Australia's Pharmaceutical Benefits Scheme
VerifiedAdded on 2020/03/01
|11
|2551
|284
AI Summary
This assignment focuses on analyzing research papers that utilize data from Australia's Pharmaceutical Benefits Scheme (PBS) for pharmacoepidemiological research. The provided list of academic publications explores various aspects of PBS data analysis, including trends in medication use, the impact of policy changes, and cost-effectiveness evaluations. The assignment requires a deep understanding of pharmacoepidemiology and the PBS system.
Contribute Materials
Your contribution can guide someone’s learning journey. Share your
documents today.

Running head: PHARMACEUTICAL BENEFITS SCHEME
Pharmaceutical Benefits Scheme
Name of the Student:
Name of the University:
Authors Note:
Pharmaceutical Benefits Scheme
Name of the Student:
Name of the University:
Authors Note:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

1PHARMACEUTICAL BENEFITS SCHEME
A publication is annually held in Australia regarding the statistics of medicine based on
the production of its drug. The utilization of drug is also taken into account. ASM estimates the
use of drugs by aggregate community by maintaining a data. The use of medicines that are
prescribed by the doctors is necessary to able to intake in Australia. ASM represents Australian
Statistics on Medicine, which maintains the procedure of publication of drugs. It is regulated by
DUSC, which is mainly referred as Drug Utilization Sub-Committee, which is a part of the
committee of pharmaceutical Advisory Benefits (Pearson et al., 2015).
It is essential to have drug utilization that is comprehensive, as they are needed for a large
number of purposes. The purposes range from evaluation and targeting the initiative of quality
use of medicines. The Pharmaceutical Industry also needs it as it helps the authorities of the
financing and regulatory authorities. The main aim of ASM is to put valid and comprehensive
statistics on the use of medicines into the public domain in Australia (Schaffer et al., 2016).
In order to enhance the healthy outcomes and the quality of medicines used in Australia,
there is encouragement for International Collaboration about utilization of drugs. It can be found
out in the publication of International data facilities available in Australia (Page et al., 2015).
PBS processing is done for providing a summary on prescriptions and maintaining its
expenditure. There are availability of various charts and tables for the processing of cost,
prescription volume and drug utilization. The Department of Government Human Resource in
Australia helps in providing wide range of statistical information about various programs on
Medicare (Currow & Sansom 2014). These programs include, MBS ( Medicare Benefits
Schedule ), PBS ( Pharmaceutical Benefits Schedule ), RPBS ( Repatriation Pharmaceutical
Benefits Scheme, AODR ( Australian Organ Donar Register ), AIR ( Australian Immunization
A publication is annually held in Australia regarding the statistics of medicine based on
the production of its drug. The utilization of drug is also taken into account. ASM estimates the
use of drugs by aggregate community by maintaining a data. The use of medicines that are
prescribed by the doctors is necessary to able to intake in Australia. ASM represents Australian
Statistics on Medicine, which maintains the procedure of publication of drugs. It is regulated by
DUSC, which is mainly referred as Drug Utilization Sub-Committee, which is a part of the
committee of pharmaceutical Advisory Benefits (Pearson et al., 2015).
It is essential to have drug utilization that is comprehensive, as they are needed for a large
number of purposes. The purposes range from evaluation and targeting the initiative of quality
use of medicines. The Pharmaceutical Industry also needs it as it helps the authorities of the
financing and regulatory authorities. The main aim of ASM is to put valid and comprehensive
statistics on the use of medicines into the public domain in Australia (Schaffer et al., 2016).
In order to enhance the healthy outcomes and the quality of medicines used in Australia,
there is encouragement for International Collaboration about utilization of drugs. It can be found
out in the publication of International data facilities available in Australia (Page et al., 2015).
PBS processing is done for providing a summary on prescriptions and maintaining its
expenditure. There are availability of various charts and tables for the processing of cost,
prescription volume and drug utilization. The Department of Government Human Resource in
Australia helps in providing wide range of statistical information about various programs on
Medicare (Currow & Sansom 2014). These programs include, MBS ( Medicare Benefits
Schedule ), PBS ( Pharmaceutical Benefits Schedule ), RPBS ( Repatriation Pharmaceutical
Benefits Scheme, AODR ( Australian Organ Donar Register ), AIR ( Australian Immunization

2PHARMACEUTICAL BENEFITS SCHEME
Register) and PIP ( Practice Incentives Programme). Online report of Groups and BS Item use
codes of PBS items, ATC classifications or patient categories.
The reports on the expenditure of the drugs that are highly specialized provides with a
summary of the National expenditure. This expenditure on drugs is dispensed through
Community Access, Private Hospitals or Public Hospitals. This data is quarterly reported in the
present financial year along with the last two financial years as well (Thai et al., 2016).
The Date of supplying tablets and the date of processing provides PSB the information
about expenditure under section 85. The information is updated once in particular month, mainly
around its second week. The ATC groups are also included in their scheme, including with the
market share and PBS sales (Vitry & Roughead 2014).
The pharmaceutical expenditure has risen faster than the economy in Australia presently.
It has leapt up to 1.1 % of gross domestic product from a mere 0.6%. The PBS expenditure has
also rose from 5 to 8 percent on a routine basis. This growth is due party by the increased
utilization and the increased price of dispensed medications (Mellish et al. 2015). The
expenditure on health per capital of different nations for 12 years from 1995 to 2017 is given
below:
Register) and PIP ( Practice Incentives Programme). Online report of Groups and BS Item use
codes of PBS items, ATC classifications or patient categories.
The reports on the expenditure of the drugs that are highly specialized provides with a
summary of the National expenditure. This expenditure on drugs is dispensed through
Community Access, Private Hospitals or Public Hospitals. This data is quarterly reported in the
present financial year along with the last two financial years as well (Thai et al., 2016).
The Date of supplying tablets and the date of processing provides PSB the information
about expenditure under section 85. The information is updated once in particular month, mainly
around its second week. The ATC groups are also included in their scheme, including with the
market share and PBS sales (Vitry & Roughead 2014).
The pharmaceutical expenditure has risen faster than the economy in Australia presently.
It has leapt up to 1.1 % of gross domestic product from a mere 0.6%. The PBS expenditure has
also rose from 5 to 8 percent on a routine basis. This growth is due party by the increased
utilization and the increased price of dispensed medications (Mellish et al. 2015). The
expenditure on health per capital of different nations for 12 years from 1995 to 2017 is given
below:
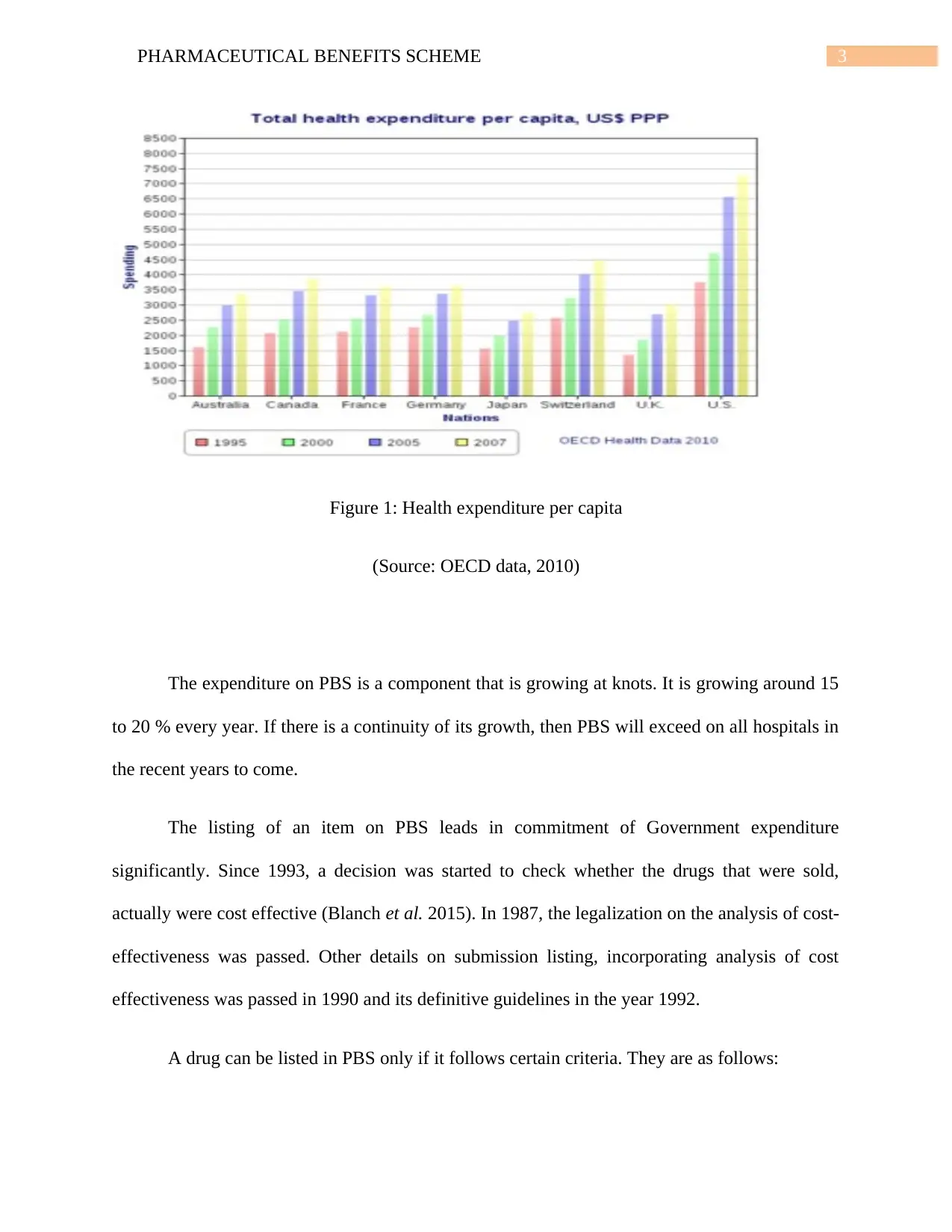
3PHARMACEUTICAL BENEFITS SCHEME
Figure 1: Health expenditure per capita
(Source: OECD data, 2010)
The expenditure on PBS is a component that is growing at knots. It is growing around 15
to 20 % every year. If there is a continuity of its growth, then PBS will exceed on all hospitals in
the recent years to come.
The listing of an item on PBS leads in commitment of Government expenditure
significantly. Since 1993, a decision was started to check whether the drugs that were sold,
actually were cost effective (Blanch et al. 2015). In 1987, the legalization on the analysis of cost-
effectiveness was passed. Other details on submission listing, incorporating analysis of cost
effectiveness was passed in 1990 and its definitive guidelines in the year 1992.
A drug can be listed in PBS only if it follows certain criteria. They are as follows:
Figure 1: Health expenditure per capita
(Source: OECD data, 2010)
The expenditure on PBS is a component that is growing at knots. It is growing around 15
to 20 % every year. If there is a continuity of its growth, then PBS will exceed on all hospitals in
the recent years to come.
The listing of an item on PBS leads in commitment of Government expenditure
significantly. Since 1993, a decision was started to check whether the drugs that were sold,
actually were cost effective (Blanch et al. 2015). In 1987, the legalization on the analysis of cost-
effectiveness was passed. Other details on submission listing, incorporating analysis of cost
effectiveness was passed in 1990 and its definitive guidelines in the year 1992.
A drug can be listed in PBS only if it follows certain criteria. They are as follows:
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

4PHARMACEUTICAL BENEFITS SCHEME
Needed for significant medical treatment or its prevention, that is not covered. It can also
be of not effective cost.
The drugs must be less toxic, more effective than the drug that is listed already for
benefiting the same issue and is accepted for its cost effectiveness.
If the drug is more effective or shows symptoms of speedy recovery besides being safe.
The legality of drugs is taken into account by PBPA on the basis of certain steps listed
below,
They look out on the cost effectiveness and criticality of drugs.
Researching on the prices of alternative brands of the same drug.
Comparing the price of drugs that are ranging in same group of therapeutic drugs.
Estimation of cost information provided by supplier.
Taking in account economies of scale, prescription volumes and all other factors like
storage requirements, date expiration, special manufacturing requirements and product
stability.
Checking the price of the particular drugs in other overseas countries which are relevant.
The PBS (Pharmaceutical Benefits Scheme) refers to a programme of the Australian
Government. Here, the Government provides subsidized prescribed drugs to the residents of
Australia. They also prescribe drugs for foreign visitors, covered by an agreement of Reciprocal
Health Care. The total benefit that has been dispensed under the scheme is given below:
Needed for significant medical treatment or its prevention, that is not covered. It can also
be of not effective cost.
The drugs must be less toxic, more effective than the drug that is listed already for
benefiting the same issue and is accepted for its cost effectiveness.
If the drug is more effective or shows symptoms of speedy recovery besides being safe.
The legality of drugs is taken into account by PBPA on the basis of certain steps listed
below,
They look out on the cost effectiveness and criticality of drugs.
Researching on the prices of alternative brands of the same drug.
Comparing the price of drugs that are ranging in same group of therapeutic drugs.
Estimation of cost information provided by supplier.
Taking in account economies of scale, prescription volumes and all other factors like
storage requirements, date expiration, special manufacturing requirements and product
stability.
Checking the price of the particular drugs in other overseas countries which are relevant.
The PBS (Pharmaceutical Benefits Scheme) refers to a programme of the Australian
Government. Here, the Government provides subsidized prescribed drugs to the residents of
Australia. They also prescribe drugs for foreign visitors, covered by an agreement of Reciprocal
Health Care. The total benefit that has been dispensed under the scheme is given below:
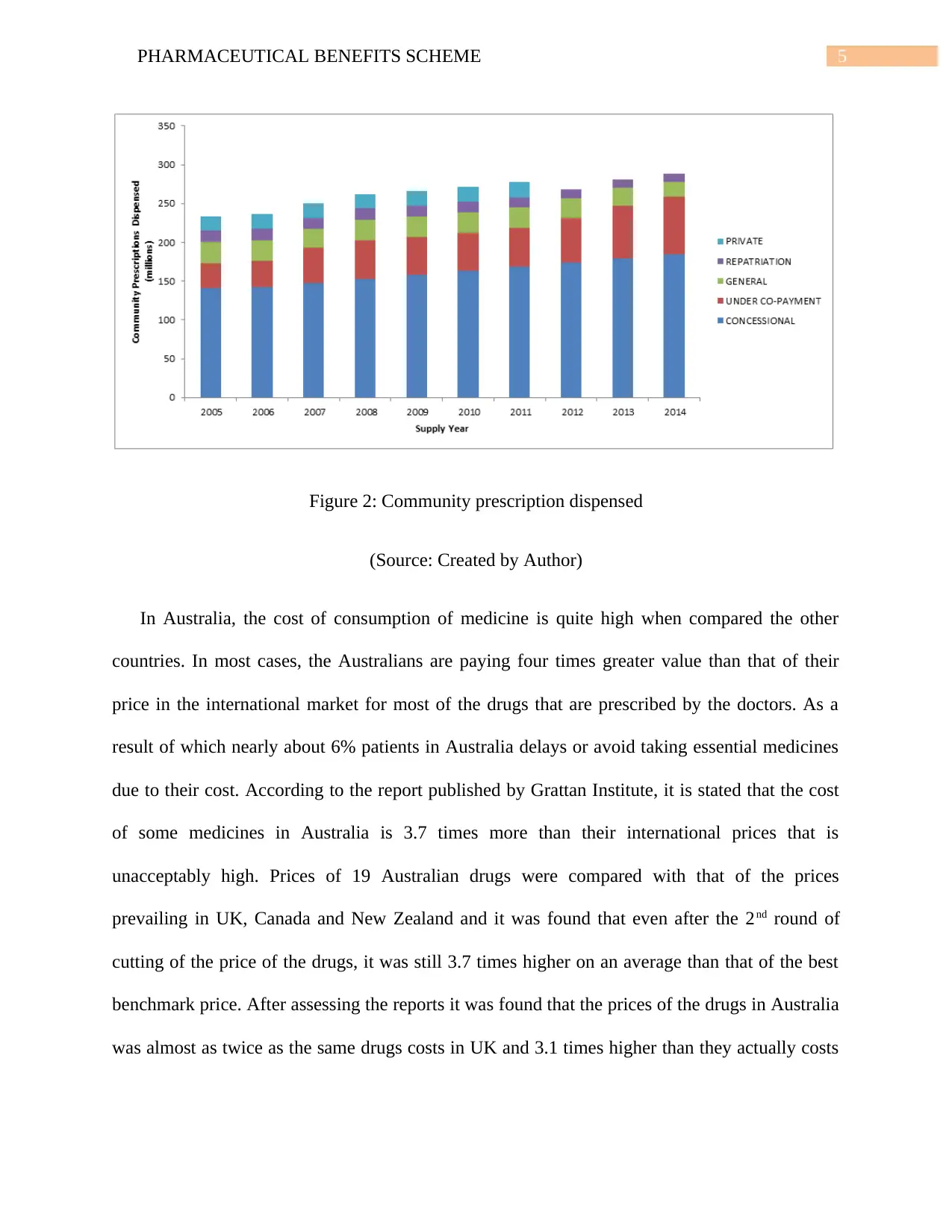
5PHARMACEUTICAL BENEFITS SCHEME
Figure 2: Community prescription dispensed
(Source: Created by Author)
In Australia, the cost of consumption of medicine is quite high when compared the other
countries. In most cases, the Australians are paying four times greater value than that of their
price in the international market for most of the drugs that are prescribed by the doctors. As a
result of which nearly about 6% patients in Australia delays or avoid taking essential medicines
due to their cost. According to the report published by Grattan Institute, it is stated that the cost
of some medicines in Australia is 3.7 times more than their international prices that is
unacceptably high. Prices of 19 Australian drugs were compared with that of the prices
prevailing in UK, Canada and New Zealand and it was found that even after the 2nd round of
cutting of the price of the drugs, it was still 3.7 times higher on an average than that of the best
benchmark price. After assessing the reports it was found that the prices of the drugs in Australia
was almost as twice as the same drugs costs in UK and 3.1 times higher than they actually costs
Figure 2: Community prescription dispensed
(Source: Created by Author)
In Australia, the cost of consumption of medicine is quite high when compared the other
countries. In most cases, the Australians are paying four times greater value than that of their
price in the international market for most of the drugs that are prescribed by the doctors. As a
result of which nearly about 6% patients in Australia delays or avoid taking essential medicines
due to their cost. According to the report published by Grattan Institute, it is stated that the cost
of some medicines in Australia is 3.7 times more than their international prices that is
unacceptably high. Prices of 19 Australian drugs were compared with that of the prices
prevailing in UK, Canada and New Zealand and it was found that even after the 2nd round of
cutting of the price of the drugs, it was still 3.7 times higher on an average than that of the best
benchmark price. After assessing the reports it was found that the prices of the drugs in Australia
was almost as twice as the same drugs costs in UK and 3.1 times higher than they actually costs

6PHARMACEUTICAL BENEFITS SCHEME
in New Zealand. A fact that the government’s price disclosure policy was performing quite
slowly was also added in the report.
According to the report of an established health economist Stephen Duckett, many years had
taken to achieve a policy that led to a considerable cut to the price of generic drugs in Australia.
It is also included in the report that savings of government, tax payers and patient would be much
more that what actually is if an efficient policy would have been taken place.
From the above discussion it is clear that the cost of prescription drugs are significantly high
is Australia. For example, Anaztrozole is a medicine that is used to cure breast cancer. In US, the
market price of 30 tablets of 1mg medicine is $2.45 whereas the same medicine of same quantity
in Australia costs $19.20 that is nearly about 10 times more that it cost in US.
There are some reasons behind high cost of drugs in Australia such as:
No Price control- The Australian government has limited or no control over majority drugs that
are supplied in the market as a result of which drug makers sets their own price without anyone’s
interference.
Competition is Limited – Majority of the drugs in Australia has no real competition to keep the
price level at a reasonable rate. Only one or two companies make those drugs as a result of which
they charge high price for those medicines.
High cost of Production – Development cost and production cost for few medicines are
increasing in Australia. Moreover the cost of conducting research is also becoming quite
expensive as a result of which the price of the medicines are also increasing.
in New Zealand. A fact that the government’s price disclosure policy was performing quite
slowly was also added in the report.
According to the report of an established health economist Stephen Duckett, many years had
taken to achieve a policy that led to a considerable cut to the price of generic drugs in Australia.
It is also included in the report that savings of government, tax payers and patient would be much
more that what actually is if an efficient policy would have been taken place.
From the above discussion it is clear that the cost of prescription drugs are significantly high
is Australia. For example, Anaztrozole is a medicine that is used to cure breast cancer. In US, the
market price of 30 tablets of 1mg medicine is $2.45 whereas the same medicine of same quantity
in Australia costs $19.20 that is nearly about 10 times more that it cost in US.
There are some reasons behind high cost of drugs in Australia such as:
No Price control- The Australian government has limited or no control over majority drugs that
are supplied in the market as a result of which drug makers sets their own price without anyone’s
interference.
Competition is Limited – Majority of the drugs in Australia has no real competition to keep the
price level at a reasonable rate. Only one or two companies make those drugs as a result of which
they charge high price for those medicines.
High cost of Production – Development cost and production cost for few medicines are
increasing in Australia. Moreover the cost of conducting research is also becoming quite
expensive as a result of which the price of the medicines are also increasing.
Paraphrase This Document
Need a fresh take? Get an instant paraphrase of this document with our AI Paraphraser

7PHARMACEUTICAL BENEFITS SCHEME
The Department of Health, Australian Government, it is found that Atorvastatin is the
most commonly prescribed drug in Australia which is used to fight against high blood
cholesterol level, curing pneumonia and also to reduce stomach disorders. Perindopril takes the
second position in the list used to treat high blood pressure that is the second most frequently
used medicine in Australia. At the third place comes Rosuvastatin that is also used to treat heart
diseases, high cholesterol level, etc.
The different other types of medicines that are consumed on daily basis in Australia are
ESOMEPRAZOLE, PARACETAMOL, PANTOPRAZOLE, PERINDOPRIL, AMOXYCILLIN,
CAFALEXIN, AMOXYCILLIN with CLAVULANIC ACID and many more.
The Several issues relating to the Pharmaceutical Benefit Scheme are listed below:
Issues regarding confidentiality – Maintaining the confidentiality of Pharmaceutical
Benefit Scheme is now a big problem. The government has legally issued clear cut
instruction to maintain transparency which include the fact that it is necessary to make
the public aware that such system of PBS exist (Daniels et al., 2017 ).
Problems of future competitors – The government anticipates that how expansion of
the proposed deed is necessary before the enactment of the deed if other medicines are to
be used in the same population. Thus, equality among the competing medicines are
confirmed by this deed (Faunce, 2015).
Problem relating to execution – Execution of the scheme is leading to some issues. The
sponsor and the department must negotiate the deed and finalize during PBAC
recommendation and PBS listing. Simultaneously with the process of finalizing the
The Department of Health, Australian Government, it is found that Atorvastatin is the
most commonly prescribed drug in Australia which is used to fight against high blood
cholesterol level, curing pneumonia and also to reduce stomach disorders. Perindopril takes the
second position in the list used to treat high blood pressure that is the second most frequently
used medicine in Australia. At the third place comes Rosuvastatin that is also used to treat heart
diseases, high cholesterol level, etc.
The different other types of medicines that are consumed on daily basis in Australia are
ESOMEPRAZOLE, PARACETAMOL, PANTOPRAZOLE, PERINDOPRIL, AMOXYCILLIN,
CAFALEXIN, AMOXYCILLIN with CLAVULANIC ACID and many more.
The Several issues relating to the Pharmaceutical Benefit Scheme are listed below:
Issues regarding confidentiality – Maintaining the confidentiality of Pharmaceutical
Benefit Scheme is now a big problem. The government has legally issued clear cut
instruction to maintain transparency which include the fact that it is necessary to make
the public aware that such system of PBS exist (Daniels et al., 2017 ).
Problems of future competitors – The government anticipates that how expansion of
the proposed deed is necessary before the enactment of the deed if other medicines are to
be used in the same population. Thus, equality among the competing medicines are
confirmed by this deed (Faunce, 2015).
Problem relating to execution – Execution of the scheme is leading to some issues. The
sponsor and the department must negotiate the deed and finalize during PBAC
recommendation and PBS listing. Simultaneously with the process of finalizing the

8PHARMACEUTICAL BENEFITS SCHEME
prices, they take place. It is also mentioned that both the parties must execute the deed
before the cut-off date (Parkinson et al., 2015).
Issues regarding timeliness – An essential step towards PBC-subsidizing of a drug is
positive recommendation for listing by PBAC. Although before a drug is to be
subsidized, there are a certain numbers of steps that are need to be followed and these
process entirely consumes a lot of time (Brett et al. 2017). Such steps includes approval
from cabinet, pricing, etc.
prices, they take place. It is also mentioned that both the parties must execute the deed
before the cut-off date (Parkinson et al., 2015).
Issues regarding timeliness – An essential step towards PBC-subsidizing of a drug is
positive recommendation for listing by PBAC. Although before a drug is to be
subsidized, there are a certain numbers of steps that are need to be followed and these
process entirely consumes a lot of time (Brett et al. 2017). Such steps includes approval
from cabinet, pricing, etc.

9PHARMACEUTICAL BENEFITS SCHEME
Reference
Blanch, B., Pearson, S. A., & Haber, P. S. (2014). An overview of the patterns of prescription
opioid use, costs and related harms in Australia. British journal of clinical
pharmacology, 78(5), 1159-1166.
Brett, J., Karanges, E. A., Daniels, B., Buckley, N. A., Schneider, C., Nassir, A., ... & Pearson, S.
A. (2017). Psychotropic medication use in Australia, 2007 to 2015: Changes in annual
incidence, prevalence and treatment exposure. Australian & New Zealand Journal of
Psychiatry, 0004867417721018.
Currow, D. C., & Sansom, L. N. (2014). Uptake of medicines and prescribing patterns in the
palliative care schedule of the Pharmaceutical Benefits Scheme. The Medical journal of
Australia, 200(10), 560-561.
Daniels, B., Lord, S. J., Kiely, B. E., Houssami, N., Haywood, P., Lu, C. Y., ... & Pearson, S. A.
(2017). Use and outcomes of targeted therapies in early and metastatic HER2-positive
breast cancer in Australia: protocol detailing observations in a whole of population
cohort. BMJ open, 7(1), e014439.
Faunce, T. (2015). How the Australia-US free trade agreement compromised the pharmaceutical
benefits scheme. Australian Journal of International Affairs, 69(5), 473-478.
Mellish, L., Karanges, E. A., Litchfield, M. J., Schaffer, A. L., Blanch, B., Daniels, B. J., ... &
Pearson, S. A. (2015). The Australian Pharmaceutical Benefits Scheme data collection: a
practical guide for researchers. BMC research notes, 8(1), 634.
Reference
Blanch, B., Pearson, S. A., & Haber, P. S. (2014). An overview of the patterns of prescription
opioid use, costs and related harms in Australia. British journal of clinical
pharmacology, 78(5), 1159-1166.
Brett, J., Karanges, E. A., Daniels, B., Buckley, N. A., Schneider, C., Nassir, A., ... & Pearson, S.
A. (2017). Psychotropic medication use in Australia, 2007 to 2015: Changes in annual
incidence, prevalence and treatment exposure. Australian & New Zealand Journal of
Psychiatry, 0004867417721018.
Currow, D. C., & Sansom, L. N. (2014). Uptake of medicines and prescribing patterns in the
palliative care schedule of the Pharmaceutical Benefits Scheme. The Medical journal of
Australia, 200(10), 560-561.
Daniels, B., Lord, S. J., Kiely, B. E., Houssami, N., Haywood, P., Lu, C. Y., ... & Pearson, S. A.
(2017). Use and outcomes of targeted therapies in early and metastatic HER2-positive
breast cancer in Australia: protocol detailing observations in a whole of population
cohort. BMJ open, 7(1), e014439.
Faunce, T. (2015). How the Australia-US free trade agreement compromised the pharmaceutical
benefits scheme. Australian Journal of International Affairs, 69(5), 473-478.
Mellish, L., Karanges, E. A., Litchfield, M. J., Schaffer, A. L., Blanch, B., Daniels, B. J., ... &
Pearson, S. A. (2015). The Australian Pharmaceutical Benefits Scheme data collection: a
practical guide for researchers. BMC research notes, 8(1), 634.
Secure Best Marks with AI Grader
Need help grading? Try our AI Grader for instant feedback on your assignments.

10PHARMACEUTICAL BENEFITS SCHEME
Page, E., Kemp-Casey, A., Korda, R., & Banks, E. (2015). Using Australian Pharmaceutical
Benefits Scheme data for pharmacoepidemiological research: challenges and
approaches. Public Health Res Pract, 25(4), e2541546.
Parkinson, B., Sermet, C., Clement, F., Crausaz, S., Godman, B., Garner, S., ... & Elshaug, A. G.
(2015). Disinvestment and value-based purchasing strategies for pharmaceuticals: an
international review. Pharmacoeconomics, 33(9), 905-924.
Pearson, S. A., Pesa, N., Langton, J. M., Drew, A., Faedo, M., & Robertson, J. (2015). Studies
using Australia's Pharmaceutical Benefits Scheme data for pharmacoepidemiological
research: a systematic review of the published literature (1987–
2013). Pharmacoepidemiology and drug safety, 24(5), 447-455.
Schaffer, A. L., Buckley, N. A., Cairns, R., & Pearson, S. A. (2016). Interrupted time series
analysis of the effect of rescheduling alprazolam in Australia: taking control of
prescription drug use. JAMA internal medicine, 176(8), 1223-1225.
Thai, L. P., Moss, J. R., Godman, B., & Vitry, A. I. (2016). Cost driver analysis of statin
expenditure on Australia’s Pharmaceutical Benefits Scheme. Expert review of
pharmacoeconomics & outcomes research, 16(3), 419-433.
Vitry, A., & Roughead, E. (2014). Managed entry agreements for pharmaceuticals in
Australia. Health Policy, 117(3), 345-352.
Page, E., Kemp-Casey, A., Korda, R., & Banks, E. (2015). Using Australian Pharmaceutical
Benefits Scheme data for pharmacoepidemiological research: challenges and
approaches. Public Health Res Pract, 25(4), e2541546.
Parkinson, B., Sermet, C., Clement, F., Crausaz, S., Godman, B., Garner, S., ... & Elshaug, A. G.
(2015). Disinvestment and value-based purchasing strategies for pharmaceuticals: an
international review. Pharmacoeconomics, 33(9), 905-924.
Pearson, S. A., Pesa, N., Langton, J. M., Drew, A., Faedo, M., & Robertson, J. (2015). Studies
using Australia's Pharmaceutical Benefits Scheme data for pharmacoepidemiological
research: a systematic review of the published literature (1987–
2013). Pharmacoepidemiology and drug safety, 24(5), 447-455.
Schaffer, A. L., Buckley, N. A., Cairns, R., & Pearson, S. A. (2016). Interrupted time series
analysis of the effect of rescheduling alprazolam in Australia: taking control of
prescription drug use. JAMA internal medicine, 176(8), 1223-1225.
Thai, L. P., Moss, J. R., Godman, B., & Vitry, A. I. (2016). Cost driver analysis of statin
expenditure on Australia’s Pharmaceutical Benefits Scheme. Expert review of
pharmacoeconomics & outcomes research, 16(3), 419-433.
Vitry, A., & Roughead, E. (2014). Managed entry agreements for pharmaceuticals in
Australia. Health Policy, 117(3), 345-352.
1 out of 11
Related Documents
Your All-in-One AI-Powered Toolkit for Academic Success.
+13062052269
info@desklib.com
Available 24*7 on WhatsApp / Email
![[object Object]](/_next/static/media/star-bottom.7253800d.svg)
Unlock your academic potential
© 2024 | Zucol Services PVT LTD | All rights reserved.



