraction) during exercise triggers calcium signaling that promotes glucose uptake via GLUT4,
muscle growth and differentiation via MEF2 and Akt-mTOR,and has a negative effect on the activity of FOXO family members (FOXO1,FOXO3a),
minimizing autophagy and muscle atrophy.Please see text for more information.Adapted from [92].Abbreviations:AMP:Adenosine monophosphate;
AMPK:Adenosine monophosphate activated kinase;ATF:activating transcription factor;CaMK:Ca2+
/calmodulin-dependent protein kinase;CREB:cAMP
response element-binding protein;ERK:extracellular signal–regulated kinase;FOXO:Forkhead box protein O;GLUT4:glucose transporter 4;HDAC:Histone
deacetylases;IL-6:interleukin 6;JNK:c-Jun N-terminal kinases;mTOR:mammalian target of rapamycin;MEF:myocyte enhancing factor;NAD/H+:Nicotinea-
mide adenine dinucleotide;NRF1/2:nuclear respiratory factor 1/2;p70 S6K:ribosomal protein S6 kinase beta-1;PGC1-α:peroxisome proliferator-activated
receptor gamma co-activator 1-alpha;PI3K:phosphatidylinositol-3-kinases;ROS:reactive oxygen species;SIRT:silent mating type information regulation
homolog;TFAM:mitochondrial transcription factor A;
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 3 of 10
remains to be determined whether altering fiber type dis-
tribution benefits individuals with T2D in this respect. It is
entirely possible that fiber type composition is irrelevant if
the cellular metabolic machinery (e.g.,glucose transport,
mitochondria, etc.) is dysfunctional. In other words, quan-
tity doesnot necessarily equalquality.Challenging the
idea that switching fiber type confers a metabolic advan-
tage,two independent studies demonstrated that chronic
RT-driven improvementsin insulin responsivenessand
high-density lipoprotein (HDL) levels in individuals with
T2D occurred without any changes in fiber type compos-
ition [29, 30], a phenomenon routinelyobservedin
healthyindividuals[31]. Metabolicadaptationswithin
muscle can therefore occur independently of a change in
muscle fiber type composition. It is important to note that
directcomparisons ofthe effectsof differentdurations
and intensities of RT on fiber type composition are virtu-
ally absent from the literature.
Chronic resistance training and mitochondrial
fitness
Resistance training effects on muscle mitochondrial
function in individuals with type 2 diabetes:how much
do we really know?
Perturbations in mitochondrialoxidative capacity play a
major role in the development and progression ofinsu-
lin resistance and T2D [32].As few as 3 days of high-fat
feeding are sufficient to induce insulin resistance and re-
duce muscle mitochondrialoxidative phosphorylation at
Fig. 1 Summarizes the physiologicalstimuli,triggered by resistance training and the specific molecular signaling events leading to a number
beneficialadaptive responses.These multifactorialbenefits induced by resistance training can either be mediated independently of an increase
muscle mass (e.g.,increased key insulin signaling proteins resulting in improved insulin action,enhanced post-exercise oxygen consumption
resulting in a decrease of adipose tissue mass,increased mitochondrialcontent positively affecting fatty acid oxidation capacity and improved
glucose homeostasis due to augmented rates of glycogen synthesis).The benefits can also be associated with an increase in muscle mass (e.g.,
improved glycemic controlvia increased glucose transporter 4 protein expression,increased resting energy expenditure and metabolic demand
via increased muscle protein turnover).Increased substrate oxidation during exercise can alter redox state and energy charge,signaling for activa-
tion of SIRT family members and AMPK.Downstream activation of PGC-1α and FOXO1 can promote fatty acid oxidation,mitochondrialbiogenesis
and increased antioxidant effects.ROS signaling during exercise can also promote mitochondrialfunction and bolster antioxidant defense via
SOD,GPX and PRDX.Mechanicalstress (e.g.,contraction) during exercise triggers calcium signaling that promotes glucose uptake via GLUT4,
muscle growth and differentiation via MEF2 and Akt-mTOR,and has a negative effect on the activity of FOXO family members (FOXO1,FOXO3a),
minimizing autophagy and muscle atrophy.Please see text for more information.Adapted from [92].Abbreviations:AMP:Adenosine monophosphate;
AMPK:Adenosine monophosphate activated kinase;ATF:activating transcription factor;CaMK:Ca2+
/calmodulin-dependent protein kinase;CREB:cAMP
response element-binding protein;ERK:extracellular signal–regulated kinase;FOXO:Forkhead box protein O;GLUT4:glucose transporter 4;HDAC:Histone
deacetylases;IL-6:interleukin 6;JNK:c-Jun N-terminal kinases;mTOR:mammalian target of rapamycin;MEF:myocyte enhancing factor;NAD/H+:Nicotinea-
mide adenine dinucleotide;NRF1/2:nuclear respiratory factor 1/2;p70 S6K:ribosomal protein S6 kinase beta-1;PGC1-α:peroxisome proliferator-activated
receptor gamma co-activator 1-alpha;PI3K:phosphatidylinositol-3-kinases;ROS:reactive oxygen species;SIRT:silent mating type information regulation
homolog;TFAM:mitochondrial transcription factor A;
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 3 of 10
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide
the transcriptionallevelin lean, sedentary individuals
[33].Individuals with T2D have reduced mitochondrial
oxidative capacity when expressed perunit of muscle
masscompared to healthy individuals;however,when
mitochondrialoxidativecapacityis normalizedto
markersof mitochondrialcontent(e.g.,mitochondrial
DNA copy number,citrate synthase activity),these dif-
ferences are insignificant[16,34].That being said,dis-
rupted mitochondrialmorphology and a 35% reduction
in mitochondrialsize reported in individuals with T2D
are indicative of functional impairment [16].
Aerobic and combined training improve mitochondrial
function [35].Mechanicalstressinducesactivation of
the mitogen-activated protein kinase (MAPK)family of
proteins(extracellular-regulatedkinase(ERK1/2) and
p38 MAPK). Activation ofp38 MAPK during contrac-
tion stimulatesactivating transcription factor(ATF) 2
and MEF2,which increase PGC-1α expression and im-
prove mitochondrialfunction in skeletalmuscle (Fig.1)
[36].Some evidence to support a role for chronic RT in
increasing muscle mitochondrialoxidative capacity ex-
ists in healthy individuals [37–39];however,similar data
in individualswith T2D are limited.RT (alone or in
combination with ET) has only recently been recognized
as a promising intervention to maintain muscle mito-
chondrial oxidative capacity [40] and improve the overall
metabolic phenotype ofindividuals with T2D [13,41].
Discrepancies in the measured outcomes and the details
of the training protocols implementedin RT
interventions have made it difficult to ascertain the value
of implementing chronic RT to improve muscle mito-
chondrial function in individuals with T2D.
Twelve weeks of RT (50–75% of 1RM) twice per week
failed to alterPGC-1α protein contentand mitochon-
drialtranscription factor A (TFAM) RNA content in in-
dividualswith T2D, indicatingthat this particular
duration and/orintensity wasnot sufficientto induce
changesin key regulatory moleculesof mitochondrial
biogenesis[42]. As mentionedpreviously,however,
quantity does not necessarily equal quality,and the topic
of mitochondrialnumber vs.function is highly debated.
According to the classicalview oftraining adaptations,
RT signals through theAkt-mTOR-S6K pathwayby
which it promotesmuscle hypertrophy though myofi-
brillar protein biosynthesis.ET leads to an activation of
adenosine monophosphate activated kinase (AMPK) and
subsequentactivation ofPGC-1α, inducing mitochon-
drialbiogenesis [43](Fig.2). On the contrary,a recent
study demonstrated two-fold higher mRNA expression
levels ofPGC-1α,PGC-1-related coactivator (PRC) and
pyruvate dehydrogenase kinase 4 (PDK4) in a group that
performed RT after ET vs.a group thatperformed ET
alone [44].This suggests that combined (RT + ET) train-
ing amplifies the molecular response to ET alone.De-
creased energy charge,or an elevated [AMP]/[ATP] ratio
in response to contraction,and subsequent AMPK activa-
tion possibly mediates changes towards an improved oxi-
dative phenotype by phosphorylating and activating the
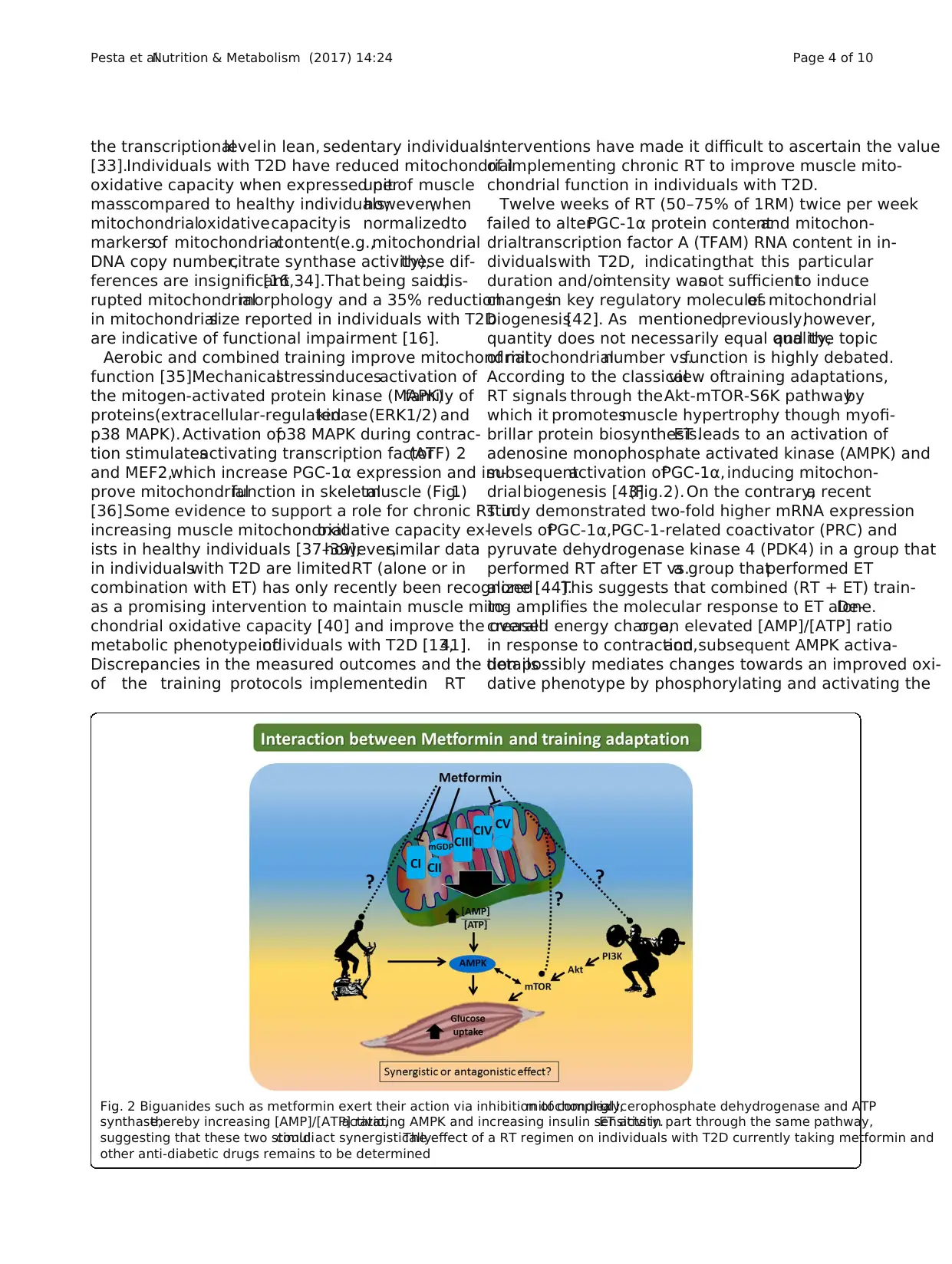
Fig. 2 Biguanides such as metformin exert their action via inhibition of complex I,mitochondrialglycerophosphate dehydrogenase and ATP
synthase,thereby increasing [AMP]/[ATP] ratio,activating AMPK and increasing insulin sensitivity.ET acts in part through the same pathway,
suggesting that these two stimulicould act synergistically.The effect of a RT regimen on individuals with T2D currently taking metformin and
other anti-diabetic drugs remains to be determined
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 4 of 10
[33].Individuals with T2D have reduced mitochondrial
oxidative capacity when expressed perunit of muscle
masscompared to healthy individuals;however,when
mitochondrialoxidativecapacityis normalizedto
markersof mitochondrialcontent(e.g.,mitochondrial
DNA copy number,citrate synthase activity),these dif-
ferences are insignificant[16,34].That being said,dis-
rupted mitochondrialmorphology and a 35% reduction
in mitochondrialsize reported in individuals with T2D
are indicative of functional impairment [16].
Aerobic and combined training improve mitochondrial
function [35].Mechanicalstressinducesactivation of
the mitogen-activated protein kinase (MAPK)family of
proteins(extracellular-regulatedkinase(ERK1/2) and
p38 MAPK). Activation ofp38 MAPK during contrac-
tion stimulatesactivating transcription factor(ATF) 2
and MEF2,which increase PGC-1α expression and im-
prove mitochondrialfunction in skeletalmuscle (Fig.1)
[36].Some evidence to support a role for chronic RT in
increasing muscle mitochondrialoxidative capacity ex-
ists in healthy individuals [37–39];however,similar data
in individualswith T2D are limited.RT (alone or in
combination with ET) has only recently been recognized
as a promising intervention to maintain muscle mito-
chondrial oxidative capacity [40] and improve the overall
metabolic phenotype ofindividuals with T2D [13,41].
Discrepancies in the measured outcomes and the details
of the training protocols implementedin RT
interventions have made it difficult to ascertain the value
of implementing chronic RT to improve muscle mito-
chondrial function in individuals with T2D.
Twelve weeks of RT (50–75% of 1RM) twice per week
failed to alterPGC-1α protein contentand mitochon-
drialtranscription factor A (TFAM) RNA content in in-
dividualswith T2D, indicatingthat this particular
duration and/orintensity wasnot sufficientto induce
changesin key regulatory moleculesof mitochondrial
biogenesis[42]. As mentionedpreviously,however,
quantity does not necessarily equal quality,and the topic
of mitochondrialnumber vs.function is highly debated.
According to the classicalview oftraining adaptations,
RT signals through theAkt-mTOR-S6K pathwayby
which it promotesmuscle hypertrophy though myofi-
brillar protein biosynthesis.ET leads to an activation of
adenosine monophosphate activated kinase (AMPK) and
subsequentactivation ofPGC-1α, inducing mitochon-
drialbiogenesis [43](Fig.2). On the contrary,a recent
study demonstrated two-fold higher mRNA expression
levels ofPGC-1α,PGC-1-related coactivator (PRC) and
pyruvate dehydrogenase kinase 4 (PDK4) in a group that
performed RT after ET vs.a group thatperformed ET
alone [44].This suggests that combined (RT + ET) train-
ing amplifies the molecular response to ET alone.De-
creased energy charge,or an elevated [AMP]/[ATP] ratio
in response to contraction,and subsequent AMPK activa-
tion possibly mediates changes towards an improved oxi-
dative phenotype by phosphorylating and activating the
Fig. 2 Biguanides such as metformin exert their action via inhibition of complex I,mitochondrialglycerophosphate dehydrogenase and ATP
synthase,thereby increasing [AMP]/[ATP] ratio,activating AMPK and increasing insulin sensitivity.ET acts in part through the same pathway,
suggesting that these two stimulicould act synergistically.The effect of a RT regimen on individuals with T2D currently taking metformin and
other anti-diabetic drugs remains to be determined
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 4 of 10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

transcription factor cAMP-response-element binding pro-
tein (CREB),which increases PGC-1α transcriptionalac-
tivity.Exercise-mediated perturbation ofredox potential
or the [NAD+
]/[NADH] ratio will also promote the deace-
tylation activity of silent mating-type information regula-
tion (SIRT)1 and 3, which furtherpotentiate PGC-1α
transcriptionalactivity [45,46] (Fig.1).SIRT1 and SIRT3
activity can lead to deacetylation of FOXO1,allowing nu-
clear translocation and increased expression of PDK4 that
triggers a switch from glucose to lipid oxidation. However,
FOXO activity (FOXO1 and FOXO3a) in the muscle has
also been implicated in increased autophagy and muscle
atrophy via increasesin atrogin-1 expression.Interest-
ingly,exercise induction ofFOXO activity via effects on
redox potentialmay face inhibitorycrosstalkfrom
exercise-mediated activation of the Akt-mTOR pathways,
as Akt is known to phosphorylate FOXOs and exclude
them from the nucleus [47].The roles of these seemingly
competing pathways in promoting mitochondrial function
via enhanced lipid metabolism butmaintaining muscle
mass remain to be examined in the contextof exercise
interventions.
One interesting theory on how to improve muscle
mitochondrialoxidativecapacityin diabetesis via
‘gene shifting’.A recent reportsuggeststhat satellite
cell activation following chronic RT leads to incorpor-
ation ofnew mitochondrialDNA into mature muscle
cells [48].This phenomenon,known as ‘gene shifting’,
may help normalize mitochondrialoxidative capacity
in individualssuffering from impaired mitochondrial
function by providing undamaged mitochondrialDNA
from which new, functionalmitochondrialproteins
can be synthesized.In support of this hypothesis,a
supervised 14-week RT regimen (three times per week
at 50–80% ofthe 1RM) increased cytochrome c oxi-
dase (COX)activity,enhanced mitochondrialcreatine
kinase levels and decreased oxidative DNA damage in
elderly men and women [49].These findingssuggest
that chronic RT up-regulatesselected componentsof
the mitochondrialelectrontransportsystem which
may convey many ofthe beneficialeffects ofthis ex-
ercise modality [50].
Collectively,these findings suggest that chronic RT is
capableof inducingmusclemitochondrialbiogenesis
and enhancingdownstream oxidativecapacity.In
addition to biogenesis,mitochondria also undergo re-
modeling (i.e.,fusion and fission) to enhance and/or pre-
serve function.RT can act as a triggerto induce
mitochondrialfission,followed by recovery-induced fu-
sion and mitochondrialbiogenesis.These RT-triggered
processes may act as naturalselection at the levelof the
mitochondrion (mito-checkpoint)and eliminate disad-
vantageous mitochondria,thereby driving adaptive selec-
tion of advantageousphenotypicvariations[51].This
conceptadds another dimension to the currentunder-
standingof chronic RT-mitigatedimprovementsin
muscle mitochondrial health.
Resistance training-induced oxidative stress in type 2 dia-
betes:is defense the best offense?
T2D is characterized by excessive reactive oxygen spe-
cies (ROS) emission which presumably leads to oxidative
stress in different tissues [52,53].There are at least 11
sites in muscle mitochondria capable of producing ROS
and their rates under different (patho)physiologicalcon-
ditions stillneed to be determined [54].Recent estima-
tions assessingex vivo mitochondrialsuperoxide/
H 2O 2 production in conditions mimicking physiological
states have reported that 0.35% ofoxygen consumed at
rest is diverted to ROS production,and that this propor-
tion is reduced to 0.03–0.01% duringexercise[55].
Given that mitochondria superoxide/H2O2 production is
likely to decrease during moderate exercise,it is import-
ant to also consider other cellular reactions and sites as
significantmediators ofROS production.Both ET and
RT increase oxidant production,which can lead to oxi-
dative stress [56,57].ROS can activate c-jun N-terminal
kinase (JNK)signaling as evidenced by a recentreport
demonstratingthat infusion of the antioxidantN-
acetylcysteine reduced JNK signaling during aerobic ex-
ercise training [58] (Fig.1).Interleukin-6 (IL-6) is associ-
ated with a variety ofmetabolic effects across different
organsand its secretion duringcontraction isJNK-
dependent [59],emphasizing the importance of JNK sig-
naling and ROS-mediated JNK activation in facilitating
metabolic adaptations to aerobic exercise training [58].
In a comparative study assessing the effects ofexhaust-
ive ET vs.RT on ROS production,both training modal-
ities increasedoxidativestress;however,lipids were
affected by peroxidation only during RT,and proteins
wereoxidized and formed carbonylsduring ET [57].
Training frequency also impactsROS production and
oxidative stress.While a single RT session was sufficient
to induce oxidative damage in untrained men [60],6
months ofRT (three times per week at50–80% ofthe
1RM) induced whole-body adaptationsresulting in de-
creased training-induced oxidative stress and homocyst-
eine levels [61].Data on RT-mediated oxidative stress are
sparse in individuals with T2D.A recentstudy reported
that 12 months of supervised ET,RT and flexibility train-
ing equally reduced oxidative stress in men with T2D [62].
In addition to promoting acute ROS formation [63],
aerobic exercise training generally improves muscle anti-
oxidantdefensemechanisms[64].Interestingly,mito-
chondrial hydrogenperoxide(H2O2) productionis
importantfor muscle differentiationin vitro, and
mitochondria-targetedantioxidant treatment with
MitoQ and MitoTEMPOL, as well as mitochondria-
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 5 of 10
tein (CREB),which increases PGC-1α transcriptionalac-
tivity.Exercise-mediated perturbation ofredox potential
or the [NAD+
]/[NADH] ratio will also promote the deace-
tylation activity of silent mating-type information regula-
tion (SIRT)1 and 3, which furtherpotentiate PGC-1α
transcriptionalactivity [45,46] (Fig.1).SIRT1 and SIRT3
activity can lead to deacetylation of FOXO1,allowing nu-
clear translocation and increased expression of PDK4 that
triggers a switch from glucose to lipid oxidation. However,
FOXO activity (FOXO1 and FOXO3a) in the muscle has
also been implicated in increased autophagy and muscle
atrophy via increasesin atrogin-1 expression.Interest-
ingly,exercise induction ofFOXO activity via effects on
redox potentialmay face inhibitorycrosstalkfrom
exercise-mediated activation of the Akt-mTOR pathways,
as Akt is known to phosphorylate FOXOs and exclude
them from the nucleus [47].The roles of these seemingly
competing pathways in promoting mitochondrial function
via enhanced lipid metabolism butmaintaining muscle
mass remain to be examined in the contextof exercise
interventions.
One interesting theory on how to improve muscle
mitochondrialoxidativecapacityin diabetesis via
‘gene shifting’.A recent reportsuggeststhat satellite
cell activation following chronic RT leads to incorpor-
ation ofnew mitochondrialDNA into mature muscle
cells [48].This phenomenon,known as ‘gene shifting’,
may help normalize mitochondrialoxidative capacity
in individualssuffering from impaired mitochondrial
function by providing undamaged mitochondrialDNA
from which new, functionalmitochondrialproteins
can be synthesized.In support of this hypothesis,a
supervised 14-week RT regimen (three times per week
at 50–80% ofthe 1RM) increased cytochrome c oxi-
dase (COX)activity,enhanced mitochondrialcreatine
kinase levels and decreased oxidative DNA damage in
elderly men and women [49].These findingssuggest
that chronic RT up-regulatesselected componentsof
the mitochondrialelectrontransportsystem which
may convey many ofthe beneficialeffects ofthis ex-
ercise modality [50].
Collectively,these findings suggest that chronic RT is
capableof inducingmusclemitochondrialbiogenesis
and enhancingdownstream oxidativecapacity.In
addition to biogenesis,mitochondria also undergo re-
modeling (i.e.,fusion and fission) to enhance and/or pre-
serve function.RT can act as a triggerto induce
mitochondrialfission,followed by recovery-induced fu-
sion and mitochondrialbiogenesis.These RT-triggered
processes may act as naturalselection at the levelof the
mitochondrion (mito-checkpoint)and eliminate disad-
vantageous mitochondria,thereby driving adaptive selec-
tion of advantageousphenotypicvariations[51].This
conceptadds another dimension to the currentunder-
standingof chronic RT-mitigatedimprovementsin
muscle mitochondrial health.
Resistance training-induced oxidative stress in type 2 dia-
betes:is defense the best offense?
T2D is characterized by excessive reactive oxygen spe-
cies (ROS) emission which presumably leads to oxidative
stress in different tissues [52,53].There are at least 11
sites in muscle mitochondria capable of producing ROS
and their rates under different (patho)physiologicalcon-
ditions stillneed to be determined [54].Recent estima-
tions assessingex vivo mitochondrialsuperoxide/
H 2O 2 production in conditions mimicking physiological
states have reported that 0.35% ofoxygen consumed at
rest is diverted to ROS production,and that this propor-
tion is reduced to 0.03–0.01% duringexercise[55].
Given that mitochondria superoxide/H2O2 production is
likely to decrease during moderate exercise,it is import-
ant to also consider other cellular reactions and sites as
significantmediators ofROS production.Both ET and
RT increase oxidant production,which can lead to oxi-
dative stress [56,57].ROS can activate c-jun N-terminal
kinase (JNK)signaling as evidenced by a recentreport
demonstratingthat infusion of the antioxidantN-
acetylcysteine reduced JNK signaling during aerobic ex-
ercise training [58] (Fig.1).Interleukin-6 (IL-6) is associ-
ated with a variety ofmetabolic effects across different
organsand its secretion duringcontraction isJNK-
dependent [59],emphasizing the importance of JNK sig-
naling and ROS-mediated JNK activation in facilitating
metabolic adaptations to aerobic exercise training [58].
In a comparative study assessing the effects ofexhaust-
ive ET vs.RT on ROS production,both training modal-
ities increasedoxidativestress;however,lipids were
affected by peroxidation only during RT,and proteins
wereoxidized and formed carbonylsduring ET [57].
Training frequency also impactsROS production and
oxidative stress.While a single RT session was sufficient
to induce oxidative damage in untrained men [60],6
months ofRT (three times per week at50–80% ofthe
1RM) induced whole-body adaptationsresulting in de-
creased training-induced oxidative stress and homocyst-
eine levels [61].Data on RT-mediated oxidative stress are
sparse in individuals with T2D.A recentstudy reported
that 12 months of supervised ET,RT and flexibility train-
ing equally reduced oxidative stress in men with T2D [62].
In addition to promoting acute ROS formation [63],
aerobic exercise training generally improves muscle anti-
oxidantdefensemechanisms[64].Interestingly,mito-
chondrial hydrogenperoxide(H2O2) productionis
importantfor muscle differentiationin vitro, and
mitochondria-targetedantioxidant treatment with
MitoQ and MitoTEMPOL, as well as mitochondria-
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 5 of 10

targeted catalase,blocks this effect [65].As such,acute
ROS formation during RT may also have beneficialef-
fectson muscle differentiation in vivo.Indeed,mito-
chondrial ROS generation might be involved in initiating
mitochondrial biogenesis [66].Transient ROS “build-up”
has been suggested to prime the cellular redox system,
which culminatesin an improved handling ofsubse-
quentpro-oxidantenvironments.Oxidative stress must
therefore be considered in connection with radicalscav-
enging activities and cellular antioxidant defense mecha-
nisms.A 12-week RT intervention (twice perweek at
50–75% ofthe 1RM) not only reduced oxidative stress,
but also significantly increased cytosolic and mitochon-
drial antioxidantproteins [superoxidedismutase-2
(SOD2),glutathione peroxidase-1 (GPX1),peroxiredoxin
isoform 5 (PRDX5),heat-shock-protein-70 (HSP70)]in
muscle of individuals with T2D [67].
Chronic RT effects on ROS production in human
muscle are relatively unexplored.Due to the absence of
reliable techniques to precisely measure ROS production
in vivo,fundamentalquestions regarding the sources of
ROS and the effects oftraining modalities on ROS sig-
naling remain unanswered.As such,more studies are re-
quired to elucidate how ROS affectsthe hypertrophic
and adaptive responses of human muscle to chronic RT,
especially in T2D.Unraveling these pathways could lead
to a betterunderstanding ofthe potentialtherapeutic
role of antioxidantsupplementsin treating metabolic
impairments.Furthermore,it is importantto consider
the interaction ofmedication orsupplementuse with
the antioxidant defense system (Fig.2).A recent review
explored the novelhypothesis that attenuation of oxida-
tive stressfrom exercise training by these exogenous
compounds blunts beneficial metabolic adaptations [68].
Chronic resistance training and medication use
Metformin and exercise training in diabetes:the good,
the bad and the unknown
In recent years,the combined effects of metformin treat-
ment and training regimens on metabolic outcomes have
gained attention,the majority of these studies conducted
in healthyand/or pre-diabeticpopulations[69–73]
(Fig.2). Metformin is the mostwidely prescribed first-
line oralanti-diabetic drug recommended by the Ameri-
can DiabetesAssociation (ADA).The exactmolecular
mechanism of action underlying this drug’s physiological
benefitsremainsmysterious,although itis often pre-
scribed together with a regular exercise training program
as part of a lifestyle intervention.Metformin is known to
havepleiotropiceffectsand multiplepotentialtarget
pathways.One possible site of metformin action is inhib-
ition ofmitochondrialrespiratory system complex I,in-
creasingthe [AMP]/[ATP] ratio (much like muscle
contraction does) and activatingAMPK. AMPK
phosphorylation ofmultiple downstream effectorscan
divert cellular metabolism towards restoration of energy
homeostasis[74],thereby improving insulin sensitivity
(Fig.2).ET-mediated increases in insulin sensitivity also
act in part through AMPK activation [75],suggesting
that exercise training and metformin could have additive
effects.In support, a recentrandomized,controlled
chronic exercise training (ET,RT, ET + RT) intervention
(22 weeks)in individuals with T2D demonstrated that
compared with controls,ET led to a significantreduc-
tion in HbA1c in metformin users (-0.57%)but not in
untreated individuals (-0.17%).However,metformin did
not have any effecton improvementsin indicatorsof
aerobic fitness,strength,body weight or waist circumfer-
ence[76].Importantly,this effectwas only observed
with ET and ET + RT. The RT only group did not
achieve significant reductions in HbA1c (with or without
metformin) compared with the control group.
Alternatively,metformin was also documented to have
opposing effects on fat oxidation during and following a
single boutof ET in healthy individuals [77],and a re-
cent reportdemonstrated thatmetformin blunted the
full effect of12 weeks ofcombined ET + RT on insulin
sensitivity by 25–30% in glucose intolerantindividuals
[72].This same investigation demonstrated that metfor-
min use blunted some ofthe beneficialeffectsof the
combined training related to a reduction in cardiovascu-
lar disease risk such as systolic blood pressure or high
sensitivity C-reactive protein (hs-CRP) [70].These find-
ings conflictwith an earlierinvestigation in insulin-
resistant individuals in which two to three weeks of met-
formin treatment blunted the effects ofa single bout of
ET on insulin sensitivity [71].The above studies suggest
some positive and negative interactions between metfor-
min use and adaptationsto both single and repeated
bouts of ET and combined ET + RT on glycemic control.
Taken together,these data suggestthat when exercise
training is implemented in a population of current met-
formin users,which is commonplace in a clinical setting,
it is importantto consider how use ofthis medication
may affectan individual’s training response in terms of
glycemic control.
Biguanides such as metformin have also been recently
identified as a new class ofmitochondrialglycerophos-
phate dehydrogenase (mGPD) [78] and ATP synthase in-
hibitors[79]. Inhibition of ATP synthasemay affect
energeticsin skeletalmuscleand recoveryof energy
charge during exercise.Inhibition ofmGPD by metfor-
min leadingto decreased cytosolic[NAD+
]/[NADH]
may alter activity ofSIRT1,decreasing deacetylation of
PGC-1α and reversing potentialpositive impact of exer-
cise on mitochondrial biogenesis.Also relevant is the re-
cent finding that metformin’seffect as a cancer
therapeutic is partially mediated by inhibition of mTOR,
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 6 of 10
ROS formation during RT may also have beneficialef-
fectson muscle differentiation in vivo.Indeed,mito-
chondrial ROS generation might be involved in initiating
mitochondrial biogenesis [66].Transient ROS “build-up”
has been suggested to prime the cellular redox system,
which culminatesin an improved handling ofsubse-
quentpro-oxidantenvironments.Oxidative stress must
therefore be considered in connection with radicalscav-
enging activities and cellular antioxidant defense mecha-
nisms.A 12-week RT intervention (twice perweek at
50–75% ofthe 1RM) not only reduced oxidative stress,
but also significantly increased cytosolic and mitochon-
drial antioxidantproteins [superoxidedismutase-2
(SOD2),glutathione peroxidase-1 (GPX1),peroxiredoxin
isoform 5 (PRDX5),heat-shock-protein-70 (HSP70)]in
muscle of individuals with T2D [67].
Chronic RT effects on ROS production in human
muscle are relatively unexplored.Due to the absence of
reliable techniques to precisely measure ROS production
in vivo,fundamentalquestions regarding the sources of
ROS and the effects oftraining modalities on ROS sig-
naling remain unanswered.As such,more studies are re-
quired to elucidate how ROS affectsthe hypertrophic
and adaptive responses of human muscle to chronic RT,
especially in T2D.Unraveling these pathways could lead
to a betterunderstanding ofthe potentialtherapeutic
role of antioxidantsupplementsin treating metabolic
impairments.Furthermore,it is importantto consider
the interaction ofmedication orsupplementuse with
the antioxidant defense system (Fig.2).A recent review
explored the novelhypothesis that attenuation of oxida-
tive stressfrom exercise training by these exogenous
compounds blunts beneficial metabolic adaptations [68].
Chronic resistance training and medication use
Metformin and exercise training in diabetes:the good,
the bad and the unknown
In recent years,the combined effects of metformin treat-
ment and training regimens on metabolic outcomes have
gained attention,the majority of these studies conducted
in healthyand/or pre-diabeticpopulations[69–73]
(Fig.2). Metformin is the mostwidely prescribed first-
line oralanti-diabetic drug recommended by the Ameri-
can DiabetesAssociation (ADA).The exactmolecular
mechanism of action underlying this drug’s physiological
benefitsremainsmysterious,although itis often pre-
scribed together with a regular exercise training program
as part of a lifestyle intervention.Metformin is known to
havepleiotropiceffectsand multiplepotentialtarget
pathways.One possible site of metformin action is inhib-
ition ofmitochondrialrespiratory system complex I,in-
creasingthe [AMP]/[ATP] ratio (much like muscle
contraction does) and activatingAMPK. AMPK
phosphorylation ofmultiple downstream effectorscan
divert cellular metabolism towards restoration of energy
homeostasis[74],thereby improving insulin sensitivity
(Fig.2).ET-mediated increases in insulin sensitivity also
act in part through AMPK activation [75],suggesting
that exercise training and metformin could have additive
effects.In support, a recentrandomized,controlled
chronic exercise training (ET,RT, ET + RT) intervention
(22 weeks)in individuals with T2D demonstrated that
compared with controls,ET led to a significantreduc-
tion in HbA1c in metformin users (-0.57%)but not in
untreated individuals (-0.17%).However,metformin did
not have any effecton improvementsin indicatorsof
aerobic fitness,strength,body weight or waist circumfer-
ence[76].Importantly,this effectwas only observed
with ET and ET + RT. The RT only group did not
achieve significant reductions in HbA1c (with or without
metformin) compared with the control group.
Alternatively,metformin was also documented to have
opposing effects on fat oxidation during and following a
single boutof ET in healthy individuals [77],and a re-
cent reportdemonstrated thatmetformin blunted the
full effect of12 weeks ofcombined ET + RT on insulin
sensitivity by 25–30% in glucose intolerantindividuals
[72].This same investigation demonstrated that metfor-
min use blunted some ofthe beneficialeffectsof the
combined training related to a reduction in cardiovascu-
lar disease risk such as systolic blood pressure or high
sensitivity C-reactive protein (hs-CRP) [70].These find-
ings conflictwith an earlierinvestigation in insulin-
resistant individuals in which two to three weeks of met-
formin treatment blunted the effects ofa single bout of
ET on insulin sensitivity [71].The above studies suggest
some positive and negative interactions between metfor-
min use and adaptationsto both single and repeated
bouts of ET and combined ET + RT on glycemic control.
Taken together,these data suggestthat when exercise
training is implemented in a population of current met-
formin users,which is commonplace in a clinical setting,
it is importantto consider how use ofthis medication
may affectan individual’s training response in terms of
glycemic control.
Biguanides such as metformin have also been recently
identified as a new class ofmitochondrialglycerophos-
phate dehydrogenase (mGPD) [78] and ATP synthase in-
hibitors[79]. Inhibition of ATP synthasemay affect
energeticsin skeletalmuscleand recoveryof energy
charge during exercise.Inhibition ofmGPD by metfor-
min leadingto decreased cytosolic[NAD+
]/[NADH]
may alter activity ofSIRT1,decreasing deacetylation of
PGC-1α and reversing potentialpositive impact of exer-
cise on mitochondrial biogenesis.Also relevant is the re-
cent finding that metformin’seffect as a cancer
therapeutic is partially mediated by inhibition of mTOR,
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 6 of 10
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

raising the question of whether metformin may influence
the positive effects of Akt-mTOR pathway activation on
muscle mass [20].It remains to be determined how a
chronic RT regimen would impact individuals with T2D
currently taking metformin and other anti-diabetic drugs
(Fig.2).More research on the detailed effects ofmetfor-
min on adaptations to ET and RT, especially in the context
of varying intensities and duration,is warranted.Further-
more,the significance of metformin effects directly in the
muscle atprescribed dosesrequiresattention,as it is
thoughtthatmetformin primarily targets liver metabol-
ism.It is possible that metformin-mediated amelioration
of whole-body glucose homeostasis via inhibition ofun-
controlled liver gluconeogenesis may be enough to restore
muscle insulin sensitivity and therefore promote beneficial
effects of exercise training in the muscle. Based on current
evidence,we speculate thatin the muscle,anti-diabetic
drugs such as metformin – due to inhibition of complex I
of the electron transportsystem and subsequent AMPK
activation – may interfere with adaptations to ET to a
greater extent than RT, which recruits a different signaling
cascade than AMPK. This could possibly establish RT as a
reasonable training modality for a cohort taking metfor-
min, particularly those individuals with T2D.
Chronic resistance training and adipose tissue
Adipose tissue is an important (and understudied) target
organ of resistance training in diabetes
Studies of exercise training,chronic RT in particular,on
white adipose tissue (WAT)remodeling in humans are
sparse.WAT secretes two major pro-inflammatory cyto-
kines:interleukin-6 (IL-6)and tumornecrosisfactor-
alpha (TNF-α).Obesity ischaracterized by a state of
chronic low-grade inflammation,which is implicated in
the pathogenesis ofT2D [80],and circulating levels of
both IL-6 and TNF-α are inversely related to glycemic
controland insulin sensitivity [81].Modulation oflow-
grade inflammation by 12 weeks ofET (40 minutes at
60–80% of the maximalheart rate) and RT (three times
per week at60–80% ofthe 1RM) wasinvestigated in
obese individuals with T2D.Although ET was more ef-
fective in reducing adipocytokinesin response to the
chronic training,RT also significantly reduced circulat-
ing levelsof TNF-α and IL-6 [82]. RT for 16 weeks
(three times per week at 60–85% ofthe 1RM) in obese
adolescentssignificantlyreduced IL-6 and TNF-α
plasma levels,and changes in muscle strength were dir-
ectly related to changes in pro-inflammatory cytokines
[83].These data suggest that chronic RT induces a sig-
naling pathway that alleviates systemic inflammation.In
another study,12 weeks ofsupervised RT (3 days per
week)in individuals with T2D decreased plasma levels
of hs-CRP,a non-specific marker ofinflammation,and
increased the beneficial adipokine Visfatin [84].
According to some studies [85,86] excess post-exercise
oxygen consumption is higher after RT than after ET.
This phase is characterized by utilization of fat as a fuel,
which could benefit weight loss [87].Although data are
limited,collectively,these studies highlight the systemic
anti-inflammatory effects ofRT (presumably via WAT)
and the potentialof chronic RT to improve body com-
position and alleviate chronic low-grade inflammation
associated with obesity and T2D.How chronic RT af-
fects WAT function (e.g.,fibrosis,angiogenesis,brown-
ing,etc.) and signaling pathways within the organ itself
are areas ofresearch thatrequire greater attention and
could shed light on exercise training targets that elicit a
positive metabolic response in individuals with T2D.
Closing remarks
Future directions – toward personalized medicine?
Due to the large inherent variability ofresponses to the
same RT program,generalrecommendationsare less
than ideal.The future of T2D research is moving in the
direction ofpersonalized medicine.Currentresearch in
this area continues to discover signaling pathways that
differ even amongst the most homogenous groups of in-
dividuals,and these differences ultimately lead to varia-
tions in their physiological responses to medications and
treatments.It is imperative thatwe exploitthese inter-
individual responses following exercise training interven-
tions in individuals with T2D to maximize each person’s
beneficialadaptation to a training program.A clear dis-
tinction of the different types of RT will be necessary for
the implementation of exercise training as a feasible life-
style modification in light of current and future progres-
sion toward personalizedmedicine.Different RT
programs (with varying intensities)will lead to diverse
results and metabolic outcomes,and this cause-effect re-
lationship must be clearly established for RT in order to
maximize the benefits for individuals with T2D.In this
effort,more research comparing supervised ET interven-
tions with RT interventions ofvarying intensities,dura-
tions and volumes including long-term training studies
using different modes ofperiodization,is required.This
will further elucidateexercise-mediatedeffects on
whole-body metabolism and muscle mitochondrial func-
tion,specifically in individuals with T2D.In addition,the
effectsof RT on other major targetorgans,such as
WAT, require deeper investigation.
Another question to be addressed iswhetherthe
underlying mechanisms by which chronic RT improves
muscle glucose regulation are the same as those utilized
by chronic ET.It is necessary to determine which path-
ways are recruited by each type of exercise training,once
again due to the possibility thatone training method
may impart greater benefit to certain individuals than to
others as a resultof their genetic makeup,physiology,
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 7 of 10
the positive effects of Akt-mTOR pathway activation on
muscle mass [20].It remains to be determined how a
chronic RT regimen would impact individuals with T2D
currently taking metformin and other anti-diabetic drugs
(Fig.2).More research on the detailed effects ofmetfor-
min on adaptations to ET and RT, especially in the context
of varying intensities and duration,is warranted.Further-
more,the significance of metformin effects directly in the
muscle atprescribed dosesrequiresattention,as it is
thoughtthatmetformin primarily targets liver metabol-
ism.It is possible that metformin-mediated amelioration
of whole-body glucose homeostasis via inhibition ofun-
controlled liver gluconeogenesis may be enough to restore
muscle insulin sensitivity and therefore promote beneficial
effects of exercise training in the muscle. Based on current
evidence,we speculate thatin the muscle,anti-diabetic
drugs such as metformin – due to inhibition of complex I
of the electron transportsystem and subsequent AMPK
activation – may interfere with adaptations to ET to a
greater extent than RT, which recruits a different signaling
cascade than AMPK. This could possibly establish RT as a
reasonable training modality for a cohort taking metfor-
min, particularly those individuals with T2D.
Chronic resistance training and adipose tissue
Adipose tissue is an important (and understudied) target
organ of resistance training in diabetes
Studies of exercise training,chronic RT in particular,on
white adipose tissue (WAT)remodeling in humans are
sparse.WAT secretes two major pro-inflammatory cyto-
kines:interleukin-6 (IL-6)and tumornecrosisfactor-
alpha (TNF-α).Obesity ischaracterized by a state of
chronic low-grade inflammation,which is implicated in
the pathogenesis ofT2D [80],and circulating levels of
both IL-6 and TNF-α are inversely related to glycemic
controland insulin sensitivity [81].Modulation oflow-
grade inflammation by 12 weeks ofET (40 minutes at
60–80% of the maximalheart rate) and RT (three times
per week at60–80% ofthe 1RM) wasinvestigated in
obese individuals with T2D.Although ET was more ef-
fective in reducing adipocytokinesin response to the
chronic training,RT also significantly reduced circulat-
ing levelsof TNF-α and IL-6 [82]. RT for 16 weeks
(three times per week at 60–85% ofthe 1RM) in obese
adolescentssignificantlyreduced IL-6 and TNF-α
plasma levels,and changes in muscle strength were dir-
ectly related to changes in pro-inflammatory cytokines
[83].These data suggest that chronic RT induces a sig-
naling pathway that alleviates systemic inflammation.In
another study,12 weeks ofsupervised RT (3 days per
week)in individuals with T2D decreased plasma levels
of hs-CRP,a non-specific marker ofinflammation,and
increased the beneficial adipokine Visfatin [84].
According to some studies [85,86] excess post-exercise
oxygen consumption is higher after RT than after ET.
This phase is characterized by utilization of fat as a fuel,
which could benefit weight loss [87].Although data are
limited,collectively,these studies highlight the systemic
anti-inflammatory effects ofRT (presumably via WAT)
and the potentialof chronic RT to improve body com-
position and alleviate chronic low-grade inflammation
associated with obesity and T2D.How chronic RT af-
fects WAT function (e.g.,fibrosis,angiogenesis,brown-
ing,etc.) and signaling pathways within the organ itself
are areas ofresearch thatrequire greater attention and
could shed light on exercise training targets that elicit a
positive metabolic response in individuals with T2D.
Closing remarks
Future directions – toward personalized medicine?
Due to the large inherent variability ofresponses to the
same RT program,generalrecommendationsare less
than ideal.The future of T2D research is moving in the
direction ofpersonalized medicine.Currentresearch in
this area continues to discover signaling pathways that
differ even amongst the most homogenous groups of in-
dividuals,and these differences ultimately lead to varia-
tions in their physiological responses to medications and
treatments.It is imperative thatwe exploitthese inter-
individual responses following exercise training interven-
tions in individuals with T2D to maximize each person’s
beneficialadaptation to a training program.A clear dis-
tinction of the different types of RT will be necessary for
the implementation of exercise training as a feasible life-
style modification in light of current and future progres-
sion toward personalizedmedicine.Different RT
programs (with varying intensities)will lead to diverse
results and metabolic outcomes,and this cause-effect re-
lationship must be clearly established for RT in order to
maximize the benefits for individuals with T2D.In this
effort,more research comparing supervised ET interven-
tions with RT interventions ofvarying intensities,dura-
tions and volumes including long-term training studies
using different modes ofperiodization,is required.This
will further elucidateexercise-mediatedeffects on
whole-body metabolism and muscle mitochondrial func-
tion,specifically in individuals with T2D.In addition,the
effectsof RT on other major targetorgans,such as
WAT, require deeper investigation.
Another question to be addressed iswhetherthe
underlying mechanisms by which chronic RT improves
muscle glucose regulation are the same as those utilized
by chronic ET.It is necessary to determine which path-
ways are recruited by each type of exercise training,once
again due to the possibility thatone training method
may impart greater benefit to certain individuals than to
others as a resultof their genetic makeup,physiology,
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 7 of 10
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

and current medication use.Differences in sex,age and
ethnicity likely contribute to the distinctoutcomesof
several studies looking at the metabolic effects of endur-
anceand resistancetraining,even when theexercise
protocol itself remains the same.While some studies re-
port beneficial effects of RT on diverse metabolic param-
eters [19,88,89],others do not [90,91].It is therefore
criticalto report the training load,duration and relative
intensity when comparing differentgroupsof individ-
uals.Addressing why discrepanciesexistin outcomes
from studies where individuals trained atsimilar inten-
sities and duration may hold the key to effectively in-
corporate exercise training in the treatment of metabolic
disease on a personalized level.
Conclusions
RT is a promising strategy to promote overallmetabolic
health in individualswith T2D via improvementsin
musclemitochondrialperformanceand increasesin
muscle mass that may positively impact insulin respon-
siveness and glucose control.Multifactorialevents con-
tribute to the beneficialadaptations elicited by chronic
RT (Fig.1).A consistent regimen of RT at moderate in-
tensity elicitsthe mostbeneficialmetabolic response-
s—in terms of HbA1c and insulin sensitivity—in
individualswith T2D. Currentexerciserecommenda-
tions should ideally be a synthesisof gains in muscle
mass (higher intensity RT) and mitochondrialoxidative
capacity (lower intensity RT or ET),as well as aim to re-
duce circulating pro-inflammatory cytokines(targeting
WAT), in order to fully exploitthe salutary effects of
training on overallmetabolic health.This can most effi-
ciently be achieved by utilizing combined (RT and ET)
training regimes.Anotherimportantconsideration for
current diabetes treatment guidelines is the use ofmet-
formin in combination with exercisetrainingas the
questionof synergism vs.antagonism remainsun-
answered (Fig.2).Combinations of lifestyle interventions
(e.g.,such as medication use,caloric restriction and
weightloss)may offeradditionalbenefits and additive
effects to a RT regime.
Acknowledgements
We want to thank Dr.Varman Samuelfor insightfuldiscussions.
Funding
This work was supported by the Austrian Science Fund (FWF),project no.J
3267 and American Diabetes Association Junior Faculty Award (#7-13-JF-53).
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or
analyzed during the current study.
Authors’contributions
DHP conceptualized and wrote the manuscript and designed the figures.
RLSG and AKM wrote the manuscript and gave input to the figures.BS,AKM
and LMS wrote and proofread the manuscript and gave input to the figures.
Allauthors read and approved the finalmanuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
Not applicable
Author details
1Department of Sport Science,MedicalSection,University of Innsbruck,
Fürstenweg 185,Innsbruck,Austria.2Department of Visceral,Transplant,and
Thoracic Surgery,D.SwarovskiResearch Laboratory,MedicalUniversity of
Innsbruck,Innsbruck,Austria.3Institute for ClinicalDiabetology,German
Diabetes Center,Leibniz Institute for Diabetes Research at Heinrich Heine
University,Düsseldorf,Germany.4German Center for Diabetes Research (DZD
e.V.),München-Neuherberg,Germany.5Department of Genetics and
Complex Diseases and SabriÜlker Center,Harvard T.H.Chan Schoolof Public
Health,677 Huntington Avenue,Boston,MA 02115,USA.6Salk Institute for
BiologicalStudies,10010N Torrey Pines Rd,La Jolla,CA 92037,USA.
7Biocenter,MedicalUniversity Innsbruck,Innrain 80-82,Innsbruck,Austria.
8TranslationalResearch Institute for Metabolism and Diabetes,Florida
Hospital,301 E.Princeton Street,Orlando,FL 32804,USA.9Sanford Burnham
Prebys MedicalDiscovery Institute,Center for Clinicaland Molecular Origins
of Disease,Orlando,FL,USA.
Received:6 October 2016 Accepted:14 February 2017
References
1. Guariguata L,Whiting DR,Hambleton I,Beagley J,Linnenkamp U,Shaw JE.
Globalestimates of diabetes prevalence for 2013 and projections for 2035.
Diabetes Res Clin Pract.2014;103:137–49.
2. Long AN,Dagogo-Jack S.Comorbidities of diabetes and hypertension:
mechanisms and approach to target organ protection.J Clin Hypertens
(Greenwich).2011;13:244–51.
3. Hordern MD,Dunstan DW,Prins JB,Baker MK,Singh MA,Coombes JS.
Exercise prescription for patients with type 2 diabetes and pre-diabetes:a
position statement from Exercise and Sport Science Australia.J SciMed
Sport.2012;15:25–31.
4. Colberg SR,SigalRJ,FernhallB,Regensteiner JG,Blissmer BJ,Rubin RR,
Chasan-Taber L,Albright AL,Braun B,American College of Sports M,
American Diabetes A.Exercise and type 2 diabetes:the American College of
Sports Medicine and the American Diabetes Association:joint position
statement executive summary.Diabetes Care.2010;33:2692–6.
5. Madden KM.Evidence for the benefit of exercise therapy in patients with
type 2 diabetes.Diabetes Metab Syndr Obes.2013;6:233–9.
6. Colberg SR,SigalRJ,Yardley JE,RiddellMC,Dunstan DW,Dempsey PC,
Horton ES,Castorino K,Tate DF.Physicalactivity/exercise and diabetes:a
position statement of the American diabetes association.Diabetes Care.
2016;39:2065–79.
7. SchwingshacklL,Missbach B,Dias S,Konig J,Hoffmann G.Impact of
different training modalities on glycaemic controland blood lipids in
patients with type 2 diabetes:a systematic review and network meta-
analysis.Diabetologia.2014;57:1789–97.
8. Mann S,Beedie C,BalducciS,Zanuso S,Allgrove J,Bertiato F,Jimenez A.
Changes in insulin sensitivity in response to different modalities of exercise:
a review of the evidence.Diabetes Metab Res Rev.2014;30:257–68.
9. Richter EA,Turcotte L,HespelP,Kiens B.Metabolic responses to exercise.
Effects of endurance training and implications for diabetes.Diabetes Care.
1992;15:1767–76.
10. Devries MC,Hamadeh MJ,McCready C,Sischek S,Tarnopolsky MA.Twelve
weeks of endurance training increases mitochondrialdensity and percent
IMCL touching mitochondria and alters IMCL storage distribution.FASEB
Journal.2008;22:753.718.
11. BacchiE,NegriC,Targher G,FaccioliN,Lanza M,ZoppiniG,Zanolin E,
Schena F,Bonora E,MoghettiP.Both resistance training and aerobic
training reduce hepatic fat content in type 2 diabetic subjects with
nonalcoholic fatty liver disease (the RAED2 randomized trial).Hepatology.
2013;58(4):1287–95.
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 8 of 10
ethnicity likely contribute to the distinctoutcomesof
several studies looking at the metabolic effects of endur-
anceand resistancetraining,even when theexercise
protocol itself remains the same.While some studies re-
port beneficial effects of RT on diverse metabolic param-
eters [19,88,89],others do not [90,91].It is therefore
criticalto report the training load,duration and relative
intensity when comparing differentgroupsof individ-
uals.Addressing why discrepanciesexistin outcomes
from studies where individuals trained atsimilar inten-
sities and duration may hold the key to effectively in-
corporate exercise training in the treatment of metabolic
disease on a personalized level.
Conclusions
RT is a promising strategy to promote overallmetabolic
health in individualswith T2D via improvementsin
musclemitochondrialperformanceand increasesin
muscle mass that may positively impact insulin respon-
siveness and glucose control.Multifactorialevents con-
tribute to the beneficialadaptations elicited by chronic
RT (Fig.1).A consistent regimen of RT at moderate in-
tensity elicitsthe mostbeneficialmetabolic response-
s—in terms of HbA1c and insulin sensitivity—in
individualswith T2D. Currentexerciserecommenda-
tions should ideally be a synthesisof gains in muscle
mass (higher intensity RT) and mitochondrialoxidative
capacity (lower intensity RT or ET),as well as aim to re-
duce circulating pro-inflammatory cytokines(targeting
WAT), in order to fully exploitthe salutary effects of
training on overallmetabolic health.This can most effi-
ciently be achieved by utilizing combined (RT and ET)
training regimes.Anotherimportantconsideration for
current diabetes treatment guidelines is the use ofmet-
formin in combination with exercisetrainingas the
questionof synergism vs.antagonism remainsun-
answered (Fig.2).Combinations of lifestyle interventions
(e.g.,such as medication use,caloric restriction and
weightloss)may offeradditionalbenefits and additive
effects to a RT regime.
Acknowledgements
We want to thank Dr.Varman Samuelfor insightfuldiscussions.
Funding
This work was supported by the Austrian Science Fund (FWF),project no.J
3267 and American Diabetes Association Junior Faculty Award (#7-13-JF-53).
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or
analyzed during the current study.
Authors’contributions
DHP conceptualized and wrote the manuscript and designed the figures.
RLSG and AKM wrote the manuscript and gave input to the figures.BS,AKM
and LMS wrote and proofread the manuscript and gave input to the figures.
Allauthors read and approved the finalmanuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
Not applicable
Author details
1Department of Sport Science,MedicalSection,University of Innsbruck,
Fürstenweg 185,Innsbruck,Austria.2Department of Visceral,Transplant,and
Thoracic Surgery,D.SwarovskiResearch Laboratory,MedicalUniversity of
Innsbruck,Innsbruck,Austria.3Institute for ClinicalDiabetology,German
Diabetes Center,Leibniz Institute for Diabetes Research at Heinrich Heine
University,Düsseldorf,Germany.4German Center for Diabetes Research (DZD
e.V.),München-Neuherberg,Germany.5Department of Genetics and
Complex Diseases and SabriÜlker Center,Harvard T.H.Chan Schoolof Public
Health,677 Huntington Avenue,Boston,MA 02115,USA.6Salk Institute for
BiologicalStudies,10010N Torrey Pines Rd,La Jolla,CA 92037,USA.
7Biocenter,MedicalUniversity Innsbruck,Innrain 80-82,Innsbruck,Austria.
8TranslationalResearch Institute for Metabolism and Diabetes,Florida
Hospital,301 E.Princeton Street,Orlando,FL 32804,USA.9Sanford Burnham
Prebys MedicalDiscovery Institute,Center for Clinicaland Molecular Origins
of Disease,Orlando,FL,USA.
Received:6 October 2016 Accepted:14 February 2017
References
1. Guariguata L,Whiting DR,Hambleton I,Beagley J,Linnenkamp U,Shaw JE.
Globalestimates of diabetes prevalence for 2013 and projections for 2035.
Diabetes Res Clin Pract.2014;103:137–49.
2. Long AN,Dagogo-Jack S.Comorbidities of diabetes and hypertension:
mechanisms and approach to target organ protection.J Clin Hypertens
(Greenwich).2011;13:244–51.
3. Hordern MD,Dunstan DW,Prins JB,Baker MK,Singh MA,Coombes JS.
Exercise prescription for patients with type 2 diabetes and pre-diabetes:a
position statement from Exercise and Sport Science Australia.J SciMed
Sport.2012;15:25–31.
4. Colberg SR,SigalRJ,FernhallB,Regensteiner JG,Blissmer BJ,Rubin RR,
Chasan-Taber L,Albright AL,Braun B,American College of Sports M,
American Diabetes A.Exercise and type 2 diabetes:the American College of
Sports Medicine and the American Diabetes Association:joint position
statement executive summary.Diabetes Care.2010;33:2692–6.
5. Madden KM.Evidence for the benefit of exercise therapy in patients with
type 2 diabetes.Diabetes Metab Syndr Obes.2013;6:233–9.
6. Colberg SR,SigalRJ,Yardley JE,RiddellMC,Dunstan DW,Dempsey PC,
Horton ES,Castorino K,Tate DF.Physicalactivity/exercise and diabetes:a
position statement of the American diabetes association.Diabetes Care.
2016;39:2065–79.
7. SchwingshacklL,Missbach B,Dias S,Konig J,Hoffmann G.Impact of
different training modalities on glycaemic controland blood lipids in
patients with type 2 diabetes:a systematic review and network meta-
analysis.Diabetologia.2014;57:1789–97.
8. Mann S,Beedie C,BalducciS,Zanuso S,Allgrove J,Bertiato F,Jimenez A.
Changes in insulin sensitivity in response to different modalities of exercise:
a review of the evidence.Diabetes Metab Res Rev.2014;30:257–68.
9. Richter EA,Turcotte L,HespelP,Kiens B.Metabolic responses to exercise.
Effects of endurance training and implications for diabetes.Diabetes Care.
1992;15:1767–76.
10. Devries MC,Hamadeh MJ,McCready C,Sischek S,Tarnopolsky MA.Twelve
weeks of endurance training increases mitochondrialdensity and percent
IMCL touching mitochondria and alters IMCL storage distribution.FASEB
Journal.2008;22:753.718.
11. BacchiE,NegriC,Targher G,FaccioliN,Lanza M,ZoppiniG,Zanolin E,
Schena F,Bonora E,MoghettiP.Both resistance training and aerobic
training reduce hepatic fat content in type 2 diabetic subjects with
nonalcoholic fatty liver disease (the RAED2 randomized trial).Hepatology.
2013;58(4):1287–95.
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 8 of 10

12. Church TS,Blair SN,Cocreham S,Johannsen N,Johnson W,Kramer K,Mikus
CR,Myers V,Nauta M,Rodarte RQ,et al.Effects of aerobic and resistance
training on hemoglobin A1c levels in patients with type 2 diabetes:a
randomized controlled trial.JAMA.2010;304:2253–62.
13. Sparks LM,Johannsen NM,Church TS,Earnest CP,Moonen-Kornips E,Moro
C,Hesselink MK,Smith SR,Schrauwen P.Nine months of combined training
improves ex vivo skeletalmuscle metabolism in individuals with type 2
diabetes.J Clin EndocrinolMetab.2013;98:1694–702.
14. DeFronzo RA,Tripathy D.Skeletalmuscle insulin resistance is the primary
defect in type 2 diabetes.Diabetes Care.2009;32 Suppl2:S157–63.
15. DeFronzo RA,Jacot E,Jequier E,Maeder E,Wahren J,Felber JP.The effect of
insulin on the disposal of intravenous glucose.Results from indirect calorimetry
and hepatic and femoral venous catheterization.Diabetes.1981;30:1000–7.
16. Kelley DE,He J,Menshikova EV,Ritov VB.Dysfunction of mitochondria in
human skeletalmuscle in type 2 diabetes.Diabetes.2002;51:2944–50.
17. Romero-Arenas S,Martinez-PascualM,Alcaraz PE.Impact of resistance
circuit training on neuromuscular,cardiorespiratory and body composition
adaptations in the elderly.Aging Dis.2013;4:256–63.
18. Goodman CA.The role of mTORC1 in regulating protein synthesis and
skeletalmuscle mass in response to various mechanicalstimuli.Rev Physiol
Biochem Pharmacol.2014;166:43–95.
19. Holten MK,Zacho M,Gaster M,JuelC,WojtaszewskiJF,Dela F.Strength
training increases insulin-mediated glucose uptake,GLUT4 content,and
insulin signaling in skeletalmuscle in patients with type 2 diabetes.
Diabetes.2004;53:294–305.
20. McKinsey TA,Zhang CL,Lu J,Olson EN:Signal-dependent nuclear export of a
histone deacetylase regulates muscle differentiation.Nature.2000;408:106–111.
21. HortobagyiT,Dempsey L,Fraser D,Zheng D,Hamilton G,Lambert J,Dohm
L.Changes in muscle strength,muscle fibre size and myofibrillar gene
expression after immobilization and retraining in humans.J Physiol.2000;
524(Pt 1):293–304.
22. Harmer AR,Elkins MR.Amount and frequency of exercise affect glycaemic
control more than exercise mode or intensity.Br J Sports Med.2015;49:1012–4.
23. Yki-Jarvinen H,Koivisto VA.Effects of body composition on insulin
sensitivity.Diabetes.1983;32:965–9.
24. IshiiT,Yamakita T,Sato T,Tanaka S,FujiiS.Resistance training improves
insulin sensitivity in NIDDM subjects without altering maximaloxygen
uptake.Diabetes Care.1998;21:1353–5.
25. Castorena CM,Arias EB,Sharma N,Bogan JS,Cartee GD.Fiber type effects
on contraction-stimulated glucose uptake and GLUT4 abundance in single
fibers from rat skeletalmuscle.Am J PhysiolEndocrinolMetab.2015;308:
E223–30.
26. MackrellJG,Cartee GD.A novelmethod to measure glucose uptake and
myosin heavy chain isoform expression of single fibers from rat skeletal
muscle.Diabetes.2012;61:995–1003.
27. Holloszy JO,Hansen PA.Regulation of glucose transport into skeletal
muscle.Rev PhysiolBiochem Pharmacol.1996;128:99–193.
28. Vollestad NK,Blom PC,Gronnerod O.Resynthesis of glycogen in different
muscle fibre types after prolonged exhaustive exercise in man.Acta Physiol
Scand.1989;137:15–21.
29. Schofield KL,Rehrer NJ,Perry TL,Ross A,Andersen JL,Osborne H.Insulin
and fiber type in the offspring of T2DM subjects with resistance training
and detraining.Med SciSports Exerc.2012;44:2331–9.
30. Geisler S,Brinkmann C,Schiffer T,Kreutz T,Bloch W,Brixius K.The influence
of resistance training on patients with metabolic syndrome–significance of
changes in muscle fiber size and muscle fiber distribution.J Strength Cond
Res.2011;25:2598–604.
31. Adams GR,Hather BM,Baldwin KM,Dudley GA.Skeletalmuscle myosin
heavy chain composition and resistance training.J ApplPhysiol(1985).
1993;74:911–5.
32. LowellBB,Shulman GI.Mitochondrialdysfunction and type 2 diabetes.
Science.2005;307:384–7.
33. Sparks LM,Xie H,Koza RA,Mynatt R,Hulver MW,Bray GA,Smith SR.A high-
fatdiet coordinately downregulates genes required for mitochondrial
oxidative phosphorylation in skeletalmuscle.Diabetes.2005;54:1926–33.
34. BoushelR,Gnaiger E,Schjerling P,Skovbro M,Kraunsoe R,Dela F.Patients
with type 2 diabetes have normalmitochondrialfunction in skeletalmuscle.
Diabetologia.2007;50:790–6.
35. Hesselink MK,Schrauwen-Hinderling V,Schrauwen P.Skeletalmuscle
mitochondria as a target to prevent or treat type 2 diabetes mellitus.
Nat Rev Endocrinol.2016;12:633–45.
36. Akimoto T,Pohnert SC,Li P,Zhang M,Gumbs C,Rosenberg PB,Williams RS,
Yan Z.Exercise stimulates Pgc-1alpha transcription in skeletal muscle through
activation of the p38 MAPK pathway.J Biol Chem.2005;280:19587–93.
37. Frank P,Andersson E,Ponten M,Ekblom B,Ekblom M,Sahlin K.Strength
training improves muscle aerobic capacity and glucose tolerance in elderly.
Scand J Med SciSports.2016;26:764–73.
38. Irving BA,Lanza IR,Henderson GC,Rao RR,Spiegelman BM,Nair KS.
Combined training enhances skeletalmuscle mitochondrialoxidative
capacity independent of age.J Clin EndocrinolMetab.2015;100:1654–63.
39. Pesta D,HoppelF,Macek C,Messner H,Faulhaber M,KobelC,Parson W,
Burtscher M,Schocke M,Gnaiger E.Similar qualitative and quantitative
changes of mitochondrialrespiration following strength and endurance
training in normoxia and hypoxia in sedentary humans.Am J PhysiolRegul
Integr Comp Physiol.2011;301:R1078–87.
40. Tang JE,Hartman JW,Phillips SM.Increased muscle oxidative potential
following resistance training induced fibre hypertrophy in young men.Appl
PhysiolNutr Metab.2006;31:495–501.
41. Colberg SR,Albright AL,Blissmer BJ,Braun B,Chasan-Taber L,Fernhall B,
Regensteiner JG,Rubin RR,Sigal RJ,American College of Sports M,American
Diabetes A.Exercise and type 2 diabetes:American College of Sports Medicine
and the American Diabetes Association:joint position statement.Exercise and
type 2 diabetes.Med Sci Sports Exerc.2010;42:2282–303.
42. Chung N,Kreutz T,Schiffer T,Opitz D,Hermann R,Gehlert S,Bloch W,
Brixius K,Brinkmann C.Training-induced alterations of skeletalmuscle
mitochondrialbiogenesis proteins in non-insulin-dependent type 2 diabetic
men.Can J PhysiolPharmacol.2012;90:1634–41.
43. Hawley JA.Molecular responses to strength and endurance training:are
they incompatible? ApplPhysiolNutr Metab.2009;34:355–61.
44. Wang L,Mascher H,Psilander N,Blomstrand E,Sahlin K.Resistance exercise
enhances the molecular signaling of mitochondrialbiogenesis induced by
endurance exercise in human skeletalmuscle.J ApplPhysiol(1985).2011;
111:1335–44.
45. Lantier L,Fentz J,Mounier R,Leclerc J,Treebak JT,Pehmoller C,Sanz N,
Sakakibara I,Saint-Amand E,Rimbaud S,et al.AMPK controls exercise
endurance,mitochondrialoxidative capacity,and skeletalmuscle integrity.
FASEB J.2014;28:3211–24.
46. McGee SL,Hargreaves M.AMPK-mediated regulation of transcription in
skeletalmuscle.Clin Sci(Lond).2010;118:507–18.
47. Gross DN vHA,Birnbaum NJ.The role of FoxO in the regulation of
metabolism.Oncogene.2008;27:2320–36.
48. Tarnopolsky MA.MitochondrialDNA shifting in older adults following
resistance exercise training.ApplPhysiolNutr Metab.2009;34:348–54.
49. Parise G,Brose AN,Tarnopolsky MA.Resistance exercise training decreases
oxidative damage to DNA and increases cytochrome oxidase activity in
older adults.Exp Gerontol.2005;40:173–80.
50. McInnes J.Mitochondrial-associated metabolic disorders:foundations,
pathologies and recent progress.Nutr Metab (Lond).2013;10:63.
51. RodellA,Rasmussen LJ,Bergersen LH,Singh KK,Gjedde A.Naturalselection
of mitochondria during somatic lifetime promotes healthy aging.Front
Neuroenergetics.2013;5:7.
52. Lefort N,Glancy B,Bowen B,Willis WT,Bailowitz Z,De Filippis EA,Brophy C,
Meyer C,Hojlund K,Yi Z,Mandarino LJ.Increased reactive oxygen species
production and lower abundance of complex Isubunits and carnitine
palmitoyltransferase 1B protein despite normalmitochondrialrespiration in
insulin-resistant human skeletalmuscle.Diabetes.2010;59:2444–52.
53. Houstis N,Rosen ED,Lander ES.Reactive oxygen species have a causalrole
in multiple forms of insulin resistance.Nature.2006;440:944–8.
54. Goncalves RL,Bunik VI,Brand MD.Production of superoxide/hydrogen
peroxide by the mitochondrial2-oxoadipate dehydrogenase complex.Free
Radic BiolMed.2016;91:247–255.
55. Goncalves RL,Quinlan CL,Perevoshchikova IV,Hey-Mogensen M,Brand MD.
Sitesof superoxide and hydrogen peroxide production by muscle
mitochondria assessed ex vivo under conditions mimicking rest and
exercise.J BiolChem.2015;290:209–27.
56. McBride JM,Kraemer WJ,Triplett-McBride T,Sebastianelli W.Effect of resistance
exercise on free radical production.Med Sci Sports Exerc.1998;30:67–72.
57. Alessio HM,Hagerman AE,Fulkerson BK,Ambrose J,Rice RE,Wiley RL.
Generation of reactive oxygen species after exhaustive aerobic and
isometric exercise.Med SciSports Exerc.2000;32:1576–81.
58. Petersen AC,McKenna MJ,Medved I,Murphy KT,Brown MJ,Della Gatta P,
Cameron-Smith D.Infusion with the antioxidant N-acetylcysteine attenuates
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 9 of 10
CR,Myers V,Nauta M,Rodarte RQ,et al.Effects of aerobic and resistance
training on hemoglobin A1c levels in patients with type 2 diabetes:a
randomized controlled trial.JAMA.2010;304:2253–62.
13. Sparks LM,Johannsen NM,Church TS,Earnest CP,Moonen-Kornips E,Moro
C,Hesselink MK,Smith SR,Schrauwen P.Nine months of combined training
improves ex vivo skeletalmuscle metabolism in individuals with type 2
diabetes.J Clin EndocrinolMetab.2013;98:1694–702.
14. DeFronzo RA,Tripathy D.Skeletalmuscle insulin resistance is the primary
defect in type 2 diabetes.Diabetes Care.2009;32 Suppl2:S157–63.
15. DeFronzo RA,Jacot E,Jequier E,Maeder E,Wahren J,Felber JP.The effect of
insulin on the disposal of intravenous glucose.Results from indirect calorimetry
and hepatic and femoral venous catheterization.Diabetes.1981;30:1000–7.
16. Kelley DE,He J,Menshikova EV,Ritov VB.Dysfunction of mitochondria in
human skeletalmuscle in type 2 diabetes.Diabetes.2002;51:2944–50.
17. Romero-Arenas S,Martinez-PascualM,Alcaraz PE.Impact of resistance
circuit training on neuromuscular,cardiorespiratory and body composition
adaptations in the elderly.Aging Dis.2013;4:256–63.
18. Goodman CA.The role of mTORC1 in regulating protein synthesis and
skeletalmuscle mass in response to various mechanicalstimuli.Rev Physiol
Biochem Pharmacol.2014;166:43–95.
19. Holten MK,Zacho M,Gaster M,JuelC,WojtaszewskiJF,Dela F.Strength
training increases insulin-mediated glucose uptake,GLUT4 content,and
insulin signaling in skeletalmuscle in patients with type 2 diabetes.
Diabetes.2004;53:294–305.
20. McKinsey TA,Zhang CL,Lu J,Olson EN:Signal-dependent nuclear export of a
histone deacetylase regulates muscle differentiation.Nature.2000;408:106–111.
21. HortobagyiT,Dempsey L,Fraser D,Zheng D,Hamilton G,Lambert J,Dohm
L.Changes in muscle strength,muscle fibre size and myofibrillar gene
expression after immobilization and retraining in humans.J Physiol.2000;
524(Pt 1):293–304.
22. Harmer AR,Elkins MR.Amount and frequency of exercise affect glycaemic
control more than exercise mode or intensity.Br J Sports Med.2015;49:1012–4.
23. Yki-Jarvinen H,Koivisto VA.Effects of body composition on insulin
sensitivity.Diabetes.1983;32:965–9.
24. IshiiT,Yamakita T,Sato T,Tanaka S,FujiiS.Resistance training improves
insulin sensitivity in NIDDM subjects without altering maximaloxygen
uptake.Diabetes Care.1998;21:1353–5.
25. Castorena CM,Arias EB,Sharma N,Bogan JS,Cartee GD.Fiber type effects
on contraction-stimulated glucose uptake and GLUT4 abundance in single
fibers from rat skeletalmuscle.Am J PhysiolEndocrinolMetab.2015;308:
E223–30.
26. MackrellJG,Cartee GD.A novelmethod to measure glucose uptake and
myosin heavy chain isoform expression of single fibers from rat skeletal
muscle.Diabetes.2012;61:995–1003.
27. Holloszy JO,Hansen PA.Regulation of glucose transport into skeletal
muscle.Rev PhysiolBiochem Pharmacol.1996;128:99–193.
28. Vollestad NK,Blom PC,Gronnerod O.Resynthesis of glycogen in different
muscle fibre types after prolonged exhaustive exercise in man.Acta Physiol
Scand.1989;137:15–21.
29. Schofield KL,Rehrer NJ,Perry TL,Ross A,Andersen JL,Osborne H.Insulin
and fiber type in the offspring of T2DM subjects with resistance training
and detraining.Med SciSports Exerc.2012;44:2331–9.
30. Geisler S,Brinkmann C,Schiffer T,Kreutz T,Bloch W,Brixius K.The influence
of resistance training on patients with metabolic syndrome–significance of
changes in muscle fiber size and muscle fiber distribution.J Strength Cond
Res.2011;25:2598–604.
31. Adams GR,Hather BM,Baldwin KM,Dudley GA.Skeletalmuscle myosin
heavy chain composition and resistance training.J ApplPhysiol(1985).
1993;74:911–5.
32. LowellBB,Shulman GI.Mitochondrialdysfunction and type 2 diabetes.
Science.2005;307:384–7.
33. Sparks LM,Xie H,Koza RA,Mynatt R,Hulver MW,Bray GA,Smith SR.A high-
fatdiet coordinately downregulates genes required for mitochondrial
oxidative phosphorylation in skeletalmuscle.Diabetes.2005;54:1926–33.
34. BoushelR,Gnaiger E,Schjerling P,Skovbro M,Kraunsoe R,Dela F.Patients
with type 2 diabetes have normalmitochondrialfunction in skeletalmuscle.
Diabetologia.2007;50:790–6.
35. Hesselink MK,Schrauwen-Hinderling V,Schrauwen P.Skeletalmuscle
mitochondria as a target to prevent or treat type 2 diabetes mellitus.
Nat Rev Endocrinol.2016;12:633–45.
36. Akimoto T,Pohnert SC,Li P,Zhang M,Gumbs C,Rosenberg PB,Williams RS,
Yan Z.Exercise stimulates Pgc-1alpha transcription in skeletal muscle through
activation of the p38 MAPK pathway.J Biol Chem.2005;280:19587–93.
37. Frank P,Andersson E,Ponten M,Ekblom B,Ekblom M,Sahlin K.Strength
training improves muscle aerobic capacity and glucose tolerance in elderly.
Scand J Med SciSports.2016;26:764–73.
38. Irving BA,Lanza IR,Henderson GC,Rao RR,Spiegelman BM,Nair KS.
Combined training enhances skeletalmuscle mitochondrialoxidative
capacity independent of age.J Clin EndocrinolMetab.2015;100:1654–63.
39. Pesta D,HoppelF,Macek C,Messner H,Faulhaber M,KobelC,Parson W,
Burtscher M,Schocke M,Gnaiger E.Similar qualitative and quantitative
changes of mitochondrialrespiration following strength and endurance
training in normoxia and hypoxia in sedentary humans.Am J PhysiolRegul
Integr Comp Physiol.2011;301:R1078–87.
40. Tang JE,Hartman JW,Phillips SM.Increased muscle oxidative potential
following resistance training induced fibre hypertrophy in young men.Appl
PhysiolNutr Metab.2006;31:495–501.
41. Colberg SR,Albright AL,Blissmer BJ,Braun B,Chasan-Taber L,Fernhall B,
Regensteiner JG,Rubin RR,Sigal RJ,American College of Sports M,American
Diabetes A.Exercise and type 2 diabetes:American College of Sports Medicine
and the American Diabetes Association:joint position statement.Exercise and
type 2 diabetes.Med Sci Sports Exerc.2010;42:2282–303.
42. Chung N,Kreutz T,Schiffer T,Opitz D,Hermann R,Gehlert S,Bloch W,
Brixius K,Brinkmann C.Training-induced alterations of skeletalmuscle
mitochondrialbiogenesis proteins in non-insulin-dependent type 2 diabetic
men.Can J PhysiolPharmacol.2012;90:1634–41.
43. Hawley JA.Molecular responses to strength and endurance training:are
they incompatible? ApplPhysiolNutr Metab.2009;34:355–61.
44. Wang L,Mascher H,Psilander N,Blomstrand E,Sahlin K.Resistance exercise
enhances the molecular signaling of mitochondrialbiogenesis induced by
endurance exercise in human skeletalmuscle.J ApplPhysiol(1985).2011;
111:1335–44.
45. Lantier L,Fentz J,Mounier R,Leclerc J,Treebak JT,Pehmoller C,Sanz N,
Sakakibara I,Saint-Amand E,Rimbaud S,et al.AMPK controls exercise
endurance,mitochondrialoxidative capacity,and skeletalmuscle integrity.
FASEB J.2014;28:3211–24.
46. McGee SL,Hargreaves M.AMPK-mediated regulation of transcription in
skeletalmuscle.Clin Sci(Lond).2010;118:507–18.
47. Gross DN vHA,Birnbaum NJ.The role of FoxO in the regulation of
metabolism.Oncogene.2008;27:2320–36.
48. Tarnopolsky MA.MitochondrialDNA shifting in older adults following
resistance exercise training.ApplPhysiolNutr Metab.2009;34:348–54.
49. Parise G,Brose AN,Tarnopolsky MA.Resistance exercise training decreases
oxidative damage to DNA and increases cytochrome oxidase activity in
older adults.Exp Gerontol.2005;40:173–80.
50. McInnes J.Mitochondrial-associated metabolic disorders:foundations,
pathologies and recent progress.Nutr Metab (Lond).2013;10:63.
51. RodellA,Rasmussen LJ,Bergersen LH,Singh KK,Gjedde A.Naturalselection
of mitochondria during somatic lifetime promotes healthy aging.Front
Neuroenergetics.2013;5:7.
52. Lefort N,Glancy B,Bowen B,Willis WT,Bailowitz Z,De Filippis EA,Brophy C,
Meyer C,Hojlund K,Yi Z,Mandarino LJ.Increased reactive oxygen species
production and lower abundance of complex Isubunits and carnitine
palmitoyltransferase 1B protein despite normalmitochondrialrespiration in
insulin-resistant human skeletalmuscle.Diabetes.2010;59:2444–52.
53. Houstis N,Rosen ED,Lander ES.Reactive oxygen species have a causalrole
in multiple forms of insulin resistance.Nature.2006;440:944–8.
54. Goncalves RL,Bunik VI,Brand MD.Production of superoxide/hydrogen
peroxide by the mitochondrial2-oxoadipate dehydrogenase complex.Free
Radic BiolMed.2016;91:247–255.
55. Goncalves RL,Quinlan CL,Perevoshchikova IV,Hey-Mogensen M,Brand MD.
Sitesof superoxide and hydrogen peroxide production by muscle
mitochondria assessed ex vivo under conditions mimicking rest and
exercise.J BiolChem.2015;290:209–27.
56. McBride JM,Kraemer WJ,Triplett-McBride T,Sebastianelli W.Effect of resistance
exercise on free radical production.Med Sci Sports Exerc.1998;30:67–72.
57. Alessio HM,Hagerman AE,Fulkerson BK,Ambrose J,Rice RE,Wiley RL.
Generation of reactive oxygen species after exhaustive aerobic and
isometric exercise.Med SciSports Exerc.2000;32:1576–81.
58. Petersen AC,McKenna MJ,Medved I,Murphy KT,Brown MJ,Della Gatta P,
Cameron-Smith D.Infusion with the antioxidant N-acetylcysteine attenuates
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 9 of 10
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

early adaptive responses to exercise in human skeletalmuscle.Acta Physiol
(Oxf).2012;204:382–92.
59. Whitham M,Chan MH,PalM,Matthews VB,Prelovsek O,Lunke S,El-Osta A,
Broenneke H,Alber J,Bruning JC,et al.Contraction-induced interleukin-6
gene transcription in skeletalmuscle is regulated by c-Jun terminalkinase/
activator protein-1.J BiolChem.2012;287:10771–9.
60. Rietjens SJ,Beelen M,Koopman R,LJ VANL,Bast A,Haenen GR.A single
session of resistance exercise induces oxidative damage in untrained men.
Med SciSports Exerc.2007;39:2145–51.
61. Vincent HK,Bourguignon C,Vincent KR.Resistance training lowers exercise-
induced oxidative stress and homocysteine levels in overweight and obese
older adults.Obesity (Silver Spring).2006;14:1921–30.
62. VinettiG,MozziniC,DesenzaniP,BoniE,Bulla L,LorenzettiI,Romano C,
PasiniA,CominaciniL,AssanelliD.Supervised exercise training reduces
oxidative stress and cardiometabolic risk in adults with type 2 diabetes:a
randomized controlled trial.SciRep.2015;5:9238.
63. Leeuwenburgh C,Hansen PA,Holloszy JO,Heinecke JW.Hydroxylradical
generation during exercise increases mitochondrialprotein oxidation and
levels of urinary dityrosine.Free Radic BiolMed.1999;27:186–92.
64. Hey-Mogensen M,Hojlund K,Vind BF,Wang L,Dela F,Beck-Nielsen H,
Fernstrom M,Sahlin K.Effect of physicaltraining on mitochondrial
respiration and reactive oxygen species release in skeletalmuscle in
patients with obesity and type 2 diabetes.Diabetologia.2010;53:1976–85.
65. Lee S,Tak E,Lee J,Rashid MA,Murphy MP,Ha J,Kim SS.Mitochondrial
H2O2 generated from electron transport chain complex Istimulates muscle
differentiation.CellRes.2011;21:817–34.
66. Sano M,Fukuda K.Activation of mitochondrialbiogenesis by hormesis.Circ
Res.2008;103:1191–3.
67. Brinkmann C,Chung N,Schmidt U,Kreutz T,Lenzen E,Schiffer T,Geisler S,
Graf C,Montiel-Garcia G,Renner R,et al.Training alters the skeletalmuscle
antioxidative capacity in non-insulin-dependent type 2 diabetic men.Scand
J Med SciSports.2012;22:462–70.
68. Merry TL,Ristow M.Do antioxidant supplements interfere with skeletal
muscle adaptation to exercise training? J Physiol.2016;594:5135–47.
69. Braun B,Eze P,Stephens BR,Hagobian TA,Sharoff CG,Chipkin SR,Goldstein
B.Impact of metformin on peak aerobic capacity.ApplPhysiolNutr Metab.
2008;33:61–7.
70. Malin SK,Nightingale J,ChoiSE,Chipkin SR,Braun B.Metformin modifies
the exercise training effects on risk factors for cardiovascular disease in
impaired glucose tolerant adults.Obesity (Silver Spring).2013;21:93–100.
71. Sharoff CG,Hagobian TA,Malin SK,Chipkin SR,Yu H,Hirshman MF,
Goodyear LJ,Braun B.Combining short-term metformin treatment and one
bout of exercise does not increase insulin action in insulin-resistant
individuals.Am J PhysiolEndocrinolMetab.2010;298:E815–23.
72. Malin SK,Gerber R,Chipkin SR,Braun B.Independent and combined effects
of exercise training and metformin on insulin sensitivity in individuals with
prediabetes.Diabetes Care.2012;35:131–6.
73. Malin SK,Braun B.Impact of metformin on exercise-induced metabolic
adaptations to lower type 2 diabetes risk.Exerc Sport SciRev.2016;44:4–11.
74. Hawley SA,Gadalla AE,Olsen GS,Hardie DG.The antidiabetic drug
metformin activates the AMP-activated protein kinase cascade via an
adenine nucleotide-independent mechanism.Diabetes.2002;51:2420–5.
75. Richter EA,Ruderman NB.AMPK and the biochemistry of exercise:
implications for human health and disease.Biochem J.2009;418:261–75.
76. Boule NG,Kenny GP,Larose J,Khandwala F,Kuzik N,SigalRJ.Does
metformin modify the effect on glycaemic controlof aerobic exercise,
resistance exercise or both? Diabetologia.2013;56:2378–82.
77. Malin SK,Stephens BR,Sharoff CG,Hagobian TA,Chipkin SR,Braun B.
Metformin’s effect on exercise and postexercise substrate oxidation.Int J
Sport Nutr Exerc Metab.2010;20:63–71.
78. Madiraju AK,Erion DM,RahimiY,Zhang XM,Braddock DT,Albright RA,
Prigaro BJ,Wood JL,Bhanot S,MacDonald MJ,et al.Metformin suppresses
gluconeogenesis by inhibiting mitochondrialglycerophosphate
dehydrogenase.Nature.2014;510:542–6.
79. Bridges HR,Jones AJ,Pollak MN,Hirst J.Effects of metformin and other
biguanides on oxidative phosphorylation in mitochondria.Biochem J.2014;
462:475–87.
80. Pereira SS,Alvarez-Leite JI.Low-grade inflammation,obesity,and diabetes.
CurrObes Rep.2014;3:422–31.
81. Kwon H,Pessin JE.Adipokines mediate inflammation and insulin resistance.
Front Endocrinol(Lausanne).2013;4:71.
82. El-Kader SMA.Aerobic versus resistance exercise training in modulation of
insulin resistance,adipocytokines and inflammatory cytokine levels in obese
type 2 diabetic patients.Journalof Advanced Research.2011;2:179–83.
83. Shultz SP,Dahiya R,Leong GM,Rowlands DS,Hills AP,Byrne NM.Muscular
strength,aerobic capacity,and adipocytokines in obese youth after
resistance training:A pilot study.Australas Med J.2015;8:113–20.
84. Jorge ML,de Oliveira VN,Resende NM,Paraiso LF,Calixto A,Diniz AL,
Resende ES,Ropelle ER,Carvalheira JB,Espindola FS,et al.The effects of
aerobic,resistance,and combined exercise on metabolic control,
inflammatory markers,adipocytokines,and muscle insulin signaling in
patients with type 2 diabetes mellitus.Metabolism.2011;60:1244–52.
85. Gillette RCB CA,Melby CL.Postexercise energy expenditure in response to
acute aerobic or resistive exercise.Int J Sport Nutr Exerc Metab.1994;4:347–60.
86. Elliot DL,Goldberg L,KuehlKS.Effect of resistance training on excess post-
exercise oxygen consumption.J ApplSport SciRes.1992;6:77–81.
87. Ormsbee MJ,Thyfault JP,Johnson EA,Kraus RM,ChoiMD,Hickner RC.Fat
metabolism and acute resistance exercise in trained men.J ApplPhysiol
(1985).2007;102:1767–72.
88. Castaneda C,Layne JE,Munoz-Orians L,Gordon PL,Walsmith J,FoldvariM,
Roubenoff R,Tucker KL,Nelson ME.A randomized controlled trialof
resistance exercise training to improve glycemic controlin older adults with
type 2 diabetes.Diabetes Care.2002;25:2335–41.
89. Cauza E,Hanusch-Enserer U,Strasser B,Ludvik B,Metz-SchimmerlS,Pacini
G,Wagner O,Georg P,Prager R,Kostner K,et al.The relative benefits of
endurance and strength training on the metabolic factors and muscle
function of people with type 2 diabetes mellitus.Arch Phys Med Rehabil.
2005;86:1527–33.
90. Kwon HR,Han KA,Ku YH,Ahn HJ,Koo BK,Kim HC,Min KW.The effects of
resistance training on muscle and body fat mass and muscle strength in
type 2 diabetic women.Korean Diabetes J.2010;34:101–10.
91. Bateman LA,Slentz CA,Willis LH,Shields AT,Piner LW,Bales CW,Houmard
JA,Kraus WE.Comparison of aerobic versus resistance exercise training
effects on metabolic syndrome (from the Studies of a Targeted Risk
Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT).Am J
Cardiol.2011;108:838–44.
92. Egan B,Zierath JR.Exercise metabolism and the molecular regulation of
skeletalmuscle adaptation.CellMetab.2013;17:162–84.
• We accept pre-submission inquiries
• Our selector tool helps you to find the most relevant journal
• We provide round the clock customer support
• Convenient online submission
• Thorough peer review
• Inclusion in PubMed and all major indexing services
• Maximum visibility for your research
Submit your manuscript at
www.biomedcentral.com/submit
Submit your next manuscript to BioMed Central
and we will help you at every step:
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 10 of 10
(Oxf).2012;204:382–92.
59. Whitham M,Chan MH,PalM,Matthews VB,Prelovsek O,Lunke S,El-Osta A,
Broenneke H,Alber J,Bruning JC,et al.Contraction-induced interleukin-6
gene transcription in skeletalmuscle is regulated by c-Jun terminalkinase/
activator protein-1.J BiolChem.2012;287:10771–9.
60. Rietjens SJ,Beelen M,Koopman R,LJ VANL,Bast A,Haenen GR.A single
session of resistance exercise induces oxidative damage in untrained men.
Med SciSports Exerc.2007;39:2145–51.
61. Vincent HK,Bourguignon C,Vincent KR.Resistance training lowers exercise-
induced oxidative stress and homocysteine levels in overweight and obese
older adults.Obesity (Silver Spring).2006;14:1921–30.
62. VinettiG,MozziniC,DesenzaniP,BoniE,Bulla L,LorenzettiI,Romano C,
PasiniA,CominaciniL,AssanelliD.Supervised exercise training reduces
oxidative stress and cardiometabolic risk in adults with type 2 diabetes:a
randomized controlled trial.SciRep.2015;5:9238.
63. Leeuwenburgh C,Hansen PA,Holloszy JO,Heinecke JW.Hydroxylradical
generation during exercise increases mitochondrialprotein oxidation and
levels of urinary dityrosine.Free Radic BiolMed.1999;27:186–92.
64. Hey-Mogensen M,Hojlund K,Vind BF,Wang L,Dela F,Beck-Nielsen H,
Fernstrom M,Sahlin K.Effect of physicaltraining on mitochondrial
respiration and reactive oxygen species release in skeletalmuscle in
patients with obesity and type 2 diabetes.Diabetologia.2010;53:1976–85.
65. Lee S,Tak E,Lee J,Rashid MA,Murphy MP,Ha J,Kim SS.Mitochondrial
H2O2 generated from electron transport chain complex Istimulates muscle
differentiation.CellRes.2011;21:817–34.
66. Sano M,Fukuda K.Activation of mitochondrialbiogenesis by hormesis.Circ
Res.2008;103:1191–3.
67. Brinkmann C,Chung N,Schmidt U,Kreutz T,Lenzen E,Schiffer T,Geisler S,
Graf C,Montiel-Garcia G,Renner R,et al.Training alters the skeletalmuscle
antioxidative capacity in non-insulin-dependent type 2 diabetic men.Scand
J Med SciSports.2012;22:462–70.
68. Merry TL,Ristow M.Do antioxidant supplements interfere with skeletal
muscle adaptation to exercise training? J Physiol.2016;594:5135–47.
69. Braun B,Eze P,Stephens BR,Hagobian TA,Sharoff CG,Chipkin SR,Goldstein
B.Impact of metformin on peak aerobic capacity.ApplPhysiolNutr Metab.
2008;33:61–7.
70. Malin SK,Nightingale J,ChoiSE,Chipkin SR,Braun B.Metformin modifies
the exercise training effects on risk factors for cardiovascular disease in
impaired glucose tolerant adults.Obesity (Silver Spring).2013;21:93–100.
71. Sharoff CG,Hagobian TA,Malin SK,Chipkin SR,Yu H,Hirshman MF,
Goodyear LJ,Braun B.Combining short-term metformin treatment and one
bout of exercise does not increase insulin action in insulin-resistant
individuals.Am J PhysiolEndocrinolMetab.2010;298:E815–23.
72. Malin SK,Gerber R,Chipkin SR,Braun B.Independent and combined effects
of exercise training and metformin on insulin sensitivity in individuals with
prediabetes.Diabetes Care.2012;35:131–6.
73. Malin SK,Braun B.Impact of metformin on exercise-induced metabolic
adaptations to lower type 2 diabetes risk.Exerc Sport SciRev.2016;44:4–11.
74. Hawley SA,Gadalla AE,Olsen GS,Hardie DG.The antidiabetic drug
metformin activates the AMP-activated protein kinase cascade via an
adenine nucleotide-independent mechanism.Diabetes.2002;51:2420–5.
75. Richter EA,Ruderman NB.AMPK and the biochemistry of exercise:
implications for human health and disease.Biochem J.2009;418:261–75.
76. Boule NG,Kenny GP,Larose J,Khandwala F,Kuzik N,SigalRJ.Does
metformin modify the effect on glycaemic controlof aerobic exercise,
resistance exercise or both? Diabetologia.2013;56:2378–82.
77. Malin SK,Stephens BR,Sharoff CG,Hagobian TA,Chipkin SR,Braun B.
Metformin’s effect on exercise and postexercise substrate oxidation.Int J
Sport Nutr Exerc Metab.2010;20:63–71.
78. Madiraju AK,Erion DM,RahimiY,Zhang XM,Braddock DT,Albright RA,
Prigaro BJ,Wood JL,Bhanot S,MacDonald MJ,et al.Metformin suppresses
gluconeogenesis by inhibiting mitochondrialglycerophosphate
dehydrogenase.Nature.2014;510:542–6.
79. Bridges HR,Jones AJ,Pollak MN,Hirst J.Effects of metformin and other
biguanides on oxidative phosphorylation in mitochondria.Biochem J.2014;
462:475–87.
80. Pereira SS,Alvarez-Leite JI.Low-grade inflammation,obesity,and diabetes.
CurrObes Rep.2014;3:422–31.
81. Kwon H,Pessin JE.Adipokines mediate inflammation and insulin resistance.
Front Endocrinol(Lausanne).2013;4:71.
82. El-Kader SMA.Aerobic versus resistance exercise training in modulation of
insulin resistance,adipocytokines and inflammatory cytokine levels in obese
type 2 diabetic patients.Journalof Advanced Research.2011;2:179–83.
83. Shultz SP,Dahiya R,Leong GM,Rowlands DS,Hills AP,Byrne NM.Muscular
strength,aerobic capacity,and adipocytokines in obese youth after
resistance training:A pilot study.Australas Med J.2015;8:113–20.
84. Jorge ML,de Oliveira VN,Resende NM,Paraiso LF,Calixto A,Diniz AL,
Resende ES,Ropelle ER,Carvalheira JB,Espindola FS,et al.The effects of
aerobic,resistance,and combined exercise on metabolic control,
inflammatory markers,adipocytokines,and muscle insulin signaling in
patients with type 2 diabetes mellitus.Metabolism.2011;60:1244–52.
85. Gillette RCB CA,Melby CL.Postexercise energy expenditure in response to
acute aerobic or resistive exercise.Int J Sport Nutr Exerc Metab.1994;4:347–60.
86. Elliot DL,Goldberg L,KuehlKS.Effect of resistance training on excess post-
exercise oxygen consumption.J ApplSport SciRes.1992;6:77–81.
87. Ormsbee MJ,Thyfault JP,Johnson EA,Kraus RM,ChoiMD,Hickner RC.Fat
metabolism and acute resistance exercise in trained men.J ApplPhysiol
(1985).2007;102:1767–72.
88. Castaneda C,Layne JE,Munoz-Orians L,Gordon PL,Walsmith J,FoldvariM,
Roubenoff R,Tucker KL,Nelson ME.A randomized controlled trialof
resistance exercise training to improve glycemic controlin older adults with
type 2 diabetes.Diabetes Care.2002;25:2335–41.
89. Cauza E,Hanusch-Enserer U,Strasser B,Ludvik B,Metz-SchimmerlS,Pacini
G,Wagner O,Georg P,Prager R,Kostner K,et al.The relative benefits of
endurance and strength training on the metabolic factors and muscle
function of people with type 2 diabetes mellitus.Arch Phys Med Rehabil.
2005;86:1527–33.
90. Kwon HR,Han KA,Ku YH,Ahn HJ,Koo BK,Kim HC,Min KW.The effects of
resistance training on muscle and body fat mass and muscle strength in
type 2 diabetic women.Korean Diabetes J.2010;34:101–10.
91. Bateman LA,Slentz CA,Willis LH,Shields AT,Piner LW,Bales CW,Houmard
JA,Kraus WE.Comparison of aerobic versus resistance exercise training
effects on metabolic syndrome (from the Studies of a Targeted Risk
Reduction Intervention Through Defined Exercise - STRRIDE-AT/RT).Am J
Cardiol.2011;108:838–44.
92. Egan B,Zierath JR.Exercise metabolism and the molecular regulation of
skeletalmuscle adaptation.CellMetab.2013;17:162–84.
• We accept pre-submission inquiries
• Our selector tool helps you to find the most relevant journal
• We provide round the clock customer support
• Convenient online submission
• Thorough peer review
• Inclusion in PubMed and all major indexing services
• Maximum visibility for your research
Submit your manuscript at
www.biomedcentral.com/submit
Submit your next manuscript to BioMed Central
and we will help you at every step:
Pesta et al.Nutrition & Metabolism (2017) 14:24 Page 10 of 10
1 out of 10
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.





