A Comprehensive Analysis of the Pharmaceutical Benefit Scheme (PBS)
VerifiedAdded on 2023/06/07
|8
|2015
|287
Report
AI Summary
This report provides a detailed analysis of the Pharmaceutical Benefit Scheme (PBS) in Australia, a government drug subsidy program established in 1948. It examines the scheme's original aim of providing affordable medication to Australians and explores the challenges and opportunities it faces, particularly the incorporation of newer medications into the PBS list. The report discusses the financial implications of the PBS on the Australian economy, including rising costs and the impact of expensive pharmaceuticals. It also addresses issues such as drug overuse and potential solutions like biosimilars and price disclosure. The report concludes with recommendations for improving the PBS, such as implementing quicker drug incorporation strategies and monitoring drug availability to enhance the scheme's reliability and benefit to the Australian population.

Pharmaceutical Benefit Scheme
Student Name
Student ID
1
Student Name
Student ID
1
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

Contents
Executive Summary....................................................................................................3
Introduction................................................................................................................. 4
General discussion of the issue..................................................................................4
Issues explored..........................................................................................................6
Conclusions................................................................................................................ 7
References.................................................................................................................8
2
Executive Summary....................................................................................................3
Introduction................................................................................................................. 4
General discussion of the issue..................................................................................4
Issues explored..........................................................................................................6
Conclusions................................................................................................................ 7
References.................................................................................................................8
2

Executive Summary
The Pharmaceutical Benefit Scheme or PBS is the Australian government’s drug
subsidy program, which was established in 1948 under the act of Pharmaceutical
Benefits Act of 1947. The primary aim of the government while implementing this
scheme was proving the common Australians with affordable and appropriate
medication in less or subsidised price so that quality healthcare could be provided to
each countrymen. However, after application it faced several opportunities and
challenges which affected the application of this scheme in recent times.
Incorporation of newer medications in the PBS lists was one of the most crucial
challenges that affected the reliability and application of this scheme. This
assignment provided a detailed analysis of all such issues related to the PBS and
provided a specific analysis of its financial implication for the Australian economy.
Finally, it provided a set of recommendations using which the government can
increase its reliability and affordability to increase its application among the
countrymen.
3
The Pharmaceutical Benefit Scheme or PBS is the Australian government’s drug
subsidy program, which was established in 1948 under the act of Pharmaceutical
Benefits Act of 1947. The primary aim of the government while implementing this
scheme was proving the common Australians with affordable and appropriate
medication in less or subsidised price so that quality healthcare could be provided to
each countrymen. However, after application it faced several opportunities and
challenges which affected the application of this scheme in recent times.
Incorporation of newer medications in the PBS lists was one of the most crucial
challenges that affected the reliability and application of this scheme. This
assignment provided a detailed analysis of all such issues related to the PBS and
provided a specific analysis of its financial implication for the Australian economy.
Finally, it provided a set of recommendations using which the government can
increase its reliability and affordability to increase its application among the
countrymen.
3
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

Introduction
Pharmaceutical benefit scheme (PBS) can be described as a program formulated by
the Australian Government that provides prescription drugs at subsidized rates to the
Australian citizens. This scheme was established in the year 1948 under the
Pharmaceutical Benefits Act of 1947. The main reason for the establishment of PBS
is to ensure that the Australian can have proper access to the reliable and affordable
and necessary drugs. Currently the cost of the of the drugs have increased and due
to this PBS is undergoing scrutiny (Cheng et al., 2012). The PBS scheme entirely
focusses at the community level instead of the hospital setting which a responsibility
of the local and the state government. Thus, it can be inferred that the PBS scheme
along with the Medicare is one of the basic component of the Australian Healthcare.
The medications that are listed only within the pharmaceutical benefit schedule
receives the Medicare. The PBS scheme faces both the opportunities and
challenges. One of the prime challenges that are faced by the PBS scheme is the
enlisting of the new pharmaceuticals. It has been the rule that the 10 million per year
is to be approved by the cabinet. However, due to budget pressures, the listing of
several pharmaceuticals like severe asthma, chronic pain, schizophrenia is deferred
(Pbs.gov.au, 2018). This study is based on the critical analysis of the Pharmaceutical
benefit scheme and consumption of medicines in Australia.
General discussion of the issue
Pharmaceutical Benefit Scheme (PBS) was implemented in the healthcare facility so
that in case of emergency or in the need of medication, without any extra
prescription or paper, medications could be provided to the patient in need. ThisPBS
chart is inclusive of PBS and non-PBS medications and is based on the best evidence
available (Clarke, 2012). Despite the fact that these PBS medication chart, which is
divided in several section depending on the demand and availability of the drug,
these charts has not been changed much in the recent times. The following chart
provides a detailed idea regarding this process (Mellish et al., 2015).
4
Pharmaceutical benefit scheme (PBS) can be described as a program formulated by
the Australian Government that provides prescription drugs at subsidized rates to the
Australian citizens. This scheme was established in the year 1948 under the
Pharmaceutical Benefits Act of 1947. The main reason for the establishment of PBS
is to ensure that the Australian can have proper access to the reliable and affordable
and necessary drugs. Currently the cost of the of the drugs have increased and due
to this PBS is undergoing scrutiny (Cheng et al., 2012). The PBS scheme entirely
focusses at the community level instead of the hospital setting which a responsibility
of the local and the state government. Thus, it can be inferred that the PBS scheme
along with the Medicare is one of the basic component of the Australian Healthcare.
The medications that are listed only within the pharmaceutical benefit schedule
receives the Medicare. The PBS scheme faces both the opportunities and
challenges. One of the prime challenges that are faced by the PBS scheme is the
enlisting of the new pharmaceuticals. It has been the rule that the 10 million per year
is to be approved by the cabinet. However, due to budget pressures, the listing of
several pharmaceuticals like severe asthma, chronic pain, schizophrenia is deferred
(Pbs.gov.au, 2018). This study is based on the critical analysis of the Pharmaceutical
benefit scheme and consumption of medicines in Australia.
General discussion of the issue
Pharmaceutical Benefit Scheme (PBS) was implemented in the healthcare facility so
that in case of emergency or in the need of medication, without any extra
prescription or paper, medications could be provided to the patient in need. ThisPBS
chart is inclusive of PBS and non-PBS medications and is based on the best evidence
available (Clarke, 2012). Despite the fact that these PBS medication chart, which is
divided in several section depending on the demand and availability of the drug,
these charts has not been changed much in the recent times. The following chart
provides a detailed idea regarding this process (Mellish et al., 2015).
4
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser
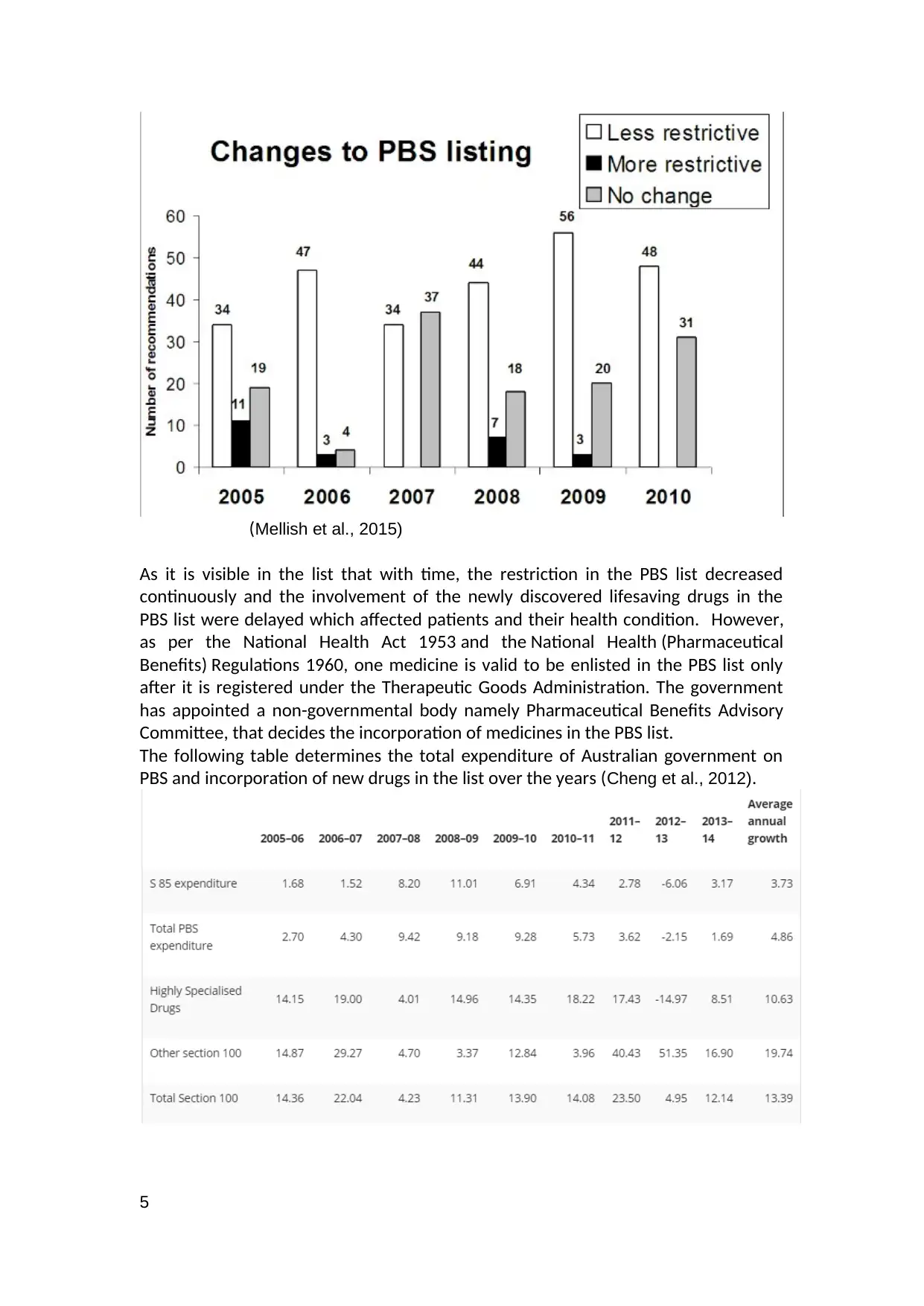
(Mellish et al., 2015)
As it is visible in the list that with time, the restriction in the PBS list decreased
continuously and the involvement of the newly discovered lifesaving drugs in the
PBS list were delayed which affected patients and their health condition. However,
as per the National Health Act 1953 and the National Health (Pharmaceutical
Benefits) Regulations 1960, one medicine is valid to be enlisted in the PBS list only
after it is registered under the Therapeutic Goods Administration. The government
has appointed a non-governmental body namely Pharmaceutical Benefits Advisory
Committee, that decides the incorporation of medicines in the PBS list.
The following table determines the total expenditure of Australian government on
PBS and incorporation of new drugs in the list over the years (Cheng et al., 2012).
5
As it is visible in the list that with time, the restriction in the PBS list decreased
continuously and the involvement of the newly discovered lifesaving drugs in the
PBS list were delayed which affected patients and their health condition. However,
as per the National Health Act 1953 and the National Health (Pharmaceutical
Benefits) Regulations 1960, one medicine is valid to be enlisted in the PBS list only
after it is registered under the Therapeutic Goods Administration. The government
has appointed a non-governmental body namely Pharmaceutical Benefits Advisory
Committee, that decides the incorporation of medicines in the PBS list.
The following table determines the total expenditure of Australian government on
PBS and incorporation of new drugs in the list over the years (Cheng et al., 2012).
5
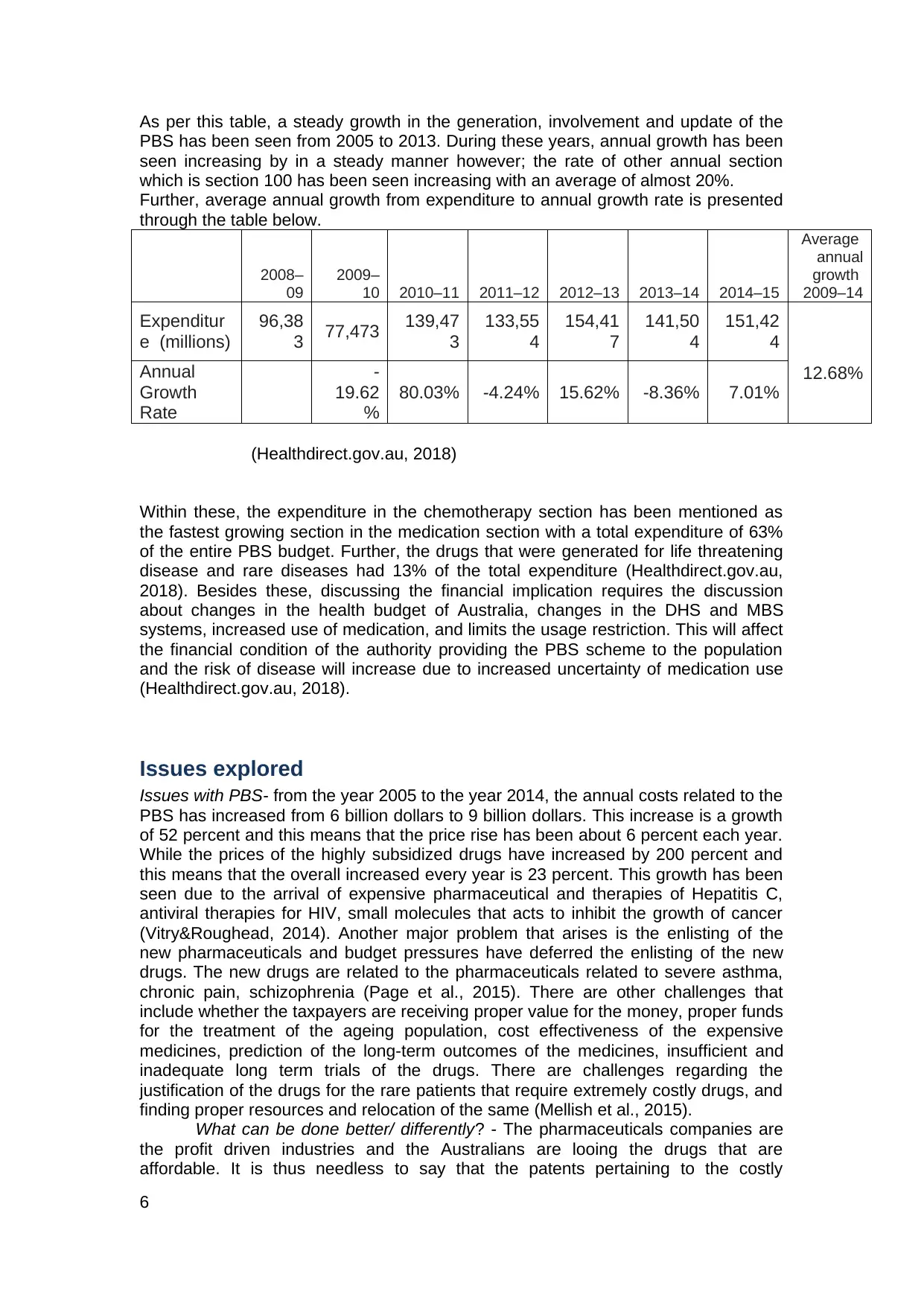
As per this table, a steady growth in the generation, involvement and update of the
PBS has been seen from 2005 to 2013. During these years, annual growth has been
seen increasing by in a steady manner however; the rate of other annual section
which is section 100 has been seen increasing with an average of almost 20%.
Further, average annual growth from expenditure to annual growth rate is presented
through the table below.
2008–
09
2009–
10 2010–11 2011–12 2012–13 2013–14 2014–15
Average
annual
growth
2009–14
Expenditur
e (millions)
96,38
3 77,473 139,47
3
133,55
4
154,41
7
141,50
4
151,42
4
12.68%Annual
Growth
Rate
-
19.62
%
80.03% -4.24% 15.62% -8.36% 7.01%
Within these, the expenditure in the chemotherapy section has been mentioned as
the fastest growing section in the medication section with a total expenditure of 63%
of the entire PBS budget. Further, the drugs that were generated for life threatening
disease and rare diseases had 13% of the total expenditure (Healthdirect.gov.au,
2018). Besides these, discussing the financial implication requires the discussion
about changes in the health budget of Australia, changes in the DHS and MBS
systems, increased use of medication, and limits the usage restriction. This will affect
the financial condition of the authority providing the PBS scheme to the population
and the risk of disease will increase due to increased uncertainty of medication use
(Healthdirect.gov.au, 2018).
Issues explored
Issues with PBS- from the year 2005 to the year 2014, the annual costs related to the
PBS has increased from 6 billion dollars to 9 billion dollars. This increase is a growth
of 52 percent and this means that the price rise has been about 6 percent each year.
While the prices of the highly subsidized drugs have increased by 200 percent and
this means that the overall increased every year is 23 percent. This growth has been
seen due to the arrival of expensive pharmaceutical and therapies of Hepatitis C,
antiviral therapies for HIV, small molecules that acts to inhibit the growth of cancer
(Vitry&Roughead, 2014). Another major problem that arises is the enlisting of the
new pharmaceuticals and budget pressures have deferred the enlisting of the new
drugs. The new drugs are related to the pharmaceuticals related to severe asthma,
chronic pain, schizophrenia (Page et al., 2015). There are other challenges that
include whether the taxpayers are receiving proper value for the money, proper funds
for the treatment of the ageing population, cost effectiveness of the expensive
medicines, prediction of the long-term outcomes of the medicines, insufficient and
inadequate long term trials of the drugs. There are challenges regarding the
justification of the drugs for the rare patients that require extremely costly drugs, and
finding proper resources and relocation of the same (Mellish et al., 2015).
What can be done better/ differently? - The pharmaceuticals companies are
the profit driven industries and the Australians are looing the drugs that are
affordable. It is thus needless to say that the patents pertaining to the costly
6
(Healthdirect.gov.au, 2018)
PBS has been seen from 2005 to 2013. During these years, annual growth has been
seen increasing by in a steady manner however; the rate of other annual section
which is section 100 has been seen increasing with an average of almost 20%.
Further, average annual growth from expenditure to annual growth rate is presented
through the table below.
2008–
09
2009–
10 2010–11 2011–12 2012–13 2013–14 2014–15
Average
annual
growth
2009–14
Expenditur
e (millions)
96,38
3 77,473 139,47
3
133,55
4
154,41
7
141,50
4
151,42
4
12.68%Annual
Growth
Rate
-
19.62
%
80.03% -4.24% 15.62% -8.36% 7.01%
Within these, the expenditure in the chemotherapy section has been mentioned as
the fastest growing section in the medication section with a total expenditure of 63%
of the entire PBS budget. Further, the drugs that were generated for life threatening
disease and rare diseases had 13% of the total expenditure (Healthdirect.gov.au,
2018). Besides these, discussing the financial implication requires the discussion
about changes in the health budget of Australia, changes in the DHS and MBS
systems, increased use of medication, and limits the usage restriction. This will affect
the financial condition of the authority providing the PBS scheme to the population
and the risk of disease will increase due to increased uncertainty of medication use
(Healthdirect.gov.au, 2018).
Issues explored
Issues with PBS- from the year 2005 to the year 2014, the annual costs related to the
PBS has increased from 6 billion dollars to 9 billion dollars. This increase is a growth
of 52 percent and this means that the price rise has been about 6 percent each year.
While the prices of the highly subsidized drugs have increased by 200 percent and
this means that the overall increased every year is 23 percent. This growth has been
seen due to the arrival of expensive pharmaceutical and therapies of Hepatitis C,
antiviral therapies for HIV, small molecules that acts to inhibit the growth of cancer
(Vitry&Roughead, 2014). Another major problem that arises is the enlisting of the
new pharmaceuticals and budget pressures have deferred the enlisting of the new
drugs. The new drugs are related to the pharmaceuticals related to severe asthma,
chronic pain, schizophrenia (Page et al., 2015). There are other challenges that
include whether the taxpayers are receiving proper value for the money, proper funds
for the treatment of the ageing population, cost effectiveness of the expensive
medicines, prediction of the long-term outcomes of the medicines, insufficient and
inadequate long term trials of the drugs. There are challenges regarding the
justification of the drugs for the rare patients that require extremely costly drugs, and
finding proper resources and relocation of the same (Mellish et al., 2015).
What can be done better/ differently? - The pharmaceuticals companies are
the profit driven industries and the Australians are looing the drugs that are
affordable. It is thus needless to say that the patents pertaining to the costly
6
(Healthdirect.gov.au, 2018)
⊘ This is a preview!⊘
Do you want full access?
Subscribe today to unlock all pages.

Trusted by 1+ million students worldwide

expensive therapies will expire in the near future. There is a probable solution and it
includes the devising of biosimilar that behave like mimic molecules. The biosimilar of
the therapies and the drugs can be used potentially to save the cost. However, it is
important to note that such biosimilar are not identical and require the procedures of
evaluation before marketing (Denaro& Martin, 2016). Another major factor of price
rise is that majority of the drugs prescribed belong to the generic category while only
a small portion of it belongs to the therapeutic classes. Thus, price disclosure can be
one method of price reductions of the drugs that are yet to receive the patent. This
will significantly bring down the price (Clarke, 2012).
Reasons for drug overuse- The most common type of drug that are overused
or abused are the sedatives and analgesics (racgp.org.au, 2018). These type of
drugs are abused due to their increased availability. The other reasons for the
misuse of drug are losing weight, being dependent on the drug, having issues with
the withdrawal symptoms, to experience the same effects, providing false information
regarding the quantity of the drug usage, neglecting activities like study and work
(Healthdirect.gov.au, 2018).
Conclusions
As the Australian government implemented the pharmaceutical benefit scheme for
the betterment and quality healthcare of countrymen, its decreasing reliability and
benefit should be revived by the government. There are several sections, which
require proper modification in PBS so that its reliability could be increased. The first
recommendation will be in the section of incorporation of drugs in the PBS list. The
general process requires ample time due to which patients who are in serious need
for the medicine are unable to avail the subsidy and had to spend huge amount of
money for their quality treatment. Therefore, implementation of quick incorporation
strategy should be present. Secondly, the availability of drug should be monitored
properly so that unavailability of PBS enlisted drug does no0t become an issue for
the people of Australia. Therefore, these are the recommendations that should be
implemented by the PBS authority to increase the reliability of the scheme.
7
includes the devising of biosimilar that behave like mimic molecules. The biosimilar of
the therapies and the drugs can be used potentially to save the cost. However, it is
important to note that such biosimilar are not identical and require the procedures of
evaluation before marketing (Denaro& Martin, 2016). Another major factor of price
rise is that majority of the drugs prescribed belong to the generic category while only
a small portion of it belongs to the therapeutic classes. Thus, price disclosure can be
one method of price reductions of the drugs that are yet to receive the patent. This
will significantly bring down the price (Clarke, 2012).
Reasons for drug overuse- The most common type of drug that are overused
or abused are the sedatives and analgesics (racgp.org.au, 2018). These type of
drugs are abused due to their increased availability. The other reasons for the
misuse of drug are losing weight, being dependent on the drug, having issues with
the withdrawal symptoms, to experience the same effects, providing false information
regarding the quantity of the drug usage, neglecting activities like study and work
(Healthdirect.gov.au, 2018).
Conclusions
As the Australian government implemented the pharmaceutical benefit scheme for
the betterment and quality healthcare of countrymen, its decreasing reliability and
benefit should be revived by the government. There are several sections, which
require proper modification in PBS so that its reliability could be increased. The first
recommendation will be in the section of incorporation of drugs in the PBS list. The
general process requires ample time due to which patients who are in serious need
for the medicine are unable to avail the subsidy and had to spend huge amount of
money for their quality treatment. Therefore, implementation of quick incorporation
strategy should be present. Secondly, the availability of drug should be monitored
properly so that unavailability of PBS enlisted drug does no0t become an issue for
the people of Australia. Therefore, these are the recommendations that should be
implemented by the PBS authority to increase the reliability of the scheme.
7
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

References
Cheng, A. C., Turnidge, J., Collignon, P., Looke, D., Barton, M., & Gottlieb, T. (2012).
Control of fluoroquinolone resistance through successful regulation, Australia.
Emerging infectious diseases, 18(9), 1453.
Clarke, P. M. (2012). Challenges and opportunities for the Pharmaceutical Benefits
Scheme. The Medical Journal of Australia, 196(3), 153-154.
Denaro, C., & Martin, J. (2016). The challenge of costly drugs. Australian prescriber,
39(3), 72.
Healthdirect.gov.au. (2018). Drug abuse. Retrieved from
https://www.healthdirect.gov.au/drug-abuse
Mellish, L., Karanges, E. A., Litchfield, M. J., Schaffer, A. L., Blanch, B., Daniels, B.
J., ... & Pearson, S. A. (2015). The Australian Pharmaceutical Benefits
Scheme data collection: a practical guide for researchers. BMC research
notes, 8(1), 634.
Page, E., Kemp-Casey, A., Korda, R., & Banks, E. (2015). Using Australian
Pharmaceutical Benefits Scheme data for pharmacoepidemiological research:
challenges and approaches. Public Health Res Pract, 25(4), e2541546.
Pbs.gov.au. (2018). Pharmaceutical Benefits Scheme (PBS) | Home. Retrieved from
http://www.pbs.gov.au/pbs/home;jsessionid=gmutx1tbsunf169wz8a83tgv6
racgp.org.au. (2018). RACGP - Prescription drug abuse – A timely update. Retrieved
from https://www.racgp.org.au/afp/2016/december/prescription-drug-abuse-a-
timely-update/
Vitry, A., & Roughead, E. (2014). Managed entry agreements for pharmaceuticals in
Australia. Health Policy, 117(3), 345-352.
8
Cheng, A. C., Turnidge, J., Collignon, P., Looke, D., Barton, M., & Gottlieb, T. (2012).
Control of fluoroquinolone resistance through successful regulation, Australia.
Emerging infectious diseases, 18(9), 1453.
Clarke, P. M. (2012). Challenges and opportunities for the Pharmaceutical Benefits
Scheme. The Medical Journal of Australia, 196(3), 153-154.
Denaro, C., & Martin, J. (2016). The challenge of costly drugs. Australian prescriber,
39(3), 72.
Healthdirect.gov.au. (2018). Drug abuse. Retrieved from
https://www.healthdirect.gov.au/drug-abuse
Mellish, L., Karanges, E. A., Litchfield, M. J., Schaffer, A. L., Blanch, B., Daniels, B.
J., ... & Pearson, S. A. (2015). The Australian Pharmaceutical Benefits
Scheme data collection: a practical guide for researchers. BMC research
notes, 8(1), 634.
Page, E., Kemp-Casey, A., Korda, R., & Banks, E. (2015). Using Australian
Pharmaceutical Benefits Scheme data for pharmacoepidemiological research:
challenges and approaches. Public Health Res Pract, 25(4), e2541546.
Pbs.gov.au. (2018). Pharmaceutical Benefits Scheme (PBS) | Home. Retrieved from
http://www.pbs.gov.au/pbs/home;jsessionid=gmutx1tbsunf169wz8a83tgv6
racgp.org.au. (2018). RACGP - Prescription drug abuse – A timely update. Retrieved
from https://www.racgp.org.au/afp/2016/december/prescription-drug-abuse-a-
timely-update/
Vitry, A., & Roughead, E. (2014). Managed entry agreements for pharmaceuticals in
Australia. Health Policy, 117(3), 345-352.
8
1 out of 8
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
Copyright © 2020–2026 A2Z Services. All Rights Reserved. Developed and managed by ZUCOL.



